Abstract
We hypothesize that maternal neurodegeneration, resulting from a chemical, infectious or physical brain injury event, can be causative in the development of autism spectrum disorders (ASD). Following a maternal brain injury event before or during gestation, maternal neural proteins escape the breached blood brain barrier (BBB), triggering the formation of IgG autoantibodies. Subsequently, the autoantibodies cross the placenta and enter the fetal brain causing ASD. We propose the circulating maternal IgG autoantibodies (1) as a potential target for prevention, as a decrease could either possibly prevent ASD or lessen its severity, and (2) as biomarkers for screening, diagnosis and treatment of ASD in infants and children. This research on ASD has the potential to affect health care policies concerning women who are pregnant or planning to become pregnant and lead to novel treatment of ASD.
Keywords
Severity, Autoantibodies
Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by difficulty in communication and repetitive behaviors [1]. ASD definition includes: atypical autism, high-functioning autism, and Asperger’s disorder. Individuals with ASD may have extraordinarily high IQ, normal intellectual abilities, or intellectual disability (ID), known as ASD with ID. Initial diagnosis is often made as early as 18 months of age, but formal diagnosis may occur at 5-8 years [2], with boys four times more likely to be diagnosed than girls [3]. In the United States, in 2014, approximately 1 in 59 children had ASD. Approximately 70 percent of ASD diagnoses are accompanied by an additional condition such as Attention Deficit and Hyperactivity Disorder (ADHD), learning impairment, depression, or anxiety. Children with ASD may also have other disorders [1] such as: intellectual disability, epilepsy, Tourette’s syndrome, difficulty sleeping, and many suffer gastrointestinal dysfunctions. Despite numerous studies on ASD there is little consensus on the mechanism, including whether the cause is genetic or environmental. Approximately 10% of ASD cases have known contributing gene mutations, while 90% are classified as idiopathic, which are thought to be caused by environmental factors.
In ASD, there is increased brain volume and an altered ratio of gray/white matter. The areas of the brain that are affected in ASD patients include the cerebellum, cortex, nuclei of the amygdala, the fusiform face. Cerebellar Purkinje cell numbers decrease while white matter neuron numbers increase [4]. The cerebellum plays an important role in fine motor control, body balance, coordination and movements and in cognition. The cerebellum in individuals with ASD has a 40–50% reduction in nicotinic cholinergic receptors such as α3, α4, and β2 [5].
Neuronal and Glial Proteins as Biomarkers for Neurodegeneration
Neuronal proteins
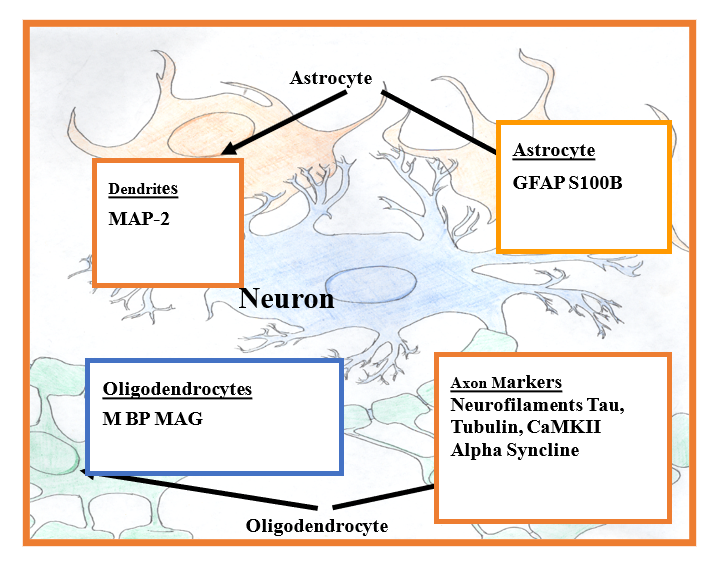
The brain has two billion nerve cells known as neurons and a trillion supporting cells including astrocytes and oligodendrocytes [6]. Neurons have two processes, a single axon and dendrites. Axonal protein components include neurofilament triplet proteins (NFP), tubulin, microtubule associated protein: (tau) is present in the axon; whereas MAP-2 is confined to the dendrites. Calcium/calmodulin kinase II (CaMKII) present in the axonal cytoskeleton phosphorylates cytoskeletal proteins (Figure 1). Although tubulin is present in virtually all eukaryotic cells, it consists of approximately 10-20% of total soluble protein in the brain. Another axonal protein, alpha-synuclein has been shown to function as a neuroprotective protein, particularly with respect to oxidative stress.
