Abstract
Introduction: This study aimed to assess the efficacy of a 16-week at-home high-intensity interval training (HIIT) program among individuals with spinal cord injury (SCI).
Method: Eight individuals (3 females) with chronic SCI below the sixth thoracic vertebrae participated in a 16-week at-home HIIT program using an arm ergometer. Participants completed baseline graded exercise tests to determine target heart rate zones. HIIT was prescribed three times per week. Each training session included six one-minute bouts with a target heart rate ~80% heart rate reserve (HRR), interspersed with two minutes of recovery (~30% HRR). A portable heart rate monitor and phone application provided visual feedback during training and allowed for measurements of adherence and compliance. Surveys were administered to assess participation, self-efficacy, and satisfaction.
Results: Participants demonstrated a decrease in submaximal cardiac output by ~17% (P=0.028) and an increase in peak power output by ~26% (P=0.027) following HIIT. An 87% adherence rate was achieved during the HIIT program. Self-reported metrics of satisfaction and self-efficacy with at-home HIIT scored moderate to high.
Conclusion: Participants demonstrated an improvement in cardiac efficiency during submaximal exercise, and maximal work capacity following at-home HIIT. Additionally, participant adherence, compliance, satisfaction, and self-efficacy metrics suggest that at-home HIIT was easily implemented and enjoyable.
Keywords
High-intensity Interval Training, Cardiovascular Health, VO2max, Arm Ergometry, Spinal Cord Injury
Introduction
Individuals with spinal cord injury (SCI) often experience motor impairments below the level of injury, leading to worsened levels of physical activity, a greater prevalence of cardiovascular disease, and reduced life expectancy [1]. Some evidence has suggested that the prevalence of heart disease may be ~3 times greater among individuals with SCI compared to able-bodied individuals (17.1% versus 4.9%, respectively) [2]. Regular exercise is critical for mitigating cardiometabolic disease risk factors such as hypertension, dyslipidemia, glucose intolerance, and obesity [3–6]. Current guidelines recommend ~90 minutes or more of moderate to vigorous aerobic exercise per week to improve cardiometabolic outcomes among individuals with SCI [7]. However, those with SCI experience substantial barriers to exercise, such as poor access to facilities, unaffordable equipment, and fear of self-injury [8].
High-intensity interval training (HIIT) is a method of exercise where individuals engage in repeated intervals of intense activity, each followed by a brief period of recovery. HIIT has previously demonstrated substantial improvements in cardiometabolic health in the able-bodied population [9–11]. Additionally, HIIT may shorten the duration of activity required to elicit similar cardiometabolic outcomes compared to continuous, moderate-intensity aerobic exercise [12,13]. Shortening the activity duration may mitigate repeated-use injuries in the upper extremities for individuals with SCI and improve adherence to a prescribed exercise routine. For these reasons, HIIT serves as a promising exercise intervention to improve cardiometabolic health among individuals with SCI.
The efficacy of HIIT in improving cardiorespiratory fitness among individuals with SCI remains unclear [12,14]. Several investigations implementing a 6-week HIIT program among individuals with chronic SCI (>1 year) have demonstrated no marked changes in cardiorespiratory fitness [13,15,16]. Due to the time and resource-intensive nature of these studies, HIIT programs were implemented for 6 weeks, which may be an insufficient duration to elicit marked changes in cardiorespiratory fitness. Thus, additional research is needed to help determine the efficacy of HIIT and program duration necessary to elicit cardiorespiratory improvements.
Overall, this study aimed to assess the efficacy of a 16-week at-home HIIT program among individuals with paraplegia due to chronic SCI. The primary outcomes included peak oxygen uptake (V̇O2peak), peak power output, and training adherence/compliance. We hypothesized that the at-home HIIT program would elicit improvements in V̇O2peak and peak power output, with favorable training adherence and compliance. A priori determined exploratory analyses were performed to investigate whether 8 weeks of at-home HIIT alone was sufficient to elicit significant changes in primary outcomes.
Materials and Methods
Study design
All experimental procedures were approved by the Mayo Clinic Institutional Review Board (IRB# 18-004972) and registered on clinicaltrials.gov (NCT04378218). Experimental procedures were performed in accordance with the ethical standards set by the Declaration of Helsinki. Participants provided written informed consent prior to enrollment. This study conforms to all STROBE guidelines and reports the required information accordingly (see Supplementary Checklist).
Participants
Ten participants diagnosed with chronic SCI below the sixth thoracic vertebrae (T6) were recruited from the Mayo Clinic SCI database and enrolled in this clinical trial. Following enrollment, one participant chose to withdraw to relocate and begin a different training program for an upcoming sporting event. Another participant completed the trial but was removed from the dataset due to discovery of a cervical (C7) lesion level (finding occurred during subsequent screening for another study, contradictory to medical record indicating a T7 lesion level). Eight eligible participants completed the trial, presented in Table 1. Due to institutional closures and restrictions during the COVID-19 pandemic, only five participants were able to return for in-person laboratory testing.
|
Participant |
Sex |
LOI |
AIS grade |
Age (years) |
Time Since Injury (years) |
Training Sessions (n) |
Adherence (%) |
Successful Intervals >80% HRR, n (%) |
Successful Intervals >70% HRR, n (%) |
Successful Intervals <30% HRR, n (%) |
|
P1 |
F |
T12 |
C |
54 |
8 |
48 |
100% |
159 (55%) |
252 (88%) |
5 (2%) |
|
P2 |
F |
T12 |
A |
56 |
28 |
48 |
100% |
253 (88%) |
286 (99%) |
87 (30%) |
|
P3 |
F |
T8 |
A |
54 |
15 |
46 |
96% |
217 (79%) |
269 (98%) |
209 (77%) |
|
P4 |
M |
T12 |
NA |
58 |
25 |
48 |
100% |
177 (62%) |
267 (93%) |
55 (19%) |
|
P5 |
M |
T7 |
A |
39 |
4 |
39 |
81% |
165 (71%) |
205 (88%) |
84 (35%) |
|
P6 |
M |
T11 |
A |
39 |
13 |
48 |
81% |
26 (9%) |
123 (43%) |
143 (53%) |
|
P7 |
M |
T10 |
A |
27 |
2 |
48 |
100% |
258 (90%) |
264 (92%) |
90 (32%) |
|
P8 |
M |
L4 |
A |
46 |
19 |
18 |
38% |
33 (31%) |
52 (48%) |
31 (29%) |
|
Mean±SD |
-- |
-- |
-- |
47±11 |
14±9 |
42±10 |
87±20% |
57±28% |
80±21% |
35±23% |
|
Adherence is defined as the percentage of training sessions undertaken out of the total 48 prescribed. Compliance is defined as the percentage of ‘successful’ intervals where the target range of heart rate reserve (HRR) was achieved. Abbreviations: F: Female; M: Male; LOI: Level of Injury; AIS: ASIA Impairment Scale; HRR: Heart Rate Reserve; SD: Standard Deviation. *NA: Data not available within medical records. |
||||||||||
To be eligible for inclusion, participants must have been at least 18 years of age and reported the use of a manual wheelchair as a primary means of mobility. Individuals were excluded if the SCI occurred at or above T6 due to confounding influences on cardiac autonomic innervation [17]. Additionally, individuals were excluded if the injury occurred less than six months prior to enrollment or if they were diagnosed with any contraindicated health condition for participation in an exercise program. No participants were taking blood pressure medications during the study.
Laboratory testing
Participants attended three laboratory testing sessions during the study: 1) at baseline, 2) after eight weeks of HIIT, and 3) after 16 weeks of HIIT. Due to COVID-19 related institutional closures, graded exercise tests performed at baseline, week 8, and week 16 of HIIT were only available in five participants. Each laboratory visit included a graded exercise test to task failure using an arm cycle ergometer (Model 891E Upper Body Ergometer, Monark Exercise AB, Vansbro, Sweden). Following familiarization with the test protocol and arm cycle, participants selected a comfortable pedaling cadence between 60 and 70 revolutions per minute and were instructed to maintain this cadence during the test within three revolutions per minute. Because of the mechanical nature of the arm cycle, starting workloads varied for each participant due to the individually selected cadence. Each graded exercise test began with two minutes of unloaded pedaling followed by gradual increases in resistance by adding 0.1–0.2 kg to the flywheel every two minutes until task failure. Starting power output and stepwise increases for all participants was 15±3 W (Range 12–20 W). The graded exercise test protocols (cadence and stepwise increases in power output) were held similar for each participant between laboratory visits. Resting and peak heart rate obtained from the baseline graded exercise test (before HIIT) were used to determine individualized target heart rates for at-home training sessions.
For each graded exercise test, participants breathed through a mouthpiece connected to a three-way T-shape non-rebreathing pneumatic sliding valve (Series 8500, Hans Rudolph, Shawnee, KS, USA) and were instrumented with a 12-lead ECG and patient monitoring system (Cardiocap/5, Datex, Louisville, CO, USA). During the graded exercise tests, breath-by-breath respiratory measurements were performed using a cardiorespiratory diagnostic system (CPXD, MGC Diagnostics, St. Paul, MN, USA) interfaced with a mass spectrometer (MGA-11000, Perkin Elmer, Waltham, MA, USA). Before each test, the metabolic cart was calibrated using a two-point procedure with pre-mixed reference gases (room air: 0.03% CO2, 21.1% O2; calibration gas: 12% O2, 5% CO2). Respiratory gas exchange measurements and heart rate were averaged across the last 30 seconds of each stage of the graded exercise test. V?O2peak was taken as the average of breaths during the last 30 seconds prior to task failure.
Cardiac output was measured during the graded exercise tests using a previously described open-circuit acetylene wash-in technique [18]. Cardiac output was assessed three times during each graded exercise test; 1) at rest, 2) during exercise at a submaximal intensity (respiratory exchange ratio ~1.0 during baseline visit), and 3) immediately prior to task failure. The power output at which the submaximal intensity measurement was taken was held constant between visits. Briefly, participants were switched from inspiring room air to a pre-mixed, commercially available wash-in gas mixture stored in a Douglas bag containing 0.9% helium, 0.6% acetylene, 21% oxygen, and balanced with nitrogen. Upon switching, participants were instructed to breathe normally for 8–12 breaths. Cardiac output was then estimated from the rate of disappearance of acetylene. Stroke volume was taken as the quotient of cardiac output and heart rate.
At-home HIIT training
A novel HIIT program was implemented in accordance with collaborating clinical guidance and a recent meta-analysis recommending high-intensity bouts of ≥2 minutes duration and total session length of ≥15 minutes [19]. After study enrollment, participants underwent an at-home training session to determine HIIT protocol characteristics and ensure proper equipment function/set-up. The instructed minimal cadence was ~40 revolutions per minute. Participants then underwent a supervised training session to ensure correct resistance adjustments and execution of high and low intensity bouts according to heart rate targets.
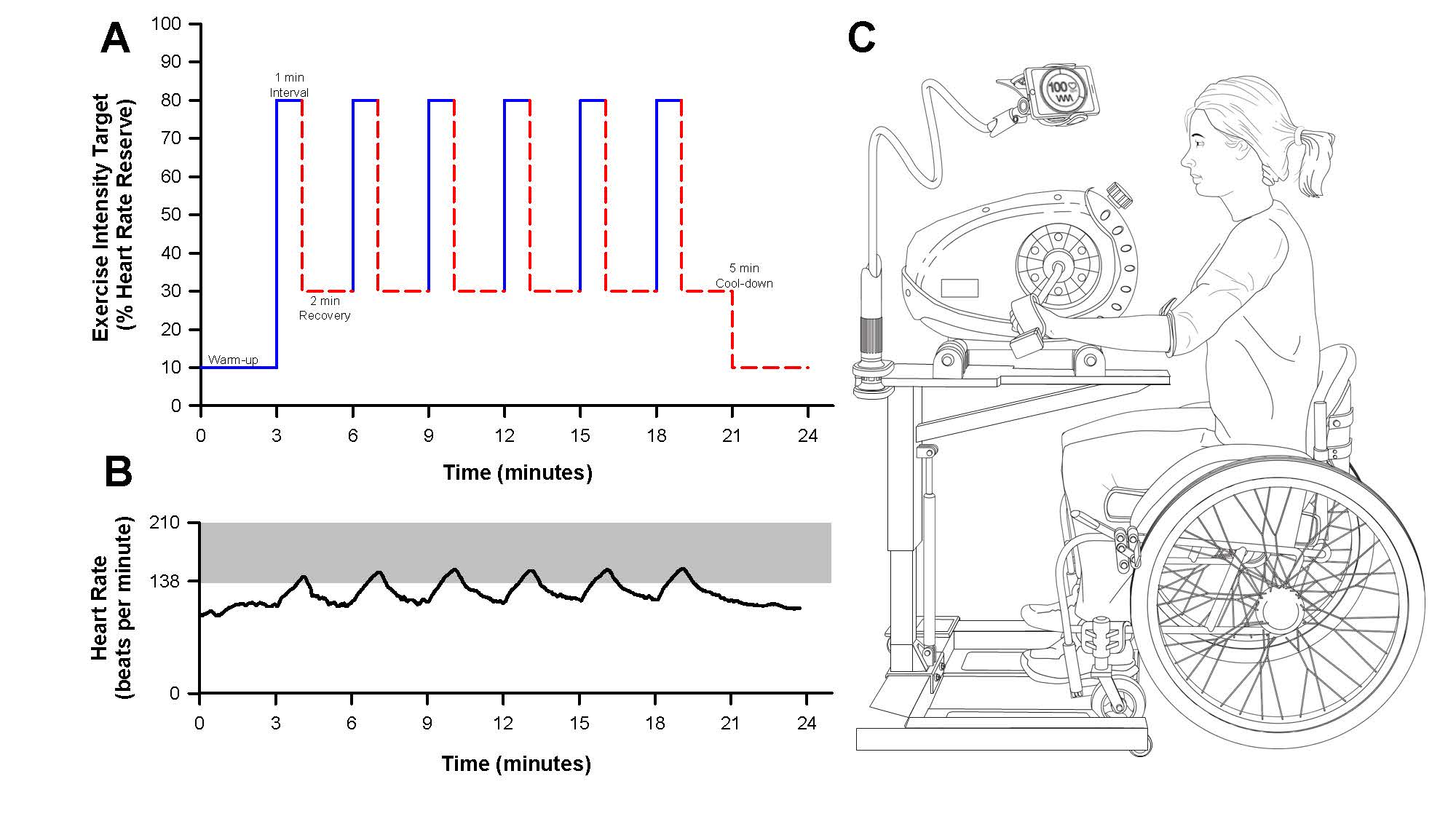
Each participant was instructed to complete three at-home HIIT training sessions per week, totaling 48 structured HIIT sessions over 16 weeks. Each training session was 24 minutes in length and structured as follows: three-minute warmup, six one-minute high-intensity intervals with a target intensity at 80% of the individuals’ heart rate reserve (HRR, calculated using the Karvonen formula) with two minutes of active recovery, and a three-minute cooldown (Figure 1A and 1B). Of note, the heart rate kinetics during the first one to two intervals were often not rapid enough to achieve the target heart rate of 80% HRR. Therefore, if a participant reached a threshold of >70% HRR, we deemed the interval acceptable in achieving the high-intensity target. Heart rate targets were kept consistent throughout the training period and thus the training program was not considered progressive.
At-home training sessions were performed using an arm cycle mounted to a wheelchair-accessible hydraulic table (PhysioTrainer UBE and Hydraulic UBE Table, HCI Fitness, Langley, WA, USA). Each in-home unit was provided and installed in the participants’ homes by the study team (Figure 1C). Participants were provided with a portable heart rate monitor (Polar H10 Heart Rate Sensor, Polar Electro Inc., Bethpage, NY, USA) that was integrated with a smartphone using a commercially available applications (Polar Beat and Polar Coach Applications, Polar Electro Inc., Bethpage, NY, USA). Training sessions were guided by real-time visual heart rate through the phone application. Heart rate and interval durations during the training sessions were measured using the phone application. From these data, participant adherence (completion of prescribed training sessions) and compliance (the achievement of target heart rate zones during bouts of high intensity and recovery) were determined.
Figure 1. At-home high-intensity interval training (HIIT) study design. A) Schematic depicting an example of an at-home HIIT session. The target exercise intensity (% of heart rate reserve) is depicted for one-minute bouts of high intensity followed by two minutes of recovery. B) Representative figure of participant heart rate data during an at-home HIIT session obtained from the Polar phone application. The shaded grey-area depicts the target heart rate zone corresponding to a value at or above ~80% HRR. C) Schematic of the participant equipment provided for at-home HIIT. Participants were given an arm cycle, hydraulic table, and Polar heart rate strap to provide visual feed-back and instruction to achieve target exercise intensity during the training sessions. The participant’s phone was mounted to the hydraulic table to allow for visual heart rate feedback.
Survey assessments
Several surveys were developed by the study team for this study to determine participant-reported levels of safety (Supplementary Table 1). Previously validated surveys did not encapsulate all areas of interest, and thus novel surveys were constructed to record areas of interest for collaborating clinicians to help guide future practice. Participants were contacted to ensure complete survey data and accurate reporting of adverse events. Participant communication excluded any coaching or encouragement to avoid confounding investigator influence. Additionally, the SCI Exercise Self-Efficacy Scale (SCI ESES) was used to assess participants’ confidence regarding carrying out regular exercise [20]. The SCI ESES consists of a 4-point rating scale (1: not at all true, 4: always true). The SCI ESES was completed at three time points (before, during, and after HIIT) to assess changes in confidence in performing exercise. A final survey, administered after the study, was developed to assess participant satisfaction with the at-home HIIT exercise program (Table 2).
|
Participant |
Q1 |
Q2 |
Q3 |
Q4 |
|
P1 |
Yes |
Yes |
Yes |
Yes |
|
P2 |
No |
Yes |
Yes |
No |
|
P3 |
Yes |
Yes |
Yes |
No |
|
P5 |
Yes |
Yes |
Yes |
No |
|
P6 |
Yes |
Yes |
Yes |
No |
|
P7 |
Yes |
Yes |
Yes |
Yes |
|
P8 |
Yes |
Yes |
No |
Yes |
|
Survey Questions: Q1: Do you like performing this HIIT exercise program? Q2: Would you recommend HIIT exercise to a friend? Q3: Would you continue performing HIIT as part of your exercise routine? Q4: Did your physical activity increase outside of the HIIT exercise program? Satisfaction survey questions and results for each participant following 16 weeks of at-home HIIT. Note, participant four (P4) did not complete the survey. Abbreviations: Q: Question. |
||||
Statistical analyses
Data normality was confirmed using Shapiro-Wilk tests. Physiological data obtained throughout the graded exercise tests (heart rate, cardiac output, stroke volume, V?O2, and O2 pulse) were examined at three intensities: 1) rest, 2) a submaximal intensity (respiratory exchange ratio ~1.0), and 3) peak intensity. One-way repeated measures analysis of variance (ANOVA) models were used to determine the effects of 8- and 16- weeks of at-home HIIT on physiological data, training adherence, training compliance, and reported measures of self-efficacy. When appropriate, a Bonferroni post-hoc test was used to correct for multiple comparisons and to determine where differences occurred (between baseline, 8-weeks, and 16-weeks specifically). Additionally, a one-way ANOVA model was used to detect differences in training compliance in terms of achieving target heart rate zones between the first eight weeks and the second eight weeks of HIIT. To aid interpretation of effect sizes, partial eta squared (ηp²) values are reported for all ANOVA models. Consistent with conventional benchmarks within the field, ηp² values of approximately 0.2, 0.4, and 0.7 are interpreted as small, medium, and large effects, respectively [21].
A linear regression was performed to examine the relationship between the age-predicted maximal heart rate and the maximal heart rate achieved during the first graded exercise test to examine if the age-predicted maximal heart rate would serve as a ‘good’ target among this patient population. All statistics were performed using SigmaStat (Version 4, Systat, Palo Alto, CA), and a priori statistical significance was set as P<0.05. Descriptive statistics are presented as mean ± standard deviation (SD).
Results
Graded exercise tests
The effects of HIIT on cardiorespiratory parameters during the graded exercise test are presented in Table 3. V̇O2peak did not significantly improve with HIIT (P=0.064, ηp2=0.498, n=5). However, peak power output improved by ~26% at 16-weeks relative to baseline (P=0.027, ηp2=0.593, n=5). There was no effect of HIIT on resting cardiac output (P=0.252, ηp2=0.368, n=4), heart rate (P=0.464, ηp2=0.175, n=5), or stroke volume (P=0.304, ηp2=0.328, n=4). Relative to baseline measures, submaximal cardiac output decreased by ~17% following 16 weeks of at-home HIIT (P=0.028, ηp2=0.695, n=4). There was no significant effect of HIIT on heart rate nor stroke volume at submaximal intensity (P>0.749, ηp2<0.065, power output: 36±6 W, n=4). There was no significant effect of at-home HIIT on peak cardiac output, stroke volume, or heart rate (P>0.361 ηp2<0.225).
|
|
Intensity |
Baseline |
Week 8 |
Week 16 |
P-value |
ηp2 |
n |
|
Heart Rate (beats per minute) |
Rest |
83±11 |
83±10 |
85±11 |
0.464 |
0.175 |
5 |
|
Submaximal |
128±27 |
129±16 |
124±20 |
0.764 |
0.065 |
5 |
|
|
Peak |
168±12 |
169±11 |
172±14 |
0.361 |
0.225 |
5 |
|
|
Stroke Volume (mL/beat) |
Rest |
106±31 |
92±9 |
86±17 |
0.304 |
0.328 |
4 |
|
Submaximal |
87±25 |
81±10 |
82±15 |
0.749 |
0.070 |
4 |
|
|
Peak |
92±14 |
91±21 |
84±10 |
0.679 |
0.121 |
4 |
|
|
Cardiac Output (L/min) |
Rest |
8.6±2.3 |
7.6±1.4 |
7.2±1.3 |
0.252 |
0.368 |
4 |
|
Submaximal |
11.7±1.5 |
10.5±0.5 |
9.7±0.2* |
0.028 |
0.695 |
4 |
|
|
Peak |
15.5±1.5 |
16.7±4.0 |
14.6±1.8 |
0.771 |
0.083 |
4 |
|
|
V̇O2 (L/min) |
Rest |
0.34±0.06 |
0.31±0.07 |
0.28±0.09 |
0.167 |
0.361 |
5 |
|
Submaximal |
0.98±0.10 |
0.99±0.10 |
1.02±0.11 |
0.582 |
0.126 |
5 |
|
|
Peak |
1.52±0.31 |
1.67±0.26 |
1.71±0.25 |
0.064 |
0.498 |
5 |
|
|
O2 Pulse (mL/beat) |
Rest |
4.1±0.3 |
3.8±0.9 |
3.3±0.9 |
0.150 |
0.378 |
5 |
|
Submaximal |
7.9±1.4 |
7.8±1.6 |
8.4±1.9 |
0.486 |
0.165 |
5 |
|
|
Peak |
9.1±2.2 |
10.0±1.8 |
10.0±1.5 |
0.063 |
0.499 |
5 |
|
|
PPO (W) |
Peak |
70±14 |
82±12 |
88±15* |
0.027 |
0.593 |
5 |
|
Cardiorespiratory data during high-intensity interval training (HIIT). Data were obtained from graded exercise tests using an arm cycle performed at baseline, after 8 weeks of HIIT, and after 16 weeks of HIIT. Data are presented for each graded exercise test at rest, a submaximal exercise intensity (corresponding to a respiratory exchange ratio of ~1.0), and at peak intensity. Note that graded exercise tests at baseline, week 8, and week 16 were only available in five participants (P2, P3, P5, P6, P7) due to intermittent institutional closures related to the COVID-19 pandemic. Additionally, some data are limited to four participants (P3, P5, P6, P7) due to aberrant gas detections during acetylene wash-in. Data are presented as mean ± SD. Significant P-values are denoted in bold text. Partial eta squared (ηp²) is reported as a measure of effect size. Effect size interpretation: small = 0.2, medium = 0.4, large = 0.7. Abbreviations: V̇O2, oxygen uptake; PPO, peak power output; ηp2, partial eta squared; n, number of participants. *Denotes statistically different from baseline measure. |
|||||||
Training adherence and compliance
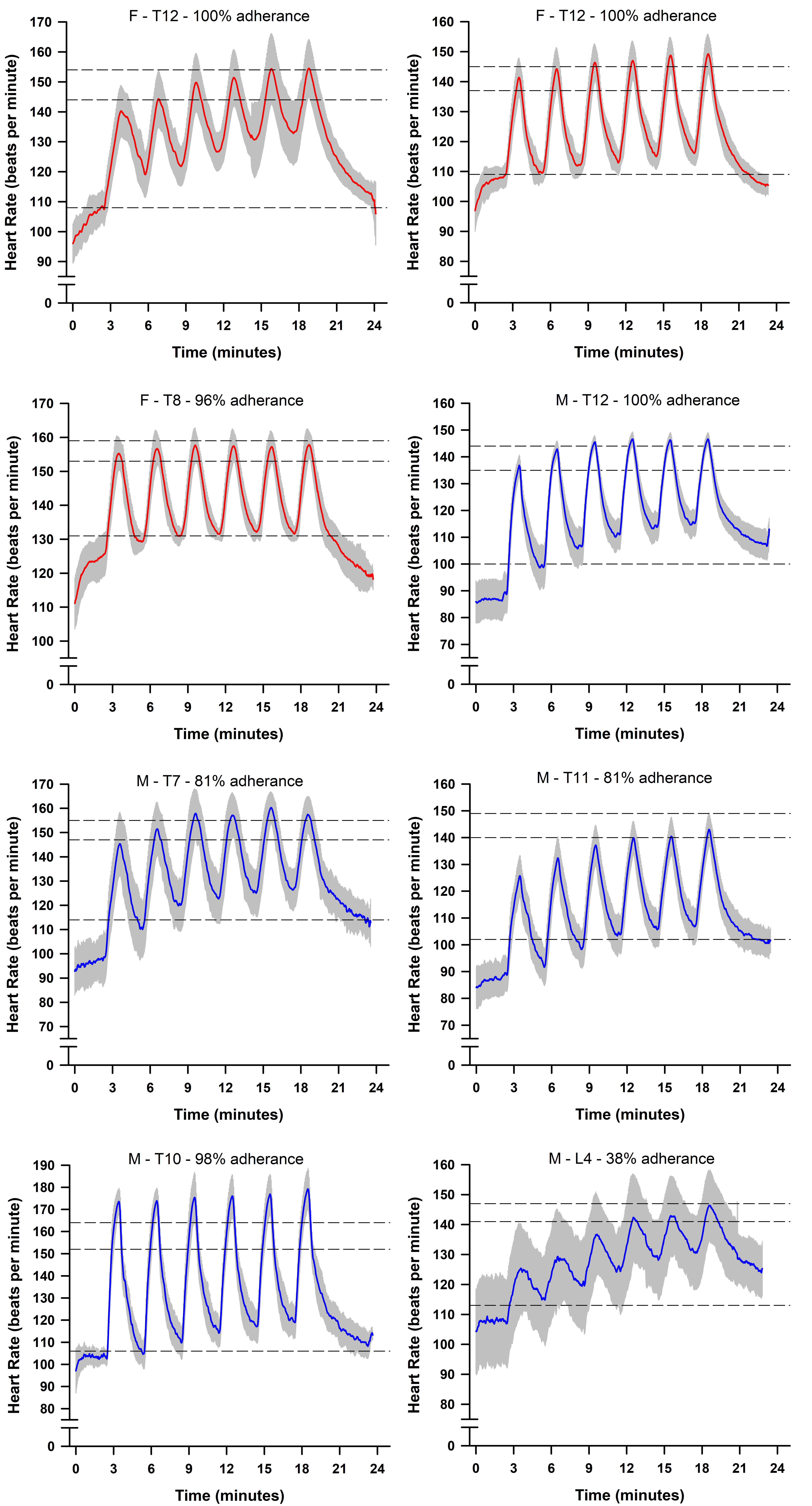
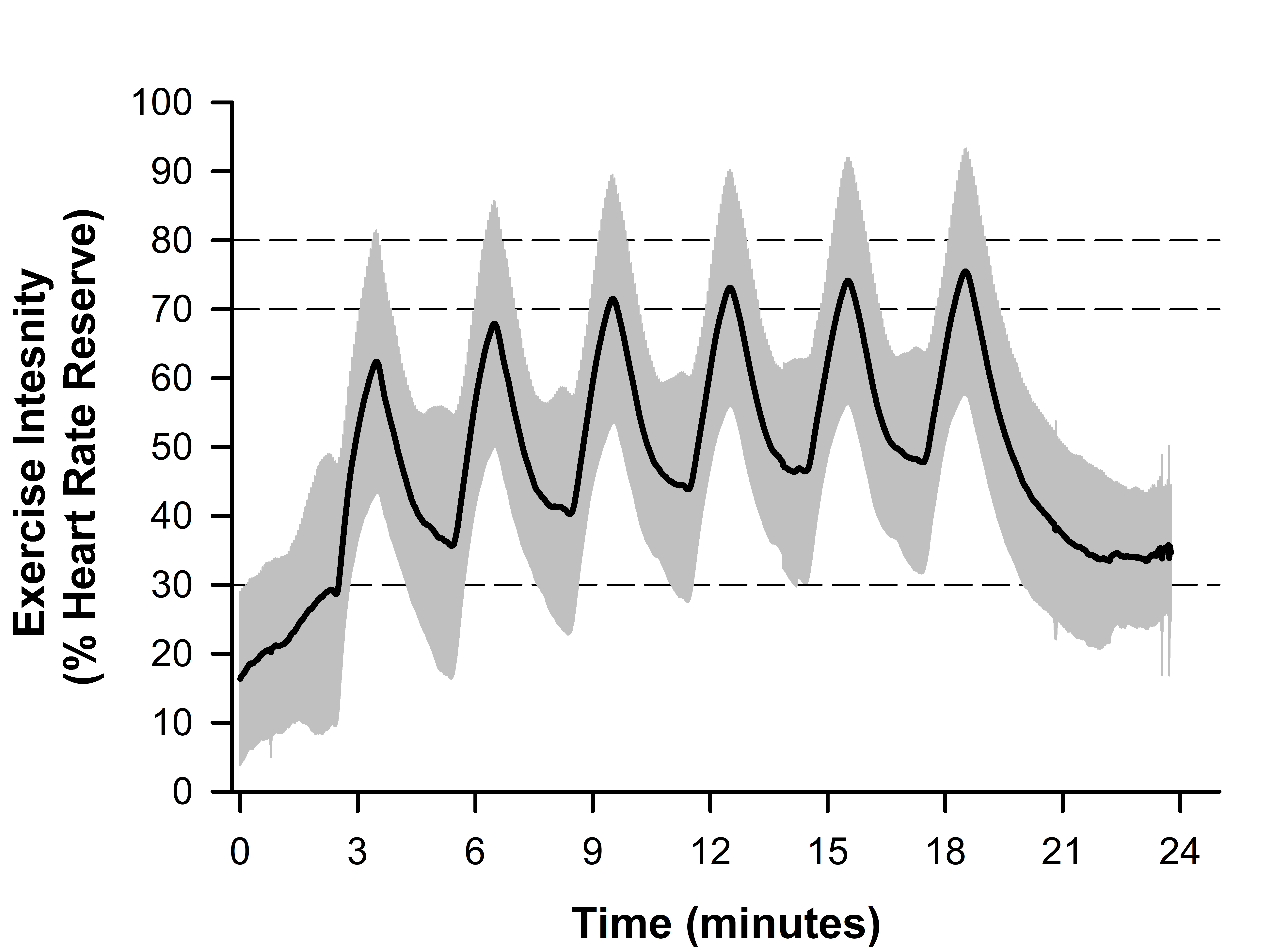
Data regarding HIIT adherence and compliance are presented in Table 1. Overall adherence to the training was 87±20% (Range: 38-100%). Figure 2 depicts the average compliance for all eight participants during the at-home HIIT sessions. Within the 24-minute HIIT sessions, participants demonstrated good compliance in achieving a heart rate corresponding to 70% HRR (80±21%, Range: 43-99%). However, participants were less compliant in achieving the target heart rate corresponding to 80% HRR during training (57±28%, Range: 9-92%). Most participants demonstrated the ability to achieve a heart rate greater than 70% HRR during the latter half of the six high-intensity intervals. Conversely, the target heart rate was frequently unachieved during the two-minute bouts of recovery. During bouts of recovery, the target heart rate was only attained ~35% of the time (Range: 1-77%). Figure 3 shows the composite heart rate data for all participant training sessions (average of 253 training intervals across all participants).
Figure 2. Line plots depicting individual participant heart rate data achieved during the at-home high-intensity interval training (HIIT) sessions. The solid line depicts the average heart rate across all completed HIIT sessions, and the grey bands depict the standard deviation. The top dashed line represents the prescribed high-intensity target of 80% heart rate reserve (HRR), the middle-dashed line represents the acceptable high-intensity threshold of 70% HRR, and bottom dashed line represents the recovery target of 30% HRR. Abbreviations: M: Male; F: Female; T: Thoracic; HRR: Heart Rate Reserve.
Figure 3. Line plot depicting the composite high-intensity interval training (HIIT) session data for all participants across all attempted workouts (n=338). Data is normalized to the percent of heart rate reserve (HRR). The solid black line depicts the average heart rate, and the grey bands depict the standard deviation. Dashed lines at 70 and 80% HRR represent acceptable and target HRR for each interval, respectively, and the dashed line at 30% HRR represents the recovery target.
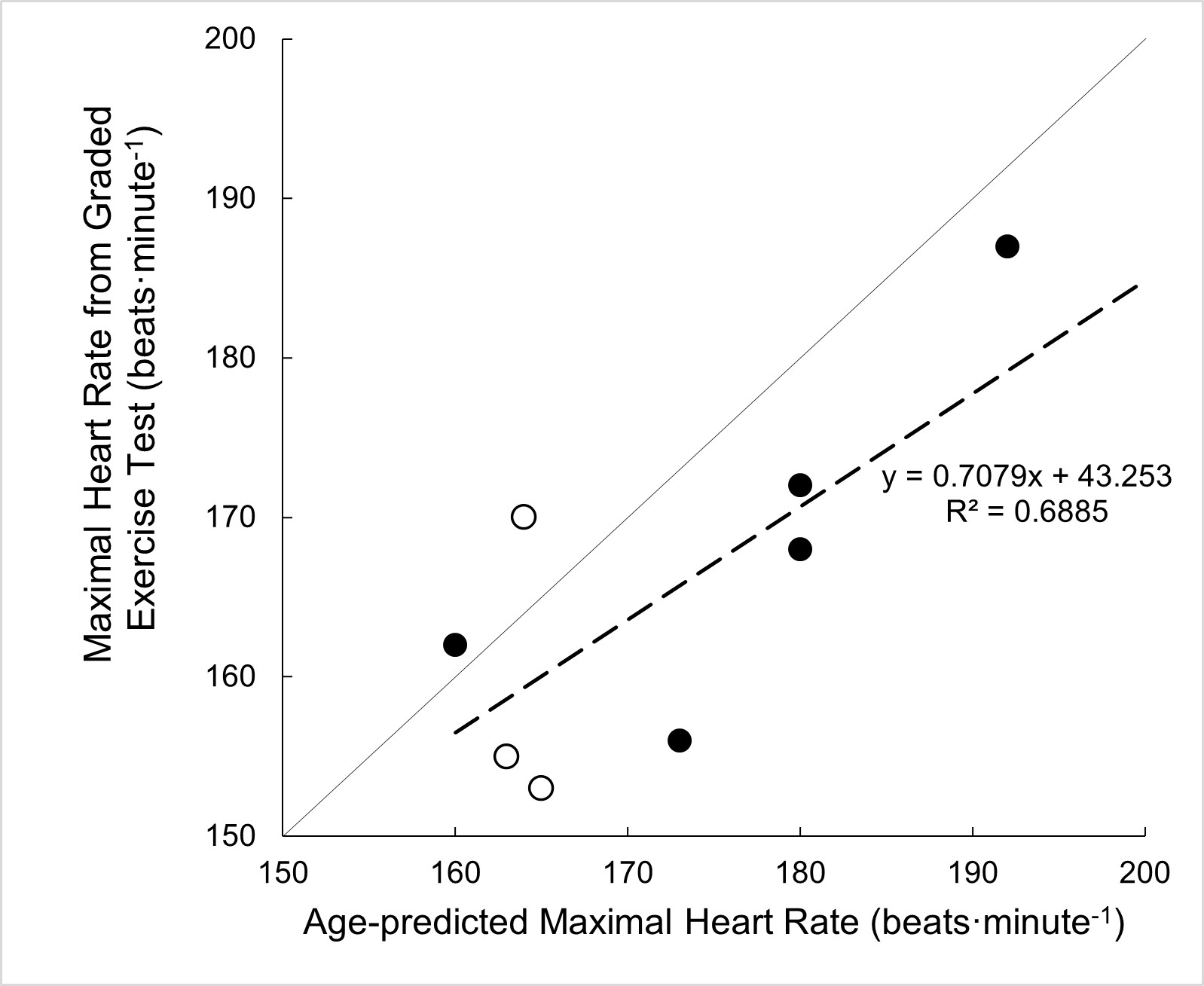
There were no significant differences in adherence rates between the first eight weeks and the last eight weeks of at-home HIIT (P=0.369, ηp2=0.102, n=8). There was no effect of training on heart rate during the recovery intervals between the first eight weeks and the last eight weeks of the training program (P=0.982, ηp2=0.002, n=8). Additionally, a linear regression demonstrated a significant relationship between the age-predicted maximal heart rate and the measured maximal heart rate during the graded exercise test (P=0.025, n=8, Figure 4).
Figure 4. Scatter plot depicting the comparison of age-predicted maximal heart rate versus the measured maximal heart rate during a graded exercise test using arm ergometry. The solid line represents the line of identity and dashed line represents line of best fit. Solid symbols indicate male data and open symbols indicate female data.
Participant safety and self-efficacy
A total of four non-serious adverse events were reported from weekly surveys during the study. Two incidents of shoulder soreness were reported after a training session, as well as two incidents of sustained tachycardia that were deemed likely related to the intervention by the principal investigator. All incidences of tachycardia resolved within 30 minutes after the session ended, and all incidences of shoulder soreness resolved within a week. Only one participant missed a week of training due to shoulder soreness. Scores determined from the SCI ESES survey did not significantly change throughout the training period (P=0.497, ηp2=0.095, n=8). Overall, participants reported high exercise self-efficacy scores at baseline (Total score: 3.4±3.3). However, the score in response to the statement “when confronted with a barrier to exercise I could usually find several solutions to overcome the barrier” was greater at week eight rather than week 16 (Total score: 3.6±0.5 vs 3.0±0.5, respectively, P=0.023, ηp2=0.701, n=8).
All participants were satisfied with the HIIT exercise program (Table 2) and agreed that they would recommend the activity to a friend, and most said they planned to continue HIIT as part of their regular exercise routine. Additionally, three participants reported a change in physical activity outside of the HIIT program. Participant four did not complete the correct survey and was lost to follow-up.
Discussion
Cardiorespiratory fitness
The data presented suggest that the HIIT program improved maximal work capacity and indicative of improved cardiac efficiency during moderate intensity exercise, as evidenced by the increase in peak power output and decrease in submaximal cardiac output, respectively. These findings are generally in good agreement with other studies examining the effects of HIIT on cardiorespiratory fitness among individuals with chronic SCI [14–16]. However, a statistically significant improvement in V̇O2peak was not observed among the participants following 16 weeks of HIIT. This observation may be explained by the relatively short duration of the high-intensity intervals (~2 minutes each) used in this study, compared to the longer duration of high-intensity intervals (~3 to 8 minutes each) used among other studies [12,22,23]. Additionally, it is likely that the presented study was underpowered to detect such differences in V̇O2peak. As such, it is plausible to suggest that a near significant improvement in V̇O2peak (p=0.064) demonstrates a strong physiological trend where a larger cohort may have reached significance.
One appealing aspect of HIIT is the potential to reduce the cumulative exercise time per week required to elicit similar improvements in cardiometabolic health compared to continuous aerobic training. This proposed benefit may be of particular interest among participants with SCI due to the high risk of stress-induced injuries in the shoulder joint. Yet, greater durations of high-intensity intervals than investigated may be required to elicit significant improvements in V̇O2peak, and theoretically other cardiometabolic health factors. Therefore, there appears to be a balance in the prescribed ‘dose’ of HIIT among this population. On one hand, too little HIIT may not elicit improvements in cardiometabolic health, yet excessive HIIT may lead to stress injuries in the upper extremities that may worsen with continuous training. Future research warrants the investigation of an increased training load of HIIT on shoulder health and function to better dissociate the limitations of prescribed HIIT among individuals with SCI. With additional efforts, we may be able to prescribe an optimal ‘dose’ of HIIT, likely contingent on individual characteristics such as strength, baseline fitness, and fatiguability.
Adherence
Several elements of this study may have contributed to the relatively high training adherence. First, in-home and wheelchair-accessible equipment served to remove reported barriers. A survey of individuals with SCI found that not having exercise equipment in the home reduced the likelihood of exercising by ~68% [24]. Additionally, the relatively short exercise durations inherent to HIIT may have also improved training adherence. Lastly, the at-home availability of the wheelchair-accessible arm cycle may have facilitated independent exercise due to enhanced availability, and incorporating technology-assisted self-monitoring through the use of an available smartphone application likely reinforced autonomy during these sessions. Finally, the at-home nature of this study design allowed scheduling flexibility for when the HIIT sessions could be performed. The removal of traveling burdens, facility access, and membership-associated fees were all potential contributors to successful adherence. Independence has been noted as a motivator for consistent activity for many with SCI [8]. However, participants who were willing to be involved in this study may have a greater internal motivation to perform regular exercise compared to the general population with SCI, leading to some degree of selection bias.
