Abstract
Purpose: To describe 3 cases of acute macular neuroretinopathy (AMN) type 2 associated with SARS-CoV-2 viral infection and migraine.
Methods: Observational case series and literature review.
Results: The three patients, which were all women in their reproductive years with a mean age of 31 (range, 22-43), were diagnosed with AMN type 2 after presenting with acute onset of uni- or bilateral persisting paracentral scotomata. On multi-modal imaging with near-infrared (NIR) reflectance imaging, wedge-shaped dark-gray lesions were discernable in a perifoveal petaloid configuration. SD-OCT through the lesions revealed initial hyperreflectivity at the level of the outer retina, beneath the OPL (outer plexiform layer) and comprising the ONL (outer nuclear layer), with disruption of the inner segment/outer segment (IS/OS) band, eventually evolving into thinning of the ONL with or without persisting disruption of the photoreceptor complex. Anterior segment, fundoscopic, angiographic and electrophysiologic examinations were unremarkable.
Conclusion: All three cases were diagnosed with a concurrent viral upper respiratory tract infection caused by the SARS-CoV-2 virus. While AMN is considered to be a rare disease, a worldwide surge in the incidence of AMN has recently been reported and the largest case series to date have been described during the latest SARS-CoV-2 viral pandemic. Thus, COVID-19 could be considered a potential risk factor for the development of AMN type 2. Two of our cases were associated with migraine, one of which experienced the onset of AMN symptoms in the immediate setting of a migraine attack and the use of triptans. This association between migraine, triptans and AMN type 2 should be considered in the context of a seemingly persisting visual aura, particularly in the presence of additional risk factors, such as female gender and oral contraceptive use.
Summary statement: An increased incidence of acute macular neuroretinopathy (AMN) type 2 is observed in the context of SARS-CoV-2 viral infection. The presently described association between AMN, migraine, and triptans should be considered when examining a patient with seemingly persisting visual aura, in particular negative scotomata, in the context of migraine.
Keywords
Acute macular neuroretinopathy (AMN), COVID-19, SARS-CoV-2, Viral infection, Migraine, Triptans, NIR imaging, OCT
Introduction
Acute macular neuroretinopathy (AMN), first described by Bos and Deutman in 1975 [1], is an infrequent yet increasingly diagnosed retinal condition, characterized by the acute onset of persisting paracentral scotomata with capricious vision loss in one or both eyes, associated with (peri)foveal petaloid (wedge-shaped) outer retinal lesions generally accepted to be caused by microvascular damage to the retinal capillary network of the macula [2,3].
Depending on the location of the lesions above or below the OPL (outer plexiform layer) on multimodal imaging with SD-OCT (spectral-domain optical coherence tomography), the disease can be classified as either type 1 AMN, also known as PAMM (paracentral acute middle maculopathy), or type 2 (classic) AMN [4]. In type 1 AMN/PAMM, vascular damage is presumed to occur at the level of the ICP (intermediate capillary plexus) and a hyperreflective band is seen internal to the OPL, at the level of the INL (inner nuclear layer) with subsequent thinning of the latter [5]. In type 2 AMN, vascular damage is presumed to occur at the level of the DCP (deep capillary plexus) and the inner choroidal vascular layers, and hyperreflectivity is seen external to the OPL, at the level of the ONL (outer nuclear layer), with subsequent thinning of the latter with or without concomitant disruption of the IS (inner segments) and OS (outer segments) [2,4,6-8].
Here, we present three cases of type 2 AMN associated with migraine and/or concurrent SARS-CoV-2 viral infection.
Case Presentation
Case 1
A 22-year-old Caucasian woman presented with a 1-day history of a persisting small paracentral scotoma in the right eye immediately after a migraine attack the previous day. Since the age of twelve, the patient is known with migraine attacks (once every two months), accompanied with a less than one-hour typical visual aura, nausea, and unilateral limb paresthesia, finally followed by unilateral headache lasting around one to two days. The patient reported the acute onset of a migraine episode, following the well-known course for the patient, but with a persisting scotoma in the right eye after resolution of all other migraine symptoms. In addition, she reported mild flu-like symptoms, which were later confirmed by PCR testing on a nasopharyngeal swab to be due to concurrent SARS-CoV-2 viral infection. During this attack she took one dose of peroral sumatriptan (Imitrex) and paracetamol.