Glial proteins
Myelinated axons contain myelin basic protein (MBP), myelin associated glycoprotein (MAG) and neurofascin 155 that are produced by oligodendrocytes in the brain and Schwan cells in peripheral nerves (Figure 1). In neurodegenerative disorders and diseases, there is a decrease of these proteins, resulting in the loss of insulating myelin sheath that consequently causes axonal destruction [7]. GFAP and S-100B, both of which are secreted by the astrocytes, are the only two antigens studied that are not present in the peripheral nervous system; they are consistent with brain injury. GFAP plays an important role in the long-term maintenance of brain cytoarchitecture, proper functioning of the blood brain barrier, and modulation of neuronal function. Loss of astrocytic structural integrity resulting from necrosis, or mechanical disruption causes disintegration of blood brain barrier (BBB) and the release of GFAP and S100B. S100B exerts both detrimental and neutrophic effects, depending on its concentration in brain tissue.
In brain neurodegenerative diseases, certain neural proteins are known to pass into the cerebrospinal fluid (CSF) or leak through the blood brain barrier (BBB) into circulating blood [8]. These proteins are used as biomarkers for these diseases and conditions. For example, in traumatic brain injury (TBI), the neurofilament triplet proteins (NFP) normally found predominately in large myelinated axons [9], are elevated in cerebrospinal fluid (CSF) and serum, as is the case with microtubuleassociated proteins tau which are normally present in both white matter and gray matter [10]. Aggregated Tau is used as a diagnostic marker for Alzheimer’s disease [11,12]. MAP-2, the most abundant microtubuleassociated protein in the mammalian brain, is found in the dendrite [13] and is a biomarker for Purkinje cell damage; it is also a sensitive biomarker for brain lesions related to seizures [14]. Tubulin, another known neurodegenerative biomarker present in almost all eukaryotic cells comprises approximately 10-20% soluble proteins in the brain [13]. Calcium/calmodulin kinase II (CaMKII) that accounts for 12% of all proteins in the brain phosphorylates cytoskeletal proteins such bas MAP-2, tau, tubulin and NFP are known neurodegenerative biomarkers [15]. Myelinated axons contain myelin basic protein (MBP) and myelin associated glycoprotein (MAG). MBP is elevated in multiple sclerosis and stroke [16]. α-synuclein acts as a neuroprotective protein against oxidative stress [17]. The astrocytic protein glial fibrillary acidic protein (GFAP) contributes to white matter architecture, myelination, and integrity of the blood-brain barrier. The S100B, an astrocytic protein, stabilizes proteins associated with microtubules, such as tau and MAP-2. An increase in S100B, in micromolar concentrations, has been observed in TBI and toxic or ischemic brain damage, and has been used as a prognostic marker. At nano concentration, S100B acts as a neurotrophic factor and is used as a biomarker for major depression [18]. Furthermore, Immunoreactivity of S100B is increased in Down’s Syndrome and Alzheimer’s disease [19]. It is important to realize that the use of blood circulated neural proteins as biomarkers for neurodegenerative diseases is limited because of their instability to proteases in blood. For example, the half-life of GFAP in serum is less than 2 days [20] and that of S100B is 2 hours [21].
Hypothesis: Neurodegeneration-Induced Autoimmunity and Autism
We hypothesize that infections, or exposures to chemicals such as air pollutants, heavy metals and pesticides. physical injury of the brain, radiation, shortly before or during pregnancy, can indirectly lead to autism by causing neurodegeneration and release of proteins across impaired BBB and subsequent formation of autoantibodies to neural proteins. Normally B lymphocytes produce antibodies, to proteins, whereas T cells are responsible for cell-mediated immune responses [22]. During pregnancy, maternal IgG antibodies protect the unborn child by crossing the placenta and the fetal BBB during development and persisting in the newborn for up to 6 months postnatal [23]; at 30 weeks it reaches 50% of circulating levels in the mother [24] and exceeds that of the mother, at birth.