Previous investigation by Koontz et al. examined the feasibility and preliminary efficacy of at-home HIIT among individuals with chronic SCI [16]. Authors found an ~89% training adherence rate, and that high-intensity heart rate targets were reached among ~46% of training sessions. Although these data are similar to our findings, Koontz and colleagues employed a study trainer to instruct each HIIT session during the 6-week program. In contrast, the presented study involved 16 weeks of HIIT without supervised training. Therefore, supervised exercise may not be required for HIIT interventions in the SCI population. Additional research is warranted to determine the behavioral and participant factors that contribute to HIIT adherence and compliance, particularly among unsupervised training sessions.
Compliance
The target heart rates during HIIT were tailored for each participant based on results from the baseline graded exercise test. Additionally, continuous heart rate feedback provided through the smartphone application may have assisted in the achievement of the high-intensity heart rate targets. The difficulty noted with participants achieving the target recovery heart rate (~30% HRR) may indicate that more than two minutes of recovery or a lightened recovery work rate is necessary. Yet, participants elicited significant improvements in cardiorespiratory fitness despite limitations in reaching target recovery heart rate. Therefore, the exact HIIT program characteristics (i.e., ability to achieve target recovery heart rate) that confer efficacious improvements in cardiorespiratory fitness remain unclear.
It is unclear whether adequate recovery influences the achievement of subsequent high-intensity bouts. Venous pooling is a known occurrence for individuals with SCI during arm cycling, which affects the hemodynamic response to exercise and may lead to an elevated heart rate between high-intensity bouts [25]. Although few participants wore compression stockings during training sessions, there is limited data to suggest a significant affect upon the venous system and cardiovascular responses [26]. In light of the presented findings, future work is needed to examine the changes in heart rate during HIIT training among individuals with SCI to determine the optimal duration of high-intensity and recovery intervals necessary to achieve the prescribed HRR targets. Additionally, these findings demonstrated a positive association between the maximal heart rate achieved during the baseline graded exercise test to the age-predicted maximal heart rate. Therefore, we suggest that age-predicted heart rate may be a useful target when prescribing exercise for this specific SCI population (injury below T6).
While originally prescribed a target of 80% HRR, we determined ≥70% HRR as sufficiently compliant due to this value qualifying as appropriate intensity to be considered HIIT. There are several potential factors that prevented participants from achieving the 80% HRR target, mainly insufficient time (1 minute) spent at high intensity for which heart rate kinetic response was not fast enough to achieve, especially in the first one to two intervals of each session. For participants that reported lower compliance, it is plausible to suggest these individuals limited their physiological adaptations to some degree.
Participant safety and self-efficacy
Infrequent incidences of adverse events were reported during the at-home HIIT program. However, indications of tachycardia were noted by primary care providers prior to study involvement, with no notable concern about participating in a regular exercise program. Reports of shoulder pain were addressed by advising rest and reduced resistance during the HIIT sessions. Additionally, some investigators have reported improvements in shoulder health among individuals with SCI following upper extremity exercise. For instance, Graham et al. noted an improvement in strength scores following arm cycle training [13]. From these results, the authors suggest that only 40 minutes of HIIT per week was sufficient to improve upper extremity strength in the chest press and lateral pull-down. Although the upper extremity adverse events were minor in our study, future studies focusing on upper extremity exercise should address shoulder health in screening and throughout the study by denoting any alterations in shoulder pain, range of motion, and strength.
Within this study, participants reported moderate confidence in exercise participation at baseline, and this confidence persisted throughout the study. Given their willingness to participate in a 16-week exercise study, it is possible that the recruited participants may have greater confidence in exercise relative to the general SCI population. Additionally, participants reported a lower barrier to exercise after eight weeks of HIIT than at the end of the study. The lower confidence in overcoming these barriers at the end of the study is an area that should be further investigated. For example, demotivation could potentially be an additional barrier due to the need to return the arm cycle and other equipment to the study staff following termination of the HIIT program.
Satisfaction survey data indicated that most participants enjoyed this at-home HIIT program. Similarly, HIIT has previously been demonstrated to be more enjoyable than moderate-intensity exercise in the SCI population [27,28]. Some have suggested that the reported enjoyment may be associated with the frequent recovery bouts breaking up the monotony of the workout, the constant change in activity, and the feeling of accomplishment achieved through more intense work [28]. Despite COVID-19 pandemic-related restrictions at our institution during the study (March 2020 through July 2021), participants continued to perform the HIIT program at home. However, a potential bias exists with the at-home sessions due to the various restrictions placed during the pandemic and may have influenced motivation for study participation, effort during the HIIT sessions, and general contentment during this time frame.
Study limitations
Presented findings should be interpreted with several pertinent caveats. In terms of study design, these findings are limited by a lack of control group. Several studies examining HIIT efficacy among patients with SCI have used various control groups, including a non-training group [16] and a continuous, moderate exercise group [13,14]. Within future studies, the inclusion of a control group may help dissociate HIIT-specific benefits compared to more conventional training programs. Additionally, the non-standardization of HIIT protocols (duration/intensity of high and low intensity bouts) may confound the comparison of findings to other HIIT studies [29,30]. Of note, the Karvonen formula, used to calculate target HRR, has not been validated in the SCI population. However, due to the exclusion of injuries above T6, we believe the blunted heart rate response was likely minimal during testing procedures. Future work is needed to determine ‘optimal’ training protocols tailored to individual patients, incorporating baseline fitness/activity levels, access to training equipment, and self-confidence in the ability to perform exercise. Such studies would particularly benefit from accounting for psychological and behavioral factors associated with effective exercise adherence and compliance among participants with chronic SCI.
This study is also limited by a small sample size, as only five participants were able to return for follow-up exercise testing due to institutional closure and restrictions during the COVID-19 pandemic. Post-hoc power analyses indicate that to achieve significant differences with the desired power of 0.8 for peak power output, and V̇O2peak, a sample size of 10 participants would be needed. Despite the limited sample size and heterogeneity of training adherence/compliance, participants still demonstrated significant improvements in cardiorespiratory fitness, suggesting good generalizability of the described HIIT protocol to the overall SCI population. However, there may be selection bias within the presented cohort due to the inclusion of generally motivated participants who completed 16-weeks of HIIT. Within the overall SCI population, training drop-out and long-term cardiorespiratory effects have yet to be determined.
Conclusion
This pilot study suggests that a 16-week at-home HIIT program was easily implemented for individuals with SCI below the sixth thoracic vertebrae. Overall, participants achieved high adherence and compliance, infrequent adverse events, a high reported self-efficacy, and improvements in cardiac efficiency during submaximal exercise, and maximal work capacity. From these observations, we propose that HIIT may provide an enjoyable long-term aerobic exercise program for individuals with SCI. Future studies warrant rigorous examination in relation to sedentary age-matched controls and other training programs, including cross over study designs.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was funded, in part, by the Mayo Clinic Rehabilitation Medicine Research Center, which received benefactor funding. KG received funding from the Mayo Clinic Center for Clinical and Translational Science Small Grants Program Fund. CW was supported by a National Institutes of Health Training Grant (T32-DK-007352-39). KW was supported by a National Institutes of Health Training Grant (T32-HL105355-10) and the Mayo Clinic Graduate School of Biomedical Sciences.
Acknowledgements
We would like to thank Andrew Miller, Technical Specialist Coordinator within the Clinical Research and Trials Unit at Mayo Clinic for conducting maximal exercise testing for all participants. We would like to thank Tyson Scrabeck and Julie Block for working with participants during the COVID-19 pandemic to navigate clinical changes. We would like to thank the Mayo Clinic Media Services Department for assisting in editing the workout recordings. We would like to sincerely thank our participants for their dedication to the 16-week project. The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
All procedures have been approved and performed in accordance with the Declaration of Helsinki and by the Mayo Clinic Institutional Review Board #18-004972. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Author Contributions
KG, ML, and DV initiated the project. ML, KW, CW and DV, communicated directly with patients and carried out patient education and testing. ML and KW drafted the manuscript and incorporated all author edits. CW and KW contributed to writing the report, extracting, analyzing, and interpretation of data, OM assisted in data analysis. DV, MG, MV, EL, OM, MJ, LB, KZ and KG contributed edits to the manuscript. KG and CW supervised all aspects of this project and provided approval for the publication and content.
References
2. Cragg JJ, Noonan VK, Krassioukov A, Borisoff J. Cardiovascular disease and spinal cord injury: results from a national population health survey. Neurology. 2013 Aug 20;81(8):723–8.
3. Groah SL, Nash MS, Ward EA, Libin A, Mendez AJ, Burns P, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011 Mar-Apr;31(2):73–80.
4. Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil. 2007 Feb;86(2):142–52.
5. Nash MS, Groah SL, Gater DR Jr, Dyson-Hudson TA, Lieberman JA, Myers J, et al. Identification and Management of Cardiometabolic Risk after Spinal Cord Injury: Clinical Practice Guideline for Health Care Providers. Top Spinal Cord Inj Rehabil. 2018 Fall;24(4):379–423.
6. Rabadi MH, Mayanna SK, Vincent AS. Predictors of mortality in veterans with traumatic spinal cord injury. Spinal Cord. 2013 Oct;51(10):784–8.
7. Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord. 2018 Apr;56(4):308–21.
8. Kehn M, Kroll T. Staying physically active after spinal cord injury: a qualitative exploration of barriers and facilitators to exercise participation. BMC Public Health. 2009 Jun 1;9:168.
9. Bacon AP, Carter RE, Ogle EA, Joyner MJ. V̇O2max trainability and high intensity interval training in humans: a meta-analysis. PLoS One. 2013 Sep 16;8(9):e73182.
10. Batacan RB Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS. Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med. 2017 Mar;51(6):494–503.
11. Laursen PB, Jenkins DG. The scientific basis for high-intensity interval training: optimising training programmes and maximising performance in highly trained endurance athletes. Sports Med. 2002;32(1):53–73.
12. de Groot PC, Hjeltnes N, Heijboer AC, Stal W, Birkeland K. Effect of training intensity on physical capacity, lipid profile and insulin sensitivity in early rehabilitation of spinal cord injured individuals. Spinal Cord. 2003 Dec;41(12):673–9.
13. Graham K, Yarar-Fisher C, Li J, McCully KM, Rimmer JH, Powell D, et al. Effects of High-Intensity Interval Training Versus Moderate-Intensity Training on Cardiometabolic Health Markers in Individuals With Spinal Cord Injury: A Pilot Study. Top Spinal Cord Inj Rehabil. 2019 Summer;25(3):248–59.
14. Wouda MF, Lundgaard E, Becker F, Strøm V. Effects of moderate- and high-intensity aerobic training program in ambulatory subjects with incomplete spinal cord injury-a randomized controlled trial. Spinal Cord. 2018 Oct;56(10):955–63.
15. Gauthier C, Brosseau R, Hicks AL, Gagnon DH. Feasibility, Safety, and Preliminary Effectiveness of a Home-Based Self-Managed High-Intensity Interval Training Program Offered to Long-Term Manual Wheelchair Users. Rehabil Res Pract. 2018 May 17;2018:8209360
16. Koontz AM, Garfunkel CE, Crytzer TM, Anthony SJ, Nindl BC. Feasibility, acceptability, and preliminary efficacy of a handcycling high-intensity interval training program for individuals with spinal cord injury. Spinal Cord. 2021 Jan;59(1):34–43.
17. Buker DB, Oyarce CC, Plaza RS. Effects of Spinal Cord Injury in Heart Rate Variability After Acute and Chronic Exercise: A Systematic Review. Top Spinal Cord Inj Rehabil. 2018 Spring;24(2):167–76.
18. Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick. J Appl Physiol (1985). 2000 May;88(5):1650–8.
19. Wen D, Utesch T, Wu J, Robertson S, Liu J, Hu G, et al. Effects of different protocols of high intensity interval training for V̇O2max improvements in adults: A meta-analysis of randomised controlled trials. J Sci Med Sport. 2019 Aug;22(8):941–47.
20. Kroll T, Kehn M, Ho PS, Groah S. The SCI Exercise Self-Efficacy Scale (ESES): development and psychometric properties. Int J Behav Nutr Phys Act. 2007 Aug 30;4:34.
21. Richardson JT. Eta squared and partial eta squared as measures of effect size in educational research. Educational research review. 2011 Jan 1;6(2):135–47.
22. Brurok B, Helgerud J, Karlsen T, Leivseth G, Hoff J. Effect of aerobic high-intensity hybrid training on stroke volume and peak oxygen consumption in men with spinal cord injury. Am J Phys Med Rehabil. 2011 May;90(5):407–14.
23. Hasnan N, Engkasan JP, Husain R, Davis GM. High-Intensity Virtual-reality Arm plus FES-leg Interval Training in Individuals with Spinal Cord Injury. Biomed Tech (Berl). 2013 Aug;58 Suppl 1:/j/bmte.2013.58.issue-s1-A/bmt-2013-4028/bmt-2013-4028.xml.
24. Cowan RE, Nash MS, Anderson KD. Exercise participation barrier prevalence and association with exercise participation status in individuals with spinal cord injury. Spinal Cord. 2013 Jan;51(1):27–32.
25. Taylor JA. The physiology of exercise in spinal cord injury. Cambridge, MA: Springer; 2016.
26. Rimaud D, Calmels P, Roche F, Mongold JJ, Trudeau F, Devillard X. Effects of graduated compression stockings on cardiovascular and metabolic responses to exercise and exercise recovery in persons with spinal cord injury. Arch Phys Med Rehabil. 2007 Jun;88(6):703–9.
27. Astorino TA, Thum JS. Interval training elicits higher enjoyment versus moderate exercise in persons with spinal cord injury. J Spinal Cord Med. 2018 Jan;41(1):77–84.
28. Thum JS, Parsons G, Whittle T, Astorino TA. High-Intensity Interval Training Elicits Higher Enjoyment than Moderate Intensity Continuous Exercise. PLoS One. 2017 Jan 11;12(1):e0166299.
29. Coates AM, Joyner MJ, Little JP, Jones AM, Gibala MJ. A Perspective on High-Intensity Interval Training for Performance and Health. Sports Med. 2023 Dec;53(Suppl 1):85-96.
30. Veith DD, Linde MB, Wiggins CC, Zhao KD, Garlanger KL. Intervention Design of High-Intensity Interval Training in Individuals With Spinal Cord Injury: Narrative Review and Future Perspectives. Top Spinal Cord Inj Rehabil. 2023 Fall;29(4):1-15.