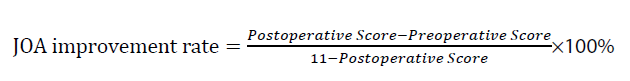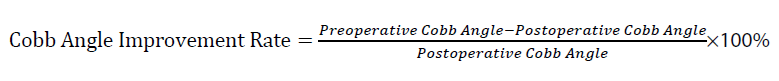Abstract
Objective: To explore the safety and clinical efficacy of using the ultrasonic bone scalpel (UBS) system for laminectomy, osteotomy of posterior longitudinal ligament (PLL) ossification, combined with correction of kyphosis deformity in the treatment of multilevel thoracic ossification of the PLL.
Methods: A retrospective analysis was conducted on patients with multilevel thoracic ossification of the PLL who underwent laminectomy, osteotomy of PLL ossification, combined with correction of kyphosis deformity using the UBS system from January 2020 to April 2023. There were 3 males and 5 females, aged 41 to 67 (mean 57.12±8.37) years; the duration of symptoms ranged from 3 to 74 (mean 33.37±23.40) months. Symptoms included progressive bilateral leg numbness and weakness, unsteady gait, and back pain in 3 cases, and urinary and fecal dysfunction in 5 cases. Seven cases showed increased muscle tone in the lower limbs, exaggerated tendon reflexes, and positive Babinski sign, while 1 case showed decreased muscle strength in the lower limbs, reduced skin sensation, decreased Achilles tendon reflex, and negative pathological signs. Preoperative Japanese Orthopaedic Association (JOA) thoracic spinal cord function score was 4.25±0.86 (3–6) points, and visual analogue scale (VAS) score was 6.87±0.99 (5–8) points. All 8 patients had multilevel thoracic ossification of the PLL, with ossification involving 4–8 segments; 5 cases were complicated by multilevel ligamentum flavum ossification. The Cobb angle of kyphosis in the segment with spinal canal stenosis was 34.62°±10.76° (24°–55°). General surgical conditions and complications were recorded, and the preoperative and postoperative JOA scores, JOA recovery rate, VAS scores, Cobb angle of kyphosis in stenotic segments, and improvement rate were statistically analyzed to evaluate the clinical efficacy and safety of the surgery.
Results: The follow-up period ranged from 12 to 26 (mean 18.25±4.68) months, with a surgical duration of 210 to 340 (mean 271.62±48.38) minutes and blood loss of 900 to 2100 (mean 1458.75±458.05) ml. The number of laminectomies ranged from 4 to 8 (mean 6.12±1.35) segments. Complications: Dural tear and cerebrospinal fluid (CSF) leakage occurred in 3 cases intraoperatively, with 2 cases undergoing tight suturing, pressure dressing, and drainage for 4–5 days, achieving primary wound healing, and 1 case undergoing intraoperative artificial dural suturing without postoperative CSF leakage; there were no complications such as loosening of internal fixation, broken nails, or rods. There was no aggravation of neurological symptoms after surgery. At the last follow-up, imaging showed no significant progression of ossification; the JOA thoracic spinal cord function score increased from 4.25±0.86 (3–6) points preoperatively to 9.75±0.70 (9–11) points postoperatively, with a statistically significant difference (t=13.015, P<0.001); JOA recovery rate was (81.06±10.93)% (67%–100%), with excellent results in 5 cases, good results in 3 cases, fair results in 0 cases, and poor results in 0 cases. VAS score decreased from 6.87±0.99 (5–8) points preoperatively to 1.37±0.74 (1–3) points postoperatively, with a statistically significant difference (t=11.881, P<0.001); Cobb angle of kyphosis in stenotic segments decreased from 34.62°±10.76° (24°–55°) to 22.12°±8.28° (8°–38°), with a statistically significant difference (t=7.395, P<0.001), and improvement rate was (36.51±14.20)% (17%–64%).
Conclusions: The application of UBS system for laminectomy, osteotomy of ossification blocks, combined with kyphosis correction technique in the treatment of long-segment thoracic ossification of the PLL is safe and effective, relatively simple to operate, and is a feasible approach.
Keywords
Thoracic vertebrae, Spinal stenosis, Ossification of PLL, Curative effect
Introduction
Thoracic ossification of posterior longitudinal ligament (T-OPLL) is a rare but severely debilitating spinal disease characterized by ossification of the posterior longitudinal ligament (PLL) in the thoracic spine, leading to spinal canal stenosis, kyphosis, compression of the spinal cord and nerve roots, and resulting in spinal cord injury and neurological dysfunction, severely impacting the patient's quality of life. The incidence of T-OPLL ranges from 0.44% to 8.92%, with a higher prevalence in Asian populations [1–3]. Multisegmental T-OPLL is commonly seen, often accompanied by ossification of the thoracic ligamentum flavum, resulting in simultaneous compression of both the anterior and posterior aspects of the spinal cord [4]. T-OPLL may be asymptomatic in the early stages, but as it progresses, neurological symptoms develop and worsen progressively. Once neurological symptoms appear, conservative treatments become ineffective, and surgical decompression is required. Traditional treatment methods include laminectomy and excision of the ossified PLL to relieve compression on the spinal cord and nerve roots. For multisegmented T-OPLL, whether using anterior or posterior approaches, such as excising the ossified PLL or performing 360° spinal decompression and ossified block anteriorization, there are disadvantages such as long operative times, significant blood loss, large surgical trauma, and high complication rates, including nerve damage. Moreover, the rate of neurological deterioration postoperatively ranges from 7% to 33% [5,6]. In recent years, with advances in medical technology, the ultrasonic bone curette, as a novel surgical tool, has been introduced into spinal surgery. The ultrasonic bone curette utilizes high-frequency vibrations to achieve precise bone tissue cutting while avoiding damage to soft tissues and neural structures. Studies have shown that the use of ultrasonic bone curette in ossified lesion excision and laminectomy has significant advantages. Furthermore, how to minimize the excision of long-segment ossified PLL while ensuring spinal cord decompression remains an unresolved issue. In this study, for thoracic spinal canal stenosis caused by long-segment ossification of the PLL, the ultrasonic bone curette was used to excise the lamina of the affected segment, and ultrasonic curette was used to sever the ossified blocks and correct the kyphosis in the areas of severe stenosis, followed by internal fixation. This indirect decompression approach aims to relieve pressure on the spinal cord and explores a comprehensive treatment plan to improve overall surgical outcomes.