Following neuronal cell death, neuronal proteins leak from the damaged neurons and glial cells into circulation, and through the breached BBB. Once in the bloodstream, these proteins act as antigens and activate B lymphocytes to form autoantibodies. Pathogenic autoantibodies (IgG) are produced when the balance between B-cell activation and inhibitory signals is disturbed [25]. IgG autoantibodies cross the placenta and fetal BBB and disrupt the neural development by binding to key neurons in the cerebellum and altering their functions [26]. This neurodevelopmental disruption in the developing fetus leads to ASD [27]. Immunologic risk factors include genetics and family history of autoimmune disease [28]. Our hypothesis is supported and is consistent with the results of many published reports that are summarized below.
Our hypothesis is in agreement with the reports of Croen et al. [29,30] showing presence of mid-pregnancy autoantibodies to fetal brain proteins and their use as early markers for ASD. Similarly, autoantibodies to cerebellum correlate with behavior in ASD children [31]. Several investigators have reported certain autoantibodies in children with ASD and their non-autistic siblings which have been shown to be against unidentified brain proteins [31-39]. The masses of the unknown proteins correspond to particular neuronal and glial proteins [40].
The results of our recent preliminary study showing significant elevation of the autoantibodies against neuronal and glial proteins in the serum of children with ASD and their mothers, compared to age-matched normal control children and their mothers is consistent with our hypothesis [40]. The results revealed a substantial increase of autoantibodies in ASD children and, to a lesser extent, in their mothers, compared to healthy controls in the following ascending order: MAP-2 > NFP > MBP > MAG > α-syncline> S100B and GFAP, CamKII, whereas tubulin and tau were not statistically different from controls. These results support the postulation that ASD is an autoimmune disease [41].
Earlier findings that ASD can be triggered by the mother’s exposure to environmental agents or toxicants shortly before or after conception is in agreement with our hypothesis. The finding that the first or second trimester is the period of neural vulnerability to environmental exposure leading to ASD [28,42] is consistent with our hypothesis. These exposures include maternal infections associated with fever [43], immune activation [44], significant bleeding during the second trimester [45] or occurrences of cytomegalovirus infection during the third trimesters [46] which cause neural degeneration.
Our hypothesis is also consistent with reports of increased development of ASD following exposure to insecticides. Although insecticides were developed to interfere with nervous system functions by causing disruption of neurotransmitters such as GABA by chlorinated hydrocarbons or acetylcholine signaling by organophosphorus compounds leading to animal death [15], recent studies showed that these chemicals also cause neuronal cell death [47,48]. Furthermore, commonly used insecticides are lipid-soluble and are able to pass through the BBB and placenta through endogenous transporters. A case-control study conducted in California found some indication of elevated risk for development of ASD exposure to p,p-DDE [49]. A recent epidemiological study showed that mothers exposed to pesticides near conception increased their likelihood of having children with ASD [50]. The associations between ASD diagnoses or symptoms and exposure to organochlorine, organophosphate, and pyrethroid pesticide during pregnancy has also been reported [51-54]. The finding of Abdel-Rahman et al. [55] of the formation of autoantibodies against neuronal and glial proteins in patients exposed to pesticides who developed neurological symptoms characteristic of those caused by organophosphate insecticides adds more support to our hypothesis.
In agreement with our hypothesis is the development of ASD following parental exposure to environmental toxicants such as diesel, lead, manganese, mercury, methylene chloride, mercury, cadmium, nickel, trichloroethylene, and vinyl chloride [56,57], volatile organic compounds [58], and plasticizers [59-61]. Many of these chemicals have been shown to cause neural cell death [6]. Similarly, the finding that in-utero exposures to valproate that causes cell death [62] and other anticonvulsants also appear to increase the risk for developing autism [63]. Further examples are sychotropic medications [64,65], thalidomide [66], as well as misoprostol in northern Brazil [67].
Neuroprotection from ASD by maternal intake of folic lends a strong support to support our assertion that neural cell death leads to the formation of autoantibodies and subsequent development of ASD. Studies have shown a folic acid-induced neuroprotective mechanism that may be due to anti-apoptotic properties of the folic acid [68]. Studies have also shown that maternal intake of folic acid resulted in diminished risk for ASD in genetically susceptible mothers [69]. It is in agreement with studies showing altered immune function such as autoimmune disorders or cytokine changes in individuals with ASD as well as their mothers [70] that contribute to ASD development [71].
Our hypothesis agrees with opinion that ASD is a neuroimmune and neurodegenerative disorder [72] that involves breakdown of the BBB [73]. It is also in agreement with the reports that in some cases, children with ASD exhibited evidence of activated microglia and astrocytes, elevated 8-oxo-guanosine levels, evidence of oxidative stress, the presence of pro-inflammatory cytokines, and neuronal cell loss [74]. This contrasts with the position of the World Health Organization that considers ASD to be a developmental disorder exclusively affected by environmental factors and genetics, rather than predominately the effect of neurodegeneration [74]. Finally, the presence of autoantibodies in ASD is similar to detection of autoantibodies in plasma from patients with Touretts’s syndrome that was reported to be present in some ASD children [75].
If this hypothesis is confirmed in large studies, ASD may be treated using therapies that render normal B cell function and eliminate pathogenic autoantibodies by selectively depleting antibody producing B cells [76]. A possible treatment is rituximab (rituxan) that is used to reduce B cells, without causing toxicity. Rituximab, a monoclonal antibody against the cell surface receptor, CD20 present in B cells [77]. Several autoimmune diseases have been treated with rituximab including rheumatoid arthritis, granulomatosis with polyangiitis, and other antineutrophil cytoplasmic antibody-associated vasculitis [77,78]. Further, the use of rituximab in treatment of patients with chronic fatigue syndrome has led to a suggestion that chronic fatigue syndrome may have an autoimmune component [79].
Declaration of Interests
The author reports no conflict of interest. The author is solely responsible for the content and writing of the article.
References
2. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Rosenberg CR, White T, Durkin MS. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries. 2018 Apr 27;67(6):1.
3. Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007 Nov 1;120(5):1183-215.
4. Casanova F, Adingupu DD, Adams F, Gooding KM, Looker HC, Aizawa K, Dove F, Elyas S, Belch JJ, Gates PE, Littleford RC. The impact of cardiovascular comorbidities and duration of diabetes on the association between microvascular function and glycaemic control. Cardiovascular diabetology. 2017 Dec;16(1):114.
5. Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, Iversen P, Bauman M, Perry E. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002 Jul 1;125(7):1483-95.
6. Abou-Donia M. Mammalian Toxicology. John Wiley & Sons; 2015 Feb 11.
7. Abou-Donia MB, Conboy LA, Kokkotou E, Jacobson E, Elmasry EM, Elkafrawy P, Neely M, Dale’Bass CR, Sullivan K. Screening for novel central nervous system biomarkers in veterans with Gulf War Illness. Neurotoxicology and teratology. 2017 May 1;61:36-46.
8. Abou-Donia MB, Abou-Donia MM, ElMasry EM, Monro JA, Mulder MF. Autoantibodies to nervous system-specific proteins are elevated in sera of flight crew members: biomarkers for nervous system injury. Journal of Toxicology and Environmental Health, Part A. 2013 Mar 1;76(6):363-80.
9. Tagliaferro P, Ramos AJ, Onaivi ES, Evrard SG, Lujilde J, Brusco A. Neuronal cytoskeleton and synaptic densities are altered after a chronic treatment with the cannabinoid receptor agonist WIN 55,212-2. Brain research. 2006 Apr 26;1085(1):163-76.
10. Liliang PC, Liang CL, Weng HC, Lu K, Wang KW, Chen HJ, Chuang JH. t proteins in serum predict outcome after severe traumatic brain injury. Journal of Surgical Research. 2010 May 15;160(2):302-7.
11. Shiiya N, Kunihara T, Miyatake T, Matsuzaki K, Yasuda K. Tau protein in the cerebrospinal fluid is a marker of brain injury after aortic surgery. The Annals of thoracic surgery. 2004 Jun 1;77(6):2034-8.
12. Salama M, Shalash A, Magdy A, Makar M, Roushdy T, Elbalkimy M, Elrassas H, Elkafrawy P, Mohamed W, Donia MB. Tubulin and Tau: Possible targets for diagnosis of Parkinson’s and Alzheimer’s diseases. PloS one. 2018;13(5).
13. McMurray CT. Neurodegeneration: diseases of the cytoskeleton?. Cell Death & Differentiation. 2000 Oct;7(10):861-5.
14. Ballough GP, Martin LJ, Cann FJ, Graham JS, Smith CD, Kling CE, Forster JS, Phann S, Filbert MG. Microtubule-associated protein 2 (MAP-2): a sensitive marker of seizure-related brain damage. Journal of neuroscience methods. 1995 Sep 1;61(1-2):23-32.
15. Abou-Donia MB. Involvement of cytoskeletal proteins in the mechanisms of organophosphorus ester-induced delayed neurotoxicity. Clinical and experimental pharmacology and physiology. 1995 May;22(5):358-9.
16. Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: the National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen activator Stroke Study. Stroke. 2006 Oct 1;37(10):2508-13.
17. Bido S, Soria FN, Fan RZ, Bezard E, Tieu K. Mitochondrial division inhibitor-1 is neuroprotective in the A53T-a-synuclein rat model of Parkinson’s disease. Scientific reports. 2017 Aug 8;7(1):1-3.
18. Grabe HJ, Ahrens N, Rose HJ, Kessler C, Freyberger HJ. Neurotrophic factor S100beta in major depression. Neuropsychobiology. 2001;44(2):88-90.
19. Griffin WS, Stanley LC, Ling CH, White L, MacLeod V, Perrot LJ, White C3, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proceedings of the National Academy of Sciences. 1989 Oct 1;86(19):7611-5.
20. Diaz-Arrastia R, Wang KK, Papa L, Sorani MD, Yue JK, Puccio AM, McMahon PJ, Inoue T, Yuh EL, Lingsma HF, Maas AI. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. Journal of neurotrauma. 2014 Jan 1;31(1):19-25.
21. Žurek J, Fedora M. The usefulness of S100B, NSE, GFAP, NF-H, secretagogin and Hsp70 as a predictive biomarker of outcome in children with traumatic brain injury. Acta neurochirurgica. 2012 Jan 1;154(1):93-103.
22. Elkon K, Casali P. Nature and functions of autoantibodies. Nature clinical practice Rheumatology. 2008 Sep;4(9):491-8.
23. Bake S, Friedman JA, Sohrabji F. Reproductive agerelated changes in the blood brain barrier: expression of IgG and tight junction proteins. Microvascular research. 2009 Dec 1;78(3):413-24.
24. Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003 Jul 28;21(24):3365-9.
25. Dekker JD, Park D, Shaffer AL, Kohlhammer H, Deng W, Lee BK, Ippolito GC, Georgiou G, Iyer VR, Staudt LM, Tucker HO. Subtype-specific addiction of the activated B-cell subset of diffuse large B-cell lymphoma to FOXP1. Proceedings of the National Academy of Sciences. 2016 Feb 2;113(5):E577-86.
26. Wills S, Rossi CC, Bennett J, Martinez-Cerdeño V, Ashwood P, Amaral DG, Van de Water J. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Molecular autism. 2011 Dec 1;2(1):5.
27. Warren RP, Singh VK, Cole P, Odell JD, Pingree CB, Warren WL, White E. Increased frequency of the null allele at the complement C4b locus in autism. Clinical & Experimental Immunology. 1991 Mar;83(3):438-40.
28. Chess S. Follow-up report on autism in congenital rubella. Journal of autism and childhood schizophrenia. 1977 Mar 1;7(1):69-81.
29. Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biological psychiatry. 2008 Oct 1;64(7):583-8.
30. Croen LA, Matevia M, Yoshida CK, Grether JK. Maternal Rh D status, anti-D immune globulin exposure during pregnancy, and risk of autism spectrum disorders. American journal of obstetrics and gynecology. 2008 Sep 1;199(3):234-e1.
31. Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain, behavior, and immunity. 2011 Mar 1;25(3):514-23.
32. Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Archives of neurology. 2012 Jun 1;69(6):693-9.
33. Braunschweig D, Ashwood P, Krakowiak P, Hertz- Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008 Mar 1;29(2):226- 31.
34. Braunschweig D, Golub MS, Koenig CM, Qi L, Pessah IN, Van de Water J, Berman RF. Maternal autismassociated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. Journal of neuroimmunology. 2012 Nov 15;252(1-2):56-65.
35. Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, Hertz-Picciotto I, Pessah IN, Van de Water J. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Translational psychiatry. 2013 Jul;3(7):e277.
36. Piras IS, Haapanen L, Napolioni V, Sacco R, Van de Water J, Persico AM. Anti-brain antibodies are associated with more severe cognitive and behavioral profiles in Italian children with Autism Spectrum Disorder. Brain, behavior, and immunity. 2014 May 1;38:91-9.
37. Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. Journal of neuroimmunology. 2006 Sep 1;178(1-2):149-55.
38. Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. Journal of neuroimmunology. 2008 Feb 1;194(1-2):165-72.
39. Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain, behavior, and immunity. 2009 Jan 1;23(1):64-74.
40. Abou-Donia MB, Suliman HB, Siniscalco D, Antonucci N, ElKafrawy P, Brahmajothi MV. De novo blood Biomarkers in autism: autoantibodies against neuronal and glial proteins. Behavioral Sciences. 2019 May;9(5):47.
41. Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Antibodies and diseases responsive to the brain. Annu Rev Immunol. 2013 Mar; 31: 345-85.
42. Dufour-Rainfray D, Vourc’h P, Tourlet S, Guilloteau D, Chalon S, Andres CR. Fetal exposure to teratogens: evidence of genes involved in autism. Neuroscience & Biobehavioral Reviews. 2011 Apr 1;35(5):1254-65.
43. Malkova NV, Collin ZY, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain, behavior, and immunity. 2012 May 1;26(4):607-16.
44. Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. Journal of autism and developmental disorders. 2013 Jan 1;43(1):25-33.
45. Torrey EF, Hersh SP, McCabe KD. Early childhood psychosis and bleeding during pregnancy. Journal of autism and childhood schizophrenia. 1975 Dec 1;5(4):287-97.
46. Ivarsson SA, Bjerre I, Vegfors P, Ahlfors K. Autism as one of several disabilities in two children with congenital cytomegalovirus infection. Neuropediatrics. 1990 May;21(02):102-3.
47. Jensen KF, Lapadula DM, Knoth Anderson J, Haykal- Coates N, Abou-Donia MB. Anomalous phosphorylated neurofilament aggregations in central and peripheral axons of hens treated with tri-ortho-cresyl phosphate (TOCP). Journal of neuroscience research. 1992 Nov;33(3):455-60.
48. Abdel-Rahman A, Shetty AK, Abou-Donia MB. Disruption of the blood–brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiology of disease. 2002 Aug 1;10(3):306-26.
49. Lyall K, Croen LA, Sjödin A, Yoshida CK, Zerbo O, Kharrazi M, Windham GC. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal midpregnancy serum samples: association with autism spectrum disorder and intellectual disability. Environmental health perspectives. 2017 Mar;125(3):474-80.
50. Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Current opinion in pediatrics. 2008 Apr 1;20(2):191-7.
51. Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental health perspectives. 2007 May;115(5):792-8.
52. Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environmental health perspectives. 2007 Oct;115(10):1482-9.
53. Roberts EM, English PB. Bayesian modeling of timedependent vulnerability to environmental hazards: an example using autism and pesticide data. Statistics in medicine. 2013 Jun 15;32(13):2308-19.
54. Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environmental health perspectives. 2014 Oct;122(10):1103-9.
55. El Rahman HA, Salama M, El-Hak SA, El-Harouny MA, ElKafrawy P, Abou-Donia MB. A Panel of Autoantibodies Against Neural Proteins as Peripheral Biomarker for Pesticide-Induced Neurotoxicity. Neurotoxicity research. 2018 Feb 1;33(2):316-36.
56. Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay area. Environmental health perspectives. 2006 Sep;114(9):1438-44.
57. von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology (Cambridge, Mass.). 2014 Nov;25(6):851.
58. McCanlies EC, Fekedulegn D, Mnatsakanova A, Burchfiel CM, Sanderson WT, Charles LE, Hertz-Picciotto I. Parental occupational exposures and autism spectrum disorder. Journal of autism and developmental disorders. 2012 Nov 1;42(11):2323-34.
59. Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Current problems in pediatric and adolescent health care. 2014 Nov 1;44(10):277-318.
60. Stein TP, Schluter MD, Steer RA, Guo L, Ming X. Bisphenol A exposure in children with autism spectrum disorders. Autism Research. 2015 Jun;8(3):272-83.
61. Testa C, Nuti F, Hayek J, De Felice C, Chelli M, Rovero P, Latini G, Papini AM. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN neuro. 2012 Apr 27;4(4):AN20120015.
62. Bollino D, Balan I, Aurelian L. Valproic acid induces neuronal cell death through a novel calpain-dependent necroptosis pathway. Journal of neurochemistry. 2015 Apr;133(2):174-86.
63. Campolongo M, Kazlauskas N, Falasco G, Urrutia L, Salgueiro N, Höcht C, Depino AM. Sociability deficits after prenatal exposure to valproic acid are rescued by early social enrichment. Molecular autism. 2018 Dec;9(1):36.
64. Christensen J, Overgaard M, Parner ET, Vestergaard M, Schendel D. Risk of epilepsy and autism in full and half siblings—A population-based cohort study. Epilepsia. 2016 Dec;57(12):2011-8.
65. Wood A. Prenatal exposure to sodium valproate is associated with increased risk of childhood autism and autistic spectrum disorder. Evidence-based nursing. 2014 Jul 1;17(3):84.
66. Strömland K, Nordin V, Miller M, Akerström B, Gillberg C. Autism in thalidomide embryopathy: a population study. Developmental Medicine & Child Neurology. 1994 Apr;36(4):351-6.
67. Miyamoto D, Hirai D, Murayama M. The roles of cortical slow waves in synaptic plasticity and memory consolidation. Frontiers in neural circuits. 2017 Nov 22; 11:92.
68. Quan FS, Yu XF, Gao Y, Ren WZ. Protective effects of folic acid against central nervous system neurotoxicity induced by lead exposure in rat pups. Genetics and Molecular Research. 2015 Jan 1;14(4):12466-71.
69. Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tassone F, Hertz- Picciotto I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. The American journal of clinical nutrition. 2012 Jul 1;96(1):80-9.
70. Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicology and teratology. 2013 Mar 1;36:67-81.
71. Goines P, Van de Water J. The immune system’s role in the biology of autism. Current opinion in neurology. 2010 Apr;23(2):111.
72. Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17(4):422-33.
73. Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Molecular autism. 2016 Dec;7(1):49.
74. Kern JK, Geier DA, Sykes LK, Geier MR. Evidence of neurodegeneration in autism spectrum disorder. Translational neurodegeneration. 2013 Dec;2(1):17.
75. Cheng YH, Zheng Y, He F, Yang JH, Li WB, Wang ML, Cui DY, Chen Y. Detection of autoantibodies and increased concentrations of interleukins in plasma from patients with Tourette’s syndrome. Journal of Molecular Neuroscience. 2012 Sep 1;48(1):219-24.
76. Isenberg DA. B cell targeted therapies in autoimmune diseases. The Journal of Rheumatology Supplement. 2006 May 1;77:24-8.
77. Edwards JC, Szczepanski L, Szechinski J, Filipowicz- Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell–targeted therapy with rituximab in patients with rheumatoid arthritis. New England Journal of Medicine. 2004 Jun 17;350(25):2572-81.
78. Edwards JC, Cambridge G, Leandro MJ. B cell depletion therapy in rheumatic disease. Best Practice & Research Clinical Rheumatology. 2006 Oct 1;20(5):915- 28.
79. Fluge Ø, Mella O. Clinical impact of B-cell depletion with the anti-CD20 antibody rituximab in chronic fatigue syndrome: a preliminary case series. BMC neurology. 2009 Dec;9(1):28.