Genetic analysis for familial hemiplegic migraine (FHM) was negative for mutations in the three core genes (i.e., CACNA1A, ATP1A2 and SCN1A), as well as in five additional genes with possible association to FHM (i.e., ATP1A3, KCNK18, PRRT2, SLC1A3 and SLC2A1). Other relevant medical history consisted of daily use of oral contraceptives and exertional asthma with occasional use of inhalational rescue medication (Ventolin and Relvar – beta-agonists with or without cortisone).
At presentation, her best-corrected visual acuity (BCVA) was 20/20 in both eyes. Amsler testing and central visual field testing (HFA 10-2 Humphrey Field Analyzer) revealed a small superiorly located paracentral relative scotoma in the right eye. Color vision, pupil reflexes and anterior segment examination were unremarkable. With careful observation on fundoscopic examination a very discrete parafoveal gray spot could be discerned as well as mild tortuosity of the retinal arteries. SD-OCT (Heidelberg Spectralis HRA+OCT; Heidelberg Engineering) through this zone revealed a lesion with initial hyperreflectivity at the level of the outer retina, beneath the OPL and comprising the ONL, with disruption of the inner segment/outer segment (IS/OS) band. On near-infrared (NIR) reflectance imaging no clear retinal lesions were discernable on the first day of presentation. However, on the fifth day after presentation, the same area was clearly demarcated and discernable as a wedge-shaped dark-gray lesion on NIR reflectance imaging. Fundus autofluorescence (FAF) was normal and fluorescein angiography (FA) showed a patchy choroidal filling, but no other abnormalities (Figure 1). All electrophysiologic studies performed, including full-field electroretinogram (ERG), multifocal ERG and electro-oculogram (EOG), were normal.
At her 5-month follow-up visit, the patient reported that the scotoma had become less dense, visual acuity had remained stable at 20/20 and no new scotoma had appeared. The wedge-shaped lesion on NIR reflectance imaging had become lighter and the hyperreflectivity on SD-OCT had dissipated, leaving residual thinning and partial atrophy of the ONL and IS/OS band.
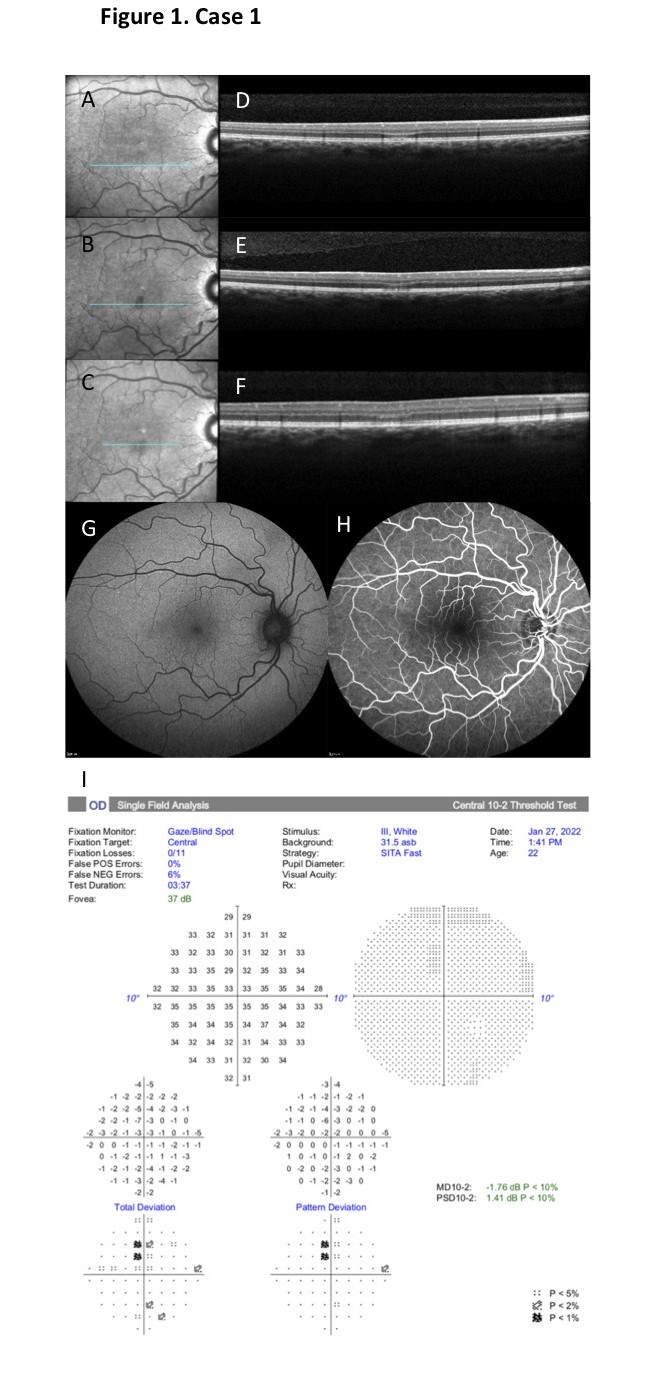
Figure 1. Case 1. A-C. On NIR reflectance imaging no retinal lesions were discernable on the first day of presentation (A). On day 5 a well- demarcated wedge-shaped dark-gray inferior parafoveal lesion was clearly discernable (B), fading to a lighter color at month 5 after presentation (C). D-F. SD-OCT through the lesion on day 1 revealed an initial hyperreflectivity at the level of the outer retina, beneath the OPL and comprising the ONL, with disruption of the inner IS/OS band (D). On day 5 the hyperreflectivity had already dissipated, leaving residual thinning and partial atrophy of the ONL and IS/OS band (E), with only partial structural reorganization through month 5 after presentation (F). G-H. Fundus autofluorescence (FAF) was normal (G) and fluorescein angiography (FA) showed a patchy choroidal filling, but no other abnormalities (H). I. Central visual field testing (HFA 10-2) revealed a small superiorly located paracentral relative scotoma in the right eye.
Case 2
An otherwise healthy 27-year-old Caucasian woman presented with a 3-week history of persistent multiple bilateral paracentral scotomata which suddenly appeared in her visual field while driving her car. Apart from the visual scotomata, she did not experience any other visual or neurological symptoms. The patient reported a concurrent symptomatic and PCR-confirmed COVID-19 upper respiratory infection two days prior to onset of her visual symptoms.
Her medical history only consisted of occasional mild migraine episodes. Prior neurological work-up at the emergency department (ED) with brain imaging using computed tomography (CT) and magnetic resonance imaging (MRI), and visual evoked potentials (VEP) were all reassuring. With the exception of daily use of oral contraceptives, the patient did not take any other medications.
At presentation BCVA was 20/20 in both eyes, with normal color vision, normal pupil reflexes and unremarkable anterior segment and fundoscopic examinations. On multimodal imaging, in particular NIR reflectance imaging, several wedge-shaped dark-gray lesions appeared in a petaloid (flower-petal) perifoveal configuration in both eyes. SD-OCT sections through the lesions revealed thinning of the ONL, attenuated IS/OS bands and slightly disrupted outer segment/retinal pigment epithelium (OS/RPE) bands. No hyperreflective bands were seen on the SD-OCT images. FA and indocyanine green angiography (ICGA), as well as multifocal ERG and central visual field testing (10- 2 HFA) were normal (Figure 2).
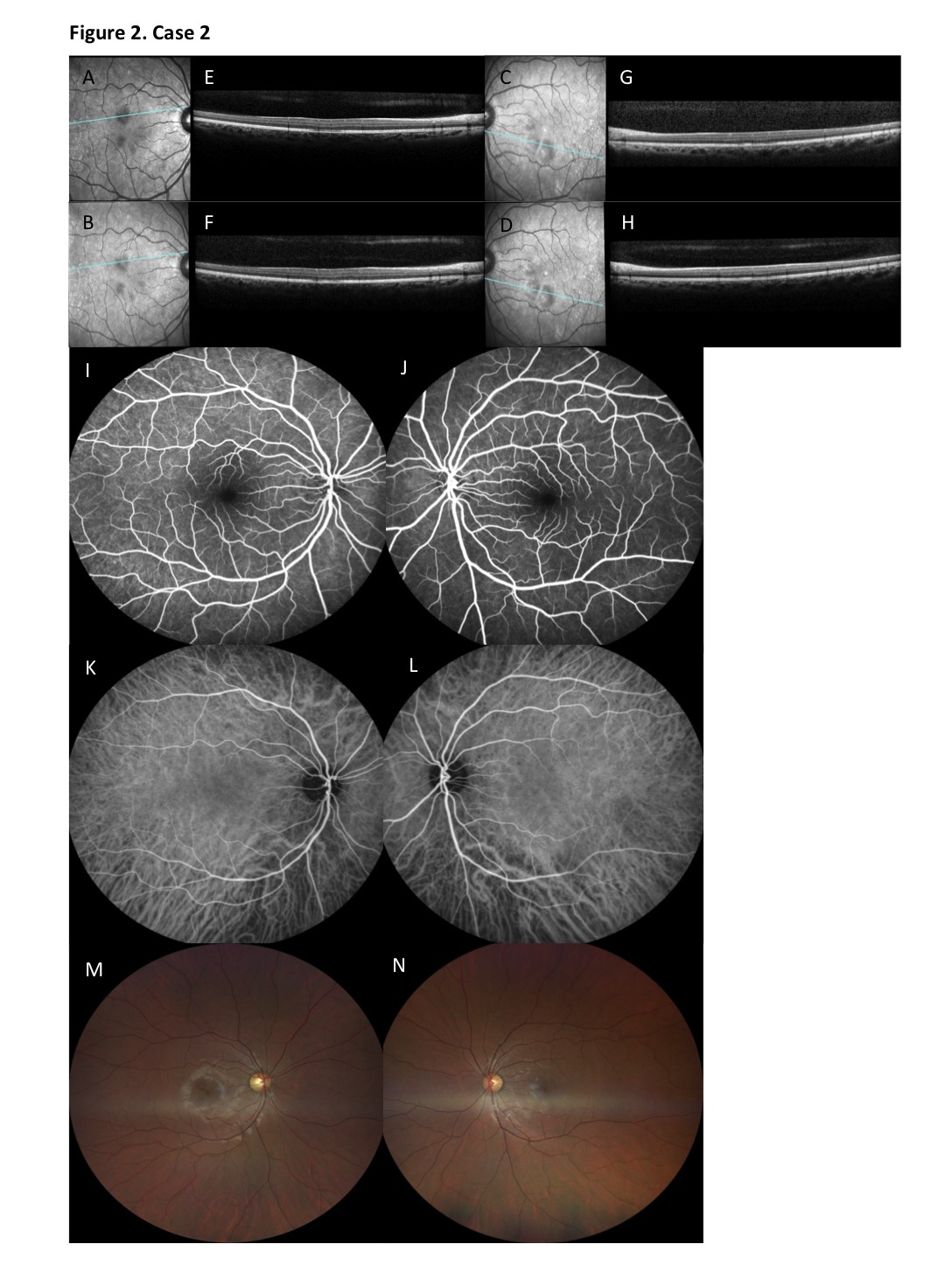
Figure 2. Case 2. A-D. On NIR reflectance imaging, several wedge-shaped dark-gray lesions were observed at presentation (3 weeks after onset) in a petaloid perifoveal configuration in both eyes (A, C). After 6 months, the lesions had faded into a lighter-gray color, but were still sharply demarcated and discernable in their perifoveal distribution (B, D). E-H. SD-OCT sections through the lesions revealed thinning of the ONL, attenuated IS/OS bands and slightly disrupted outer segment/retinal pigment epithelium (OS/RPE) bands. No hyperreflective bands were seen on the SD-OCT images at presentation (E, G). Thinning of the ONL persisted through month 6 after presentation, with only partial reconstitution of the IS/OS and OS/RPE bands (F, H). I-L. FA (I, J) and indocyanine green angiography (ICGA) (K, L) of both eyes showed no abnormalities. M-N. On color ophthalmoscopic imaging at 6 months after presentation reddish-brown perifoveal lesions were more discretely visible in the right eye (M) than in the left eye (N).
Six months after her initial presentation the patient stated that the scotomata had remained unaltered, but were less bothering in daily life, while visual acuity remained stable at 20/20 in both eyes. On NIR reflectance imaging the lesions had faded into a lighter-gray color, but were still sharply demarcated and discernable in their perifoveal distribution. SD-OCT imaging revealed persisting thinning of the ONL, with only partial reconstitution of the IS/OS and OS/RPE bands. On color ophthalmoscopic imaging reddish-brown perifoveal lesions were now more discretely visible.
Case 3
A 43-year-old Caucasian woman presented at our clinic complaining of a paracentral scotoma in the left eye, which had suddenly occurred after waking up earlier that day. She reported having developed flu-like symptoms three days earlier, consisting of malaise, a headache and light fever (38°C), which were under control with paracetamol 1000mg twice per day. She experienced no other visual or systemic symptoms. PCR on a nasopharyngeal swap taken at the emergency department (ED) demonstrated a strong positivity for the SARS-CoV-2 virus.
The patient was an otherwise healthy women in her reproductive years, not taking any contraceptive medication. She was known with a hitherto untreated borderline arterial hypertension. There was no history of migraine or other diseases.
At presentation BCVA was 20/20 OU, with unremarkable neuro-ophthalmological, biomicroscopic and ophthalmoscopic examinations in both eyes. Central visual field testing (HFA 10-2) revealed a dense paracentral scotoma in the left eye, with no abnormalities in the right eye. On multimodal imaging with SD-OCT a dense hyperreflectivity of the OPL and disruption of the underlying ONL was evident in the superior papillomacular bundle, with an associated subtle shadowing effect on the outer retinal layers and RPE. On NIR reflectance imaging no clear retinal lesions were discernable on the first day of presentation. At her 1-month follow-up visit, however, a sharply demarcated dark-gray lesion at the level of the papillomacular bundle of the left eye had developed. On follow-up SD-OCT at 1 month, the initial hyperreflectivity had evolved into thinning and atrophy of the ONL and significant disruption of the IS/OS bands, with dissipation of the shadowing effect on the outer retinal layers (Figure 3). Visual acuity remained stable at 20/20 in both eyes, while the scotoma remained unaltered, but was reportedly less bothering to the patient.
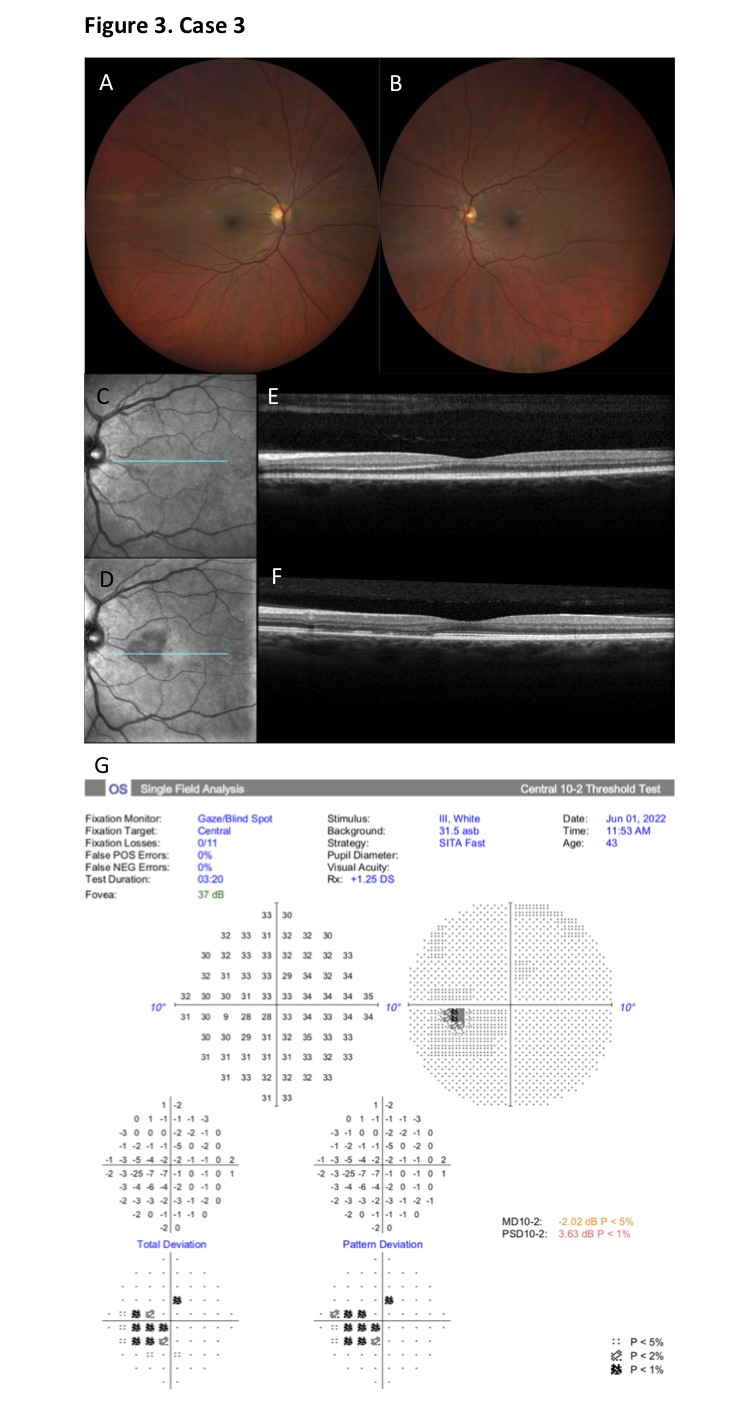
Figure 3. Case 3. A-B. Fundoscopic examination of both eyes was unremarkable. C-D. On NIR reflectance imaging no clear retinal lesions were discernable on the first day of presentation (C). At her 1-month follow-up visit, however, a sharply demarcated dark-gray lesion at the level of the papillomacular bundle of the left eye had developed (D). E-F. On SD-OCT at presentation, a dense hyperreflectivity of the OPL and disruption of the underlying ONL was evident in the superior papillomacular bundle, with an associated subtle shadowing effect on the outer retinal layers and RPE (E). At 1 month follow-up, the initial hyperreflectivity had evolved into thinning and atrophy of the ONL and significant disruption of the IS/OS bands, with dissipation of the shadowing effect on the outer retinal layers (F). G. Central visual field testing (HFA 10-2) revealed a dense paracentral scotoma in the left eye consistent with the location of the retinal lesion.
Discussion
The 3 cases (comprising 4 eyes) described above, may all be categorized as presentations of AMN type 2 with damage occurring in the outer retinal layers. As mentioned in the introduction, the etiopathogenesis of AMN is now largely accepted to be due to a vascular insult to the DCP (deep capillary plexus) of the retina and the inner choroidal layers [2,4,7,9]. In line with this, a focal ischemic hyperreflectivity was seen on SD-OCT only in the immediate period after onset of symptoms, with no abnormalities on FA, reflecting the superposition of all capillary plexi (SCP, ICP and DCP) using classic angiographic imaging.
Several associations and risk factors for AMN have been suggested, although a clear causative relation has yet to be determined. The most common demographic associations include gender, as 86% of patients are female, and relatively young age, with over half of the patients in their third decade of life and a mean age of thirty at presentation [9,10]. Since the majority of patients with AMN consist of younger women in their reproductive years, the high association of the disease with the use of oral contraceptives (in 37% of cases) could either present a confounding factor, or a real association [10]. The most commonly associated variable risk factor overall is a viral infection, in particular a flu-like, upper respiratory prodrome or concurrent infection, which is described in 46% of cases [9-11]. Other less commonly associated risk factors include the use of sympathomimetics (such as epinephrine and ephedrine), idiosyncratic reactions to intravenous contrast agents, (postpartum) hypotension, systemic shock, caffeine consumption, antecedent trauma and (migraine) headache [9,10,12].
Considering the fact that at the time of writing the thorough reviews of Turbeville et al. and of Aziz et al., only 41 cases of AMN (from its first description in 1975 up until 2002) and another 44 cases (from 2002 until 2012), respectively, had been reported in the English-language medical literature, it is very clear that the reporting of AMN has significantly increased in the past two decades [10,12]. Whether this is due to a decreased threshold for diagnosis with the advent of widespread use of multimodal imaging, an increased awareness of the disease, or due to a definite increase in incidence of AMN, remains to be determined.
More recently, during the 2020 and 2021 SARS-CoV-2 viral pandemic, however, a worldwide increase in COVID-19- related or vaccination-related AMN cases was described. This observation is corroborated by the present description, with all 3 cases having presented to our ophthalmology department between December 2020 and April 2021, in the midst of the pandemic crisis, at a time when the Omicron variant was prevalently circulating in Western Europe. We could deduce that worldwide, large-scale, increased incidence of viral infection, which is the most widely accepted associated risk factor for AMN, subsequently could lead to an increased incidence of all viral infection- associated syndromes. An inflammatory or direct cytopathic insult by the viral infection could induce the presumed capillary microvascular damage.
A recently published review of Ahmed et al. revises this reported increased incidence related to the novel SARS-CoV- 2 virus [13]. Jalink et al. reported four cases of AMN in 2021 in one hospital in the south of the Netherlands, all linked to COVID-19 infection or vaccination [14]. In the Paris Rotschild Foundation Hospital, Azar et al. reported a significant increase in AMN diagnoses in 2020, with 13 new cases (only 4 of them were tested, but all tests returned positive for COVID-19 infection) as opposed to only 1 new case in 2019. The latter department did not see a similar increase in incidence of other white dot syndromes, such as PAMM or MEWDS (multiple evanescent white dot syndrome) [15]. Furthermore, several case reports were published linking AMN to vaccination against the SARS-CoV-2 virus. In all reports, symptoms occurred in the immediate post-vaccination period of 1-3 days [16-20]. In the latter, no direct viral insult is plausible to cause the AMN.
Consistent with current understanding of the risk factors for AMN, the three cases described in our case series experienced a concurrent viral upper respiratory infection, all of them consisting of COVID-19. Case 2 and 3 were not vaccinated against the SARS-CoV-2 virus, while case 1 received three doses of the Comirnaty® vaccine (Pfizer/BioNTech), respectively 8, 7, and 3 months prior to presentation. As such, no vaccine administration took place in the immediate period before the onset of AMN symptoms.
Migraine was a common characteristic in the medical histories of the patients in case 1 and 2 of this article. This is a very rarely described association in literature, with only three cases in the initial review of Turbeville et al. [12] and one more recently described case by Drayer Turner et al. [21], none of which had an onset of AMN during or closely concurrent with a migraine attack. The patient in case 1, however, experienced a heavy migraine attack with visual aura around the time of onset of the AMN symptoms and attempted to interrupt the attack with triptans (5-HT1B/D agonists). Triptans effectively relieve migraine headache in 20-30% of cases, but are also known to potentially increase the risk of cardiovascular adverse effects in a low percentage of patients with a high cardiovascular risk profile [22,23]. Considering the fact that migraine itself has an associated increased cardiovascular risk [24], we argue it is safer to avoid triptans in the case of migraine attacks in women of childbearing age taking oral contraceptives, especially in the setting of a concurrent viral upper respiratory infection. This newly described association between migraine, triptans, and AMN type 2 should be considered when examining a patient with seemingly persisting visual aura, and in particular negative scotomata, in the context of migraine.
Conclusion
In this article we report three cases of AMN type 2 with similar demographic and clinical characteristics. Elaborating on the risk factors, we could assume that women in their reproductive years and known with migraine have an increased risk of AMN type 2 in the context of a viral upper respiratory infection, such as COVID-19. The association between migraine, triptans, and AMN type 2 should be considered when examining a patient with a seemingly persisting visual aura, and in particular negative scotomata, in the context of migraine.
Conflict of Interest Disclosure
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
No financial support was received for the conduction of this study.
Author Contributions
Depasse is the first author.
Study concept and design: Depasse, Kuijpers, ten Tusscher and De La Porte.
Analysis and interpretation of data: Depasse, Kuijpers, ten Tusscher and De La Porte.
Drafting of the manuscript: Depasse.
Critical revision of the manuscript and study supervision: Kuijpers, ten Tusscher and De La Porte.
Patient examinations: Depasse, Kuijpers.
References
2. Casalino G, Arrigo A, Romano F, Munk MR, Bandello F, Parodi MB. Acute macular neuroretinopathy: pathogenetic insights from optical coherence tomography angiography. Br J Ophthalmol. 2019;103(3):410-4.
3. Fawzi AA, Pappuru RR, Sarraf D, Le PP, McCannel CA, Sobrin L, et al. Acute macular neuroretinopathy: long- term insights revealed by multimodal imaging. Retina. 2012;32(8):1500-13.
4. Sarraf D, Rahimy E, Fawzi AA, Sohn E, Barbazetto I, Zacks DN, et al. Paracentral acute middle maculopathy: a new variant of acute macular neuroretinopathy associated with retinal capillary ischemia. JAMA Ophthalmol. 2013;131(10):1275-87.
5. Rahimy E, Kuehlewein L, Sadda SR, Sarraf D. Paracentral Acute Middle Maculopathy: What We Knew Then and What We Know Now. Retina. 2015;35(10):1921-30.
6. Tan PE, Yu PK, Balaratnasingam C, Cringle SJ, Morgan WH, McAllister IL, et al. Quantitative confocal imaging of the retinal microvasculature in the human retina. Invest Ophthalmol Vis Sci. 2012;53(9):5728-36.
7. Lee SY, Cheng JL, Gehrs KM, Folk JC, Sohn EH, Russell SR, et al. Choroidal Features of Acute Macular Neuroretinopathy via Optical Coherence Tomography Angiography and Correlation With Serial Multimodal Imaging. JAMA Ophthalmol. 2017;135(11):1177-83.
8. Chu S, Nesper PL, Soetikno BT, Bakri SJ, Fawzi AA. Projection-Resolved OCT Angiography of Microvascular Changes in Paracentral Acute Middle Maculopathy and Acute Macular Neuroretinopathy. Invest Ophthalmol Vis Sci. 2018;59(7):2913-22.
9. Bhavsar KV, Lin S, Rahimy E, Joseph A, Freund KB, Sarraf D, et al. Acute macular neuroretinopathy: A comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538-65.
10. Aziz HA, Kheir WJ, Young RC, Isom RF, Dubovy SR. Acute macular neuroretinopathy: a case report and review of the literature, 2002-2012. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):114-24.
11. Miller MH, Spalton DJ, Fitzke FW, Bird AC. Acute macular neuroretinopathy. Ophthalmology. 1989;96(2):265- 9.
12. Turbeville SD, Cowan LD, Gass JD. Acute macular neuroretinopathy: a review of the literature. Surv Ophthalmol. 2003;48(1):1-11.
13. Ahmed W, Suri A, Ahmed A. COVID-19 and Acute Macular Neuroretinopathy - An underlying association? Ann Med Surg (Lond). 2022;78:103847.
14. Jalink MB, Bronkhorst IHG. A Sudden Rise of Patients with Acute Macular Neuroretinopathy during the COVID- 19 Pandemic. Case Rep Ophthalmol. 2022;13(1):96-103.
15. Azar G, Bonnin S, Vasseur V, Faure C, Salviat F, Clermont CV, et al. Did the COVID-19 Pandemic Increase the Incidence of Acute Macular Neuroretinopathy? J Clin Med. 2021;10(21).
16. Drüke D, Pleyer U, Hoerauf H, Feltgen N, Bemme S. Acute macular neuroretinopathy (AMN) following COVID- 19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101207.
17. Franchi A, Rauchegger T, Palme C, Frede K, Haas G, Blatsios G, et al. Two Cases of Acute Macular Neuroretinopathy Associated with the Adenovirus-based COVID-19 Vaccine Vaxzevria (Astrazeneca). Ocul Immunol Inflamm. 2022;30(5):1234-9.
18. Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G. Acute Macular Neuroretinopathy following Coronavirus Disease 2019 Vaccination. Ocul Immunol Inflamm. 2021;29(4):730-3.
19. Gabrielle PH, Baudin F, Ben Ghezala I, Meillon C, Bron AM, Arnould L, et al. Bilateral acute macular neuroretinopathy in a young woman after the first dose of Oxford-AstraZeneca COVID-19 vaccine. Am J Ophthalmol Case Rep. 2022;25:101281.
20. Rennie AT, DeWeerd AJ, Martinez MG, Kay CN. Acute Macular Neuroretinopathy Following COVID-19 mRNA Vaccination. Cureus. 2022;14(7):e27502.
21. Drayer Turner LCE, Coebergh JA, Banerjee PJ. Maculopathy Masquerading as Migraine. Vision (Basel). 2021;5(3).
22. Negro A, Martelletti P. Gepants for the treatment of migraine. Expert Opin Investig Drugs. 2019;28(6):555-67.
23. Dodick D, Lipton RB, Martin V, Papademetriou V, Rosamond W, MaassenVanDenBrink A, et al. Consensus statement: cardiovascular safety profile of triptans (5-HT agonists) in the acute treatment of migraine. Headache. 2004;44(5):414-25.
24. Vargas BB, Dodick DW, Wingerchuk DM, Demaerschalk BM. Migraine with and without aura and risk for cardiovascular disease. Curr Atheroscler Rep. 2008;10(5):427-33.