The objectives of this study are:
- To explore the clinical efficacy and safety of using the ultrasonic bone scalpel (UBS) system for laminectomy, excision of ossified PLL blocks, and combined kyphosis correction in the treatment of multisegmental T-OPLL.
- To evaluate the advantages and feasibility of the UBS system in the management of multisegmental T-OPLL, and to investigate the key points of surgery and potential technical improvements for indirect decompression using the UBS system.
Methods
General information
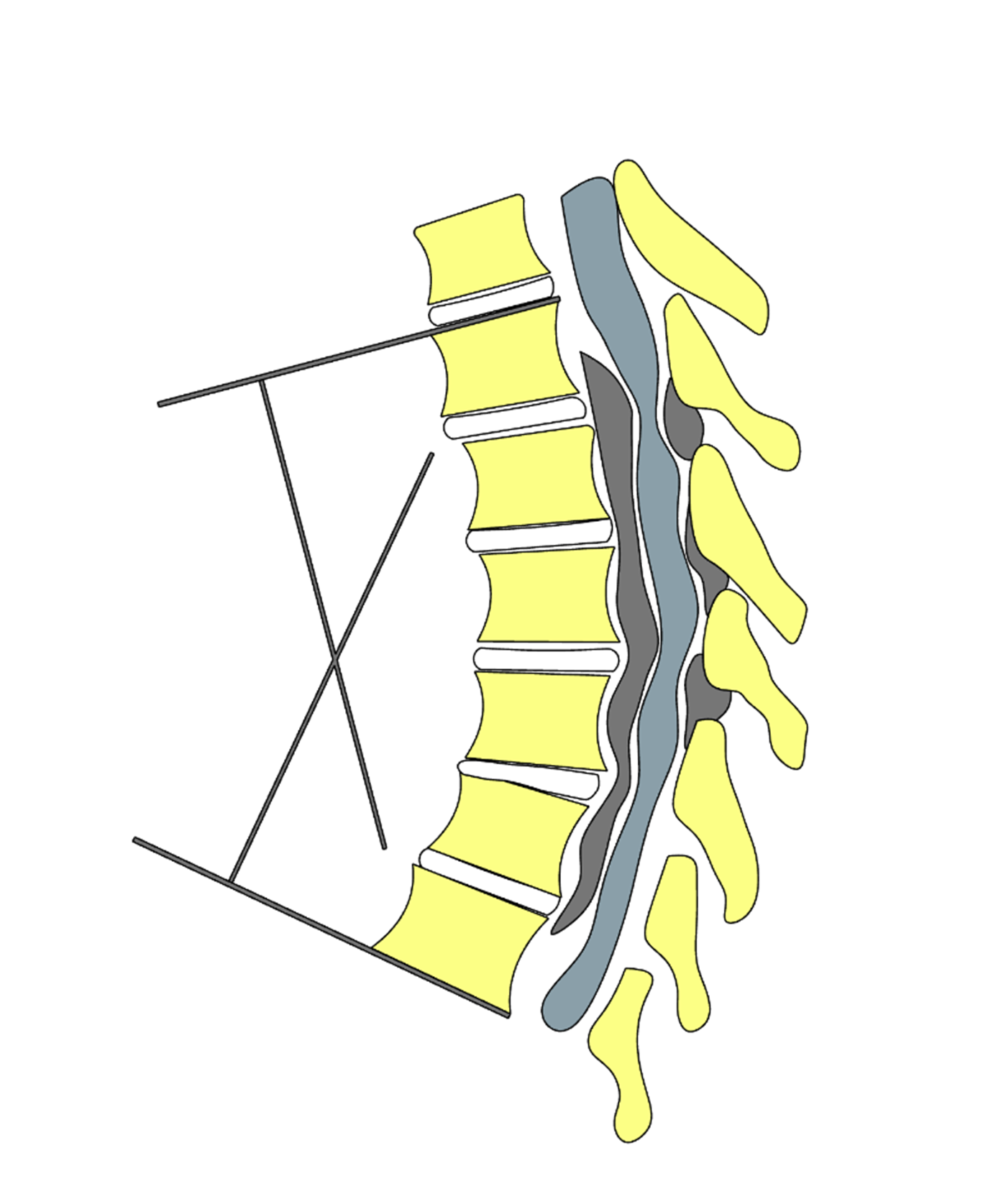
From January 2020 to April 2023, data of patients with multisegmental T-OPLL treated with UBS system for laminectomy, excision of ossified PLL blocks, and combined kyphosis correction with internal fixation were collected. A total of 8 patients were included: 3 male and 5 female, aged 41 to 67 years (mean age 57.12±8.37 years); disease duration ranged from 3 to 74 months (mean duration 33.37±23.40 months). Symptoms included progressive bilateral lower limb numbness and weakness, instability while walking, and back pain in 3 patients. Five patients had urinary and bowel dysfunction. Seven patients exhibited increased muscle tone in the lower limbs, hyperactive tendon reflexes, and positive Babinski signs, while 1 patient had reduced muscle strength, diminished skin sensation, and weakened knee and Achilles tendon reflexes, with negative pathological signs. Preoperative Japanese Orthopaedic Association (JOA) thoracic spinal cord function score was 4.25±0.86 (range: 3–6), and visual analogue scale (VAS) pain score was 6.87±0.99 (range: 5–8). All 8 patients had multisegmental thoracic ossification of the PLL, with ossified segments ranging from 4 to 8. Five patients also had multisegmental ossification of the ligamentum flavum. The Cobb angle for kyphosis in the stenotic segments was 34.62°±10.76° (range: 22°–55°). The segmental kyphosis Cobb angle at a specific motion segment was determined on the sagittal plane radiograph (or sagittal reconstruction of CT/MRI). The inferior endplate of the cranial vertebra and the superior endplate of the caudal vertebra at the target motion segment were identified. Straight reference lines were drawn along each of these endplates, and the angle formed by the intersection of these lines was measured. When necessary, perpendiculars to the endplate lines were constructed, and the acute angle between the perpendiculars was recorded. By convention, kyphotic angulation was expressed as a positive value. If endplate margins were indistinct due to degenerative changes, a best-fit line through the vertebral body margins was used. All measurements were performed in a standardized fashion to ensure reproducibility across time points and observers (Figure 1). Detailed data for the 8 patients are shown in Table 1.
Figure 1. Schematic illustration of the measurement of the Cobb angle of the stenotic segment. The angle between the superior margin of the ossified mass of the continuous posterior longitudinal ligament (PLL), which corresponds to the vertebral body, and the inferior margin of the lower margin of the ossified mass, which corresponds to the vertebral body, is the angle of the stenotic segment Cobb angle.
|
Patient ID# |
Ossified segment |
Age (year) |
Duration of illness (months) |
Surgical time (minutes) |
Blood loss (ml) |
Follow-up time (months) |
Pre-op Cobb angle |
Post-op Cobb angle |
Pre-op JOA score |
Post-op JOA score |
Pre-op VAS score |
Post-op VAS score |
|
1 |
4 |
41 |
20 |
230 |
1,360 |
18 |
22 |
8 |
3 |
10 |
6 |
2 |
|
2 |
7 |
60 |
3 |
263 |
1,520 |
12 |
41 |
26 |
4 |
11 |
7 |
1 |
|
3 |
8 |
56 |
74 |
340 |
2,100 |
21 |
24 |
20 |
4 |
9 |
5 |
1 |
|
4 |
5 |
51 |
40 |
210 |
900 |
18 |
55 |
38 |
6 |
10 |
7 |
3 |
|
5 |
7 |
57 |
46 |
290 |
1,150 |
16 |
36 |
22 |
5 |
9 |
7 |
1 |
|
6 |
5 |
67 |
44 |
223 |
920 |
26 |
26 |
20 |
4 |
10 |
8 |
1 |
|
7 |
6 |
52 |
15 |
327 |
1,750 |
13 |
37 |
23 |
4 |
9 |
8 |
1 |
|
8 |
7 |
67 |
57 |
290 |
1,970 |
22 |
36 |
20 |
4 |
10 |
7 |
1 |
Surgical method
General anesthesia was administered, and the patient was positioned prone with the chest and abdomen suspended, using electrophysiological monitoring (US Medtronic, NIM-ECLIPSE® E4). The objective of intraoperative monitoring is to utilize multimodal techniques for real-time assessment of the integrity of spinal cord conduction pathways (both sensory and motor) and nerve root function. This approach enables early detection of potential neural injury and guides intraoperative intervention, thereby reducing the risk of postoperative neurological deficits. A posterior midline incision was made to expose the affected segment and the lamina and facet joints of the two adjacent vertebral segments. Thoracic pedicle screws were implanted under O-arm navigation. An UBS was used to cut the lamina on both sides of the affected segment at the outer edge (in the laminar groove). The lamina were then lifted from the upper and lower ends, and the ligamentum flavum was separated from the dura mater, extending to the most narrowed point. The “cap removal method” was employed to excise the lamina as a whole, to complete the posterior spinal cord decompression. For continuous ossification blocks of the PLL at the apex of the kyphosis and the most severely compressed intervertebral space, the ultrasonic curette was used to scrape away the bone on the lateral side of the spinal cord, all the way down to the posterior wall of the vertebral body. The procedure exposed dural sac to protect the dural sac and spinal cord. The ultrasonic curette was used to remove the ossified PLL blocks at the intervertebral space, scraping the ossification in a V-shape (Figure 2). Then, the contralateral side was accessed to enlarge the spinal cord's lateral exposure, and the ossification at the intervertebral space was scraped off until the ossified block was disconnected. A straight connecting rod was installed, and pressure was applied between the screws at the head and tail of the osteotomy point. The procedure closed the intervertebral space and completed the "kyphosis correction" procedure. During the kyphosis correction, electrophysiological nerve monitoring (somatosensory evoked potentials [SEP]) was used to detect and avoid spinal cord and nerve injury. The transverse process cortical bone of the affected segment was removed, and granulated autograft bone was implanted for transverse process interbody fusion. All soft tissue layers were sutured tightly. Dexamethasone 20 mg was administered on the day of surgery, and 10 mg/day on the 2nd and 3rd days. Mannitol 250 ml was given 2–3 times a day. The drainage tube was removed 48–72 hours post-surgery or when the drainage volume was less than 50 ml. For patients who developed cerebrospinal fluid (CSF) leaks postoperatively, a local pressure dressing was applied for 24–48 hours, and the drainage tube was connected to a non-reflux drainage bag to maintain constant pressure drainage for 4–5 days post-surgery. The drainage tube was removed once the fluid became clear. Immediately after surgery, patients were guided in lower limb functional exercises. Early-stage postoperative lower-limb rehabilitation following spinal surgery primarily aims to prevent deconditioning, promote circulation, reduce the risk of deep vein thrombosis, and maintain basic mobility. Recommended exercises include frequent active ankle pumps with dorsiflexion-plantarflexion, gentle passive or assisted knee flexion-extension within the patient’s comfort range, and isometric contractions of the quadriceps and gluteal muscles. Short, frequent bouts of ambulation, such as transfers from bed to chair, are encouraged as tolerated, alongside supported standing balance tasks and gentle supine hip abduction-adduction movements. Key examples encompass quadriceps sets (holding contraction for 5–10 seconds), gluteal squeezes, straight leg raises, bridging, mini-squats, resisted hip abduction/adduction, step-up and step-down drills, single-leg stance balance training, controlled lunges, progressive gait training with increasing distance and cadence, and low-resistance cycling on a recumbent bike to enhance endurance. and early ambulation was encouraged once the drainage tube was removed.
Figure 2. Ultrasonic curettes and various types of curette heads.
Decompression techniques
Initial incision: Make an incision along the outer edge of the lamina, parallel to the spinal cord. Begin the procedure at the non-ossified parts of the ossified blocks at the upper and lower ends.
Lamina elevation: Lift the lamina from the non-ossified areas and separate adhesions to remove the entire posterior wall structure of the spinal canal.
Lateral expansion: At the designated apex of the ossified block, expand the lateral part of the spinal canal, moving away from the spinal cord to create sufficient space for operation.
Bone grinding and cutting: Grind and cut bone from both sides of the spinal cord to avoid stimulating the dura mater.
Ultrasonic curette: Use the non-vibrating ultrasonic curette to scrape and sever part of the ossified block from the outside in, avoiding the use of bone forceps for intra-canal operations to prevent compression or irritation of the spinal cord.
Avoid spinal cord traction: Do not traction spinal cord nerves to remove the ossified block. Resection of the pedicle and facet joints is performed on the lateral side of the spinal cord, with an angled ultrasonic curette used to scrape and remove ossified blocks on the ventral side of the spinal cord. The intervertebral disc is then removed.
Observation indicators
General indicators: Including operation time, intraoperative blood loss, and complications.
JOA score: Evaluates thoracic spinal cord function. This evaluation includes lower limb motor function (4 points), sensation (4 points), and bladder function (3 points), with a maximum score of 11. The JOA improvement rate is calculated as:
An improvement rate of ≥75% is considered excellent, 50%–75% is good, 25%–50% is fair, and <25% is poor.
VAS score: Evaluates the patient's lumbar and back pain.
Kyphosis cobb angle of the stenotic segment [7]: The Cobb angle of kyphosis in the stenotic segment is defined as the angle formed by the upper edge of the vertebral body corresponding to the upper edge of the ossified PLL block and the lower edge of the vertebral body corresponding to the lower edge of the ossified PLL block, as shown in Figure 1. The improvement rate of the Cobb angle is calculated as:
Statistical methods
Statistical analysis was performed using IBM SPSS 19.0 software. First, the Shapiro-Wilk test was used to assess the normality of the data. Data that followed a normal distribution and had equal variances were expressed as mean ± standard deviation (x±s). Preoperative and postoperative data were analysed using paired t-tests. A p-value of <0.05 was considered statistically significant.
Results
The follow-up period ranged from 12 to 26 months (18.25±4.68 months) (Mean ± SD). The surgical time ranged from 210 to 340 minutes (271.62±48.38 minutes), and the blood loss ranged from 900 to 2100 mL (1458.75±458.05 mL). The number of laminae removed ranged from 4 to 8 segments (6.12±1.35 segments).
Complications
Three patients experienced dural tears and CSF leaks during surgery. Two of them underwent careful suturing, pressure dressing, and drainage for 4–5 days, and the incision healed in one stage. One patient received artificial dural suturing during the procedure and did not experience a CSF leak postoperatively. There were no complications such as internal fixation loosening, broken screws, or rods. No significant progression of ossification was observed.
At the last follow-up, imaging showed no significant progression of ossification. The JOA score for thoracic spinal cord function increased from a preoperative value of 4.25±0.86 (range: 3–6) to 9.75±0.70 (range: 9–11), with a statistically significant difference (t=13.015, P<0.001). The JOA improvement rate was 81.06±10.93% (range: 67%–100%), with 5 patients showing excellent results, 3 showing good results, 0 showing fair results, and 0 showing poor results.
The VAS score for back pain decreased from 6.87±0.99 (range: 5–8) preoperatively to 1.37±0.74 (range: 1–3) postoperatively, with a statistically significant difference (t=11.887, P<0.001). The Cobb angle for kyphosis at the stenotic segment decreased from 34.62°±10.76° (range: 24°–55°) to 22.12°±8.28° (range: 8°–38°), with a statistically significant difference (t=7.395, P<0.001). The improvement rate was 36.51±14.20% (range: 17%–64%). A typical case is shown in Figure 3 A–L.
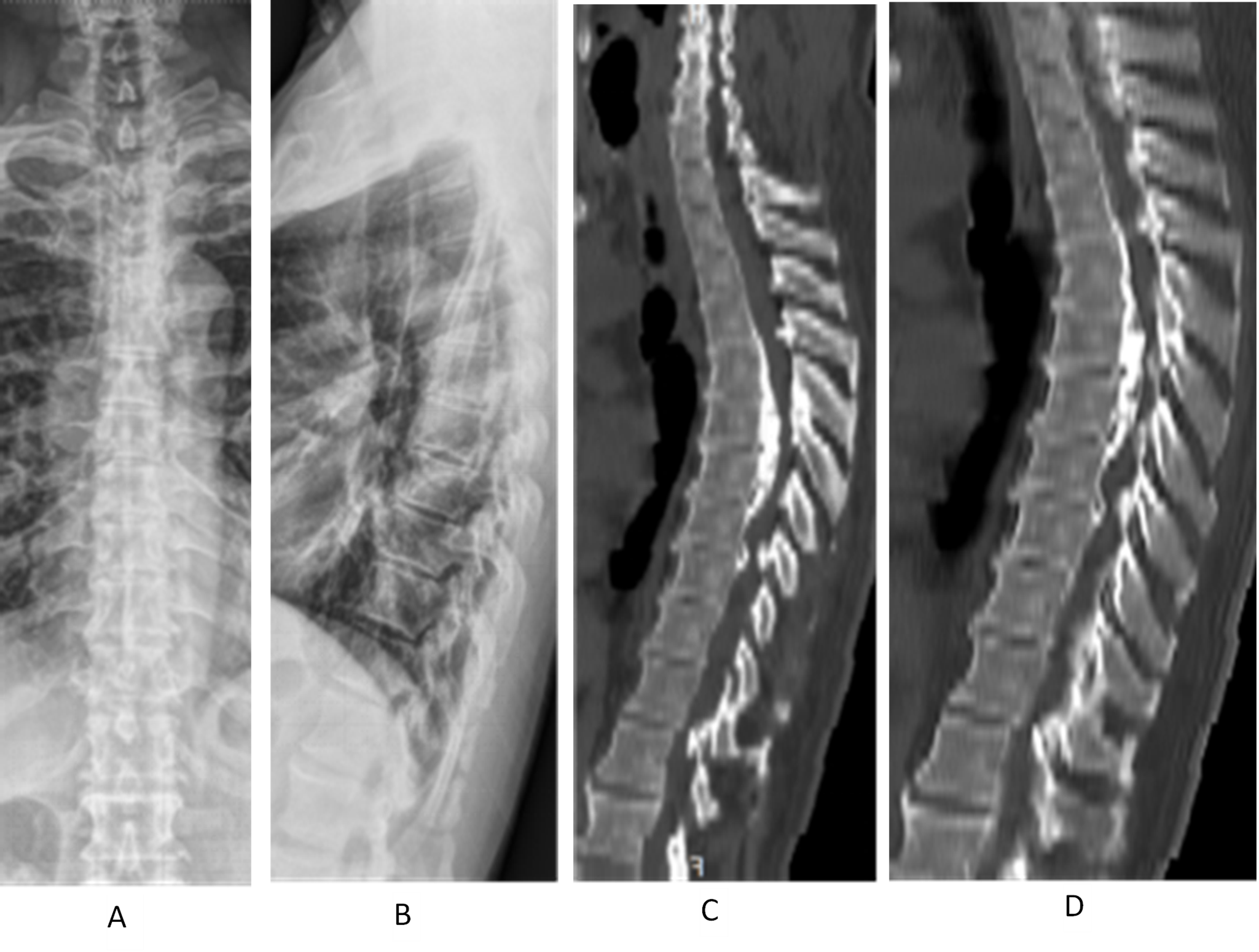
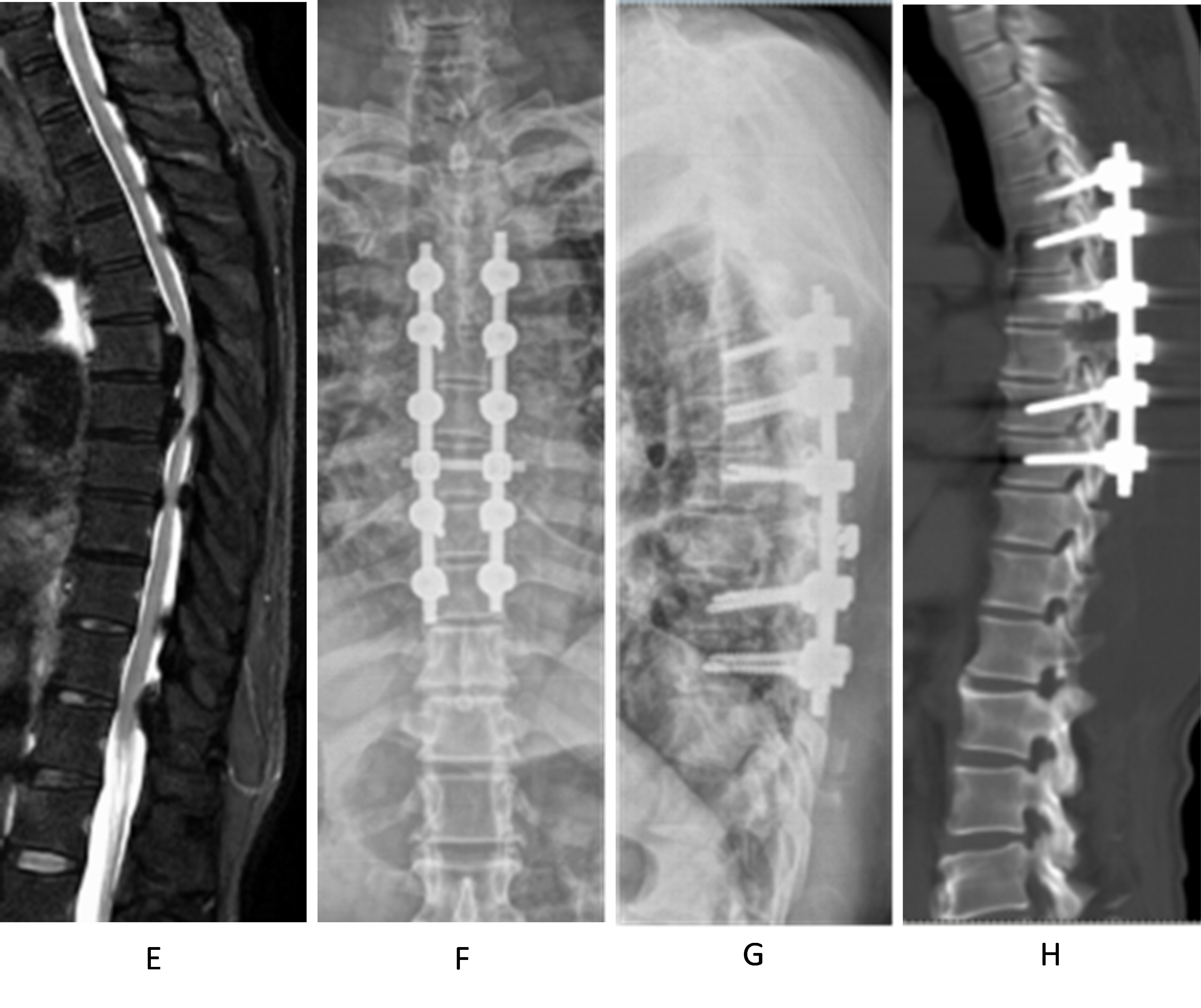
Figure 3 (A–D). Demonstrates a case of a male, 57 years old, thoracic posterior longitudinal ligament (PLL) ossification (continuous type), laminectomy, PLL ossification block cutting with ultrasonic bone knife system, and de-posteriorization orthopedic internal fixation. A and B show the frontal and lateral view of preoperative thoracic spine X-ray; C and D show the preoperative thoracic spine CT sagittal radiograph. T4–7 have continuous PLL ossification, protruding into the spinal canal. The stenosis segment has 37° of Cobb angle with posterior convexity.
Figure 3 (E–H). E shows the preoperative thoracic MRI: Ossification of the posterior longitudinal ligament (PLL) of T5–7 and ossification of the ligamentum flavum of T4–7 can be seen together with anterior and posterior compression of the segmental spinal cord. F and G show the postoperative thoracic X-ray: T3–8 pedicle pins were well positioned; H shows the postoperative thoracic CT sagittal view: T4–7 laminectomy was performed, and T5–6 PLL ossification was cut off to de-convex the kyphosis. Both facet joints were partially removed. The preoperative and postoperative Cobb's angles were 30° and 15°, respectively.
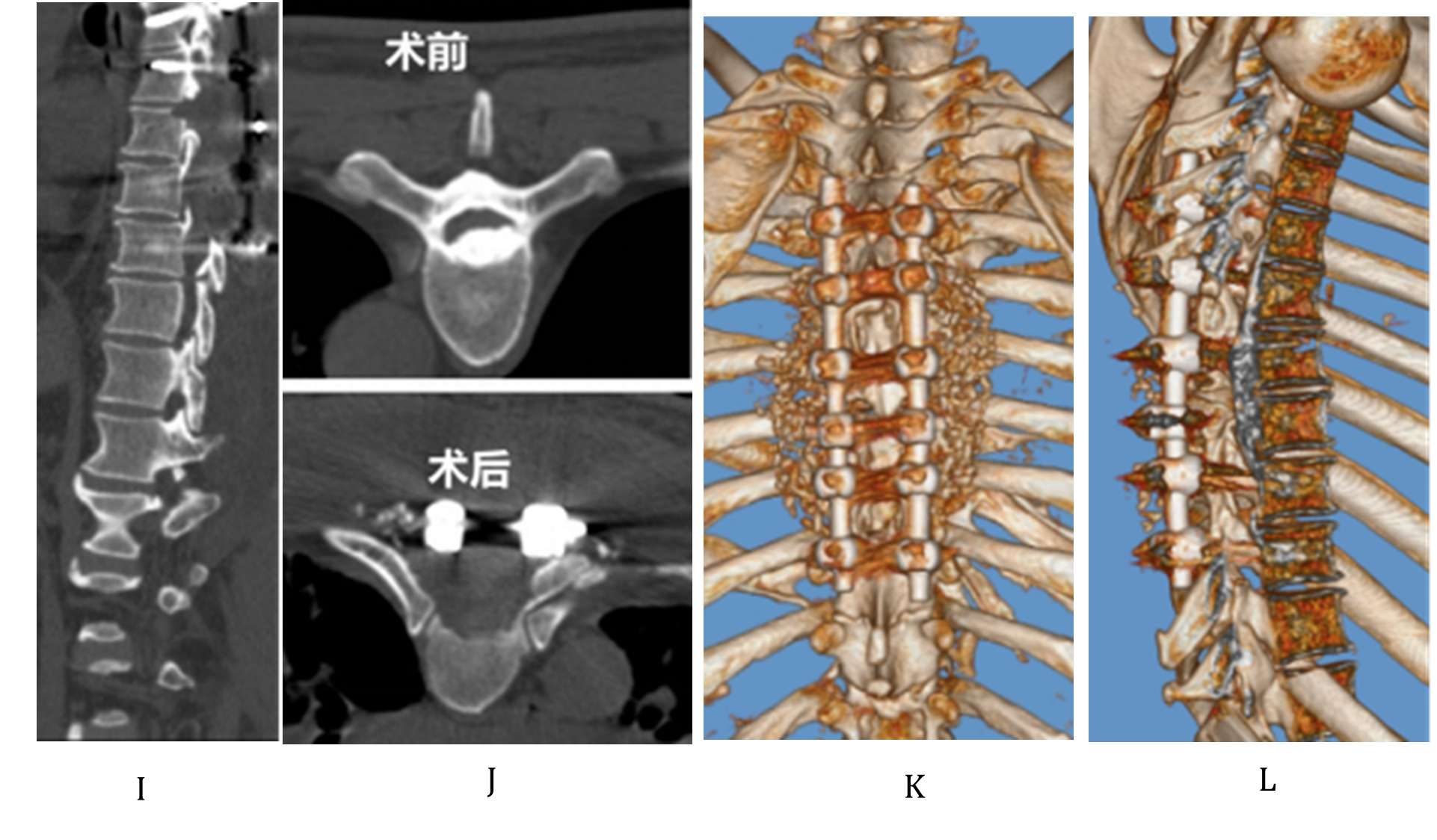
Figure 3 (I–L). I shows the postoperative thoracic spine CT sagittal view, demonstrating laminectomy of T4–7, resection of the ossified posterior longitudinal ligament (OPLL) at T5–6, and correction of kyphosis with a Cobb angle of 15° in the stenotic segment; J Comparison of preoperative (upper) and postoperative (lower) thoracic spine CT transverse views. Upper figure shows the OPLL within the spinal canal; lower figure shows the resection of the OPLL and circumferential decompression. K and L show the three-dimensional reconstruction images of thoracic spine CT at the final follow-up, demonstrating posterolateral spinal fusion and the condition within the spinal canal.
Discussion
Surgical methods for multisegmental T-OPLL
For the surgical treatment of multisegmental continuous ossification of the PLL, one approach is the anterior thoracic route for vertebral body resection and excision of ossified blocks to achieve direct decompression. Due to the physiological kyphotic structure of the thoracic spine, this approach facilitates anterior migration of the thoracic spinal cord, thereby achieving an ideal decompression effect. However, the anterior approach is complex, requiring the resection of multiple vertebrae in cases of continuous T-OPLL, leading to larger trauma. Moreover, the internal fixation may be prone to displacement and loosening due to the longer length of the implants, resulting in a high incidence of postoperative complications [8,9].
Another common surgical method is the posterior thoracic approach for 360° spinal cord decompression. This approach first removes the lamina and medial portions of the bilateral facet joints, followed by the use of the tunnel collapse technique to excise the ossified blocks. However, this method is associated with a high risk of spinal cord injury and bleeding [8–12]. Min et al. reported a study on a group of T-OPLL patients, including 18 cases of thoracic and 1 case of lumbar involvement, with 2 cases of neurological deterioration and 6 cases of CSF leakage [13].
In contrast, this study adopted the posterior approach for lamina resection of the affected segments. At the most narrowed point near the PLL ossification apex, only the ossified PLL was cut. Then, a rod-and-screw system was used to correct the kyphosis, moving the spinal cord posteriorly to achieve indirect decompression. This method minimized manipulation of the spinal cord and resulted in less bleeding. None of the 8 patients in this study experienced spinal cord injury or worsening of lower limb paralysis, indicating that this surgical approach improved the safety of multisegmental T-OPLL surgery. The average follow-up period was 17 months, and the results showed a significant improvement in spinal cord function postoperatively. At the final follow-up, the average symptom improvement rate was 85.6%, with a 100% excellent-good rate, demonstrating the favorable clinical outcomes of this surgical technique.
Application of ultrasonic curette to enhance spinal cord decompression safety
Continuous thoracic PLL ossification is often accompanied by ossification of the ligamentum flavum, and the ossified PLL and ligamentum flavum may adhere to the dura mater, increasing the risk of dural injury and CSF leakage, potentially leading to spinal cord injury. In this study, an UBS was used to perform the "cap removal" technique for complete excision of the thoracic spinal canal posterior wall. This involved making a longitudinal incision along the outer edges of the lamina and facet joints without causing vibration to the spinal cord, followed by disconnecting the non-adherent ossification at the head and tail ends. The entire posterior wall of the spinal canal was lifted, and adhesions between the dura mater and the surrounding structures were separated. When necessary, a sharp knife was used to cut through adhesions close to the lamina, completing the excision of the posterior wall.
The UBS has emerged as a transformative tool in spine surgery, leveraging high-frequency vibrations to achieve precise and selective bone cutting while minimizing damage to surrounding soft tissues, such as the spinal cord, dura mater, and nerve roots. This technology operates based on the piezoelectric effect, allowing for a level of accuracy and safety that traditional cutting tools, such as high-speed drills, cannot match. UBS offers significant advantages in terms of reducing thermal damage, enhancing surgical precision, and promoting a quicker recovery for patients [14].
The application of UBS in various spinal surgeries has been widely documented, including procedures like laminectomy, vertebrectomy, spinal tumor resections, and spinal fusion. Numerous studies have shown that UBS can significantly reduce intraoperative blood loss, shorten operation times, and lower the incidence of postoperative complications [15]. For example, a comparative study demonstrated that the mean blood loss in the UBS group was 166.0±64.3 mL, compared to 221.2±93.4 mL in the high-speed drill group, a statistically significant difference [16].
Furthermore, the versatility of UBS makes it highly effective in challenging surgical environments, such as cervical and thoracic spine surgeries, where precision and manoeuvre ability are paramount. Its variety of available tips allows for flexibility in difficult-to-reach anatomical areas, improving overall surgical outcomes [17]. However, despite its many benefits, the use of UBS is not without its limitations. High equipment costs and the need for specialized training for surgeons are key considerations, which may affect its widespread adoption. Therefore, UBS should be considered a valuable adjunct in spine surgery, chosen according to the specific needs of the procedure and the patient’s condition [18].
For the ossified PLL at the kyphosis apex or the most compressed part of the spinal cord, the ultrasonic curette was used to scrape the bone from the lateral side of the spinal cord, exposing the lateral aspect of the spinal cord. Bilateral parts of the facet joints were removed to expose the lateral edges of the intervertebral spaces. The ultrasonic curette was then used to excise and sever the ossified blocks, gradually exposing the intervertebral space. This process was performed carefully to avoid any compression or traction on the spinal cord, thus preventing spinal cord injury. Furthermore, ultrasonic cutting device coagulates the bleed while cutting bone, which make surgical field clear thus avoid potential injury to the adjacent nerves and spinal cord. After the intervertebral disc was scraped, an appropriate amount of bone graft was implanted into the intervertebral space, and compression screws were used to close the space and achieve kyphosis correction. Previous study showed that there was no significant difference with regard to the recovery rate of the JOA score between patients with and without sufficient spinal cord decompression [19], our study showed an improvement in clinical outcomes. Our study differs from others by focusing on the use of the UBS in combination with kyphosis correction for long-segment thoracic ossification of the PLL, an approach that was not widely covered in prior research. While previous studies have primarily focused on either the safety or efficacy of ultrasonic cutting devices alone, we incorporated the analysis of combined surgical techniques, which showed significant improvements in both functional outcomes and spinal alignment. The JOA score and VAS scores demonstrated substantial improvements post-surgery, and the Cobb angle of kyphosis significantly decreased, highlighting the comprehensive benefits of the UBS system in treating complex spinal conditions. These findings underline the potential of UBS systems as a reliable and effective option for spine surgeons dealing with ossification of the PLL and associated deformities.
The entire procedure was performed using the UBS system, along with electrophysiological nerve monitoring (SEP), ensuring no vibration or stimulation to the spinal cord, effectively ensuring surgical safety.
The use of the UBS and ultrasonic curette significantly reduced intraoperative blood loose, improved ability of the surgical field, and lowered the risk of transfusion. A comparative study showed that the mean blood loss in the UBS group was 166.0±64.3 mL, a statistically significant lower compared to high-speed drill group (221.2±93.4 mL), In this study, the average intraoperative blood loss was 1,458.75±458.05 mL. The blood loss is more in other surgeries. However, there is no available data to compare blood loss in surgeries without the use of the UBS or other methods such as high-speed drills in similar procedures. Further in-depth research and comparisons are needed to draw definitive conclusions on this kind of surgery. One of benefits of ultrasonic cutting device is it cut tissue and coagulate the bleeding immediately. The safe distance of ultrasonic blade from the nerve has been measured to be 1 mm away from the nerve [20]. It is safer to the nerve compared with monopolar electrosurgery coagulating device [21–23]. Due to their selective cutting properties, the UBS and ultrasonic curette prevented damage to surrounding soft tissues and spinal cord nerves, reducing the incidence of postoperative neurological complications. The high-frequency vibration of the UBS system enabled precise bone cutting, aiding in the thorough removal of ossified lesions and improving surgical outcomes.
Advantages of the surgical technique and surgical skills
The use of the UBS system for laminectomy, excision of ossified blocks, and combined kyphosis correction in the treatment of multisegmental T-OPLL offers several technical advantages:
Simplified surgical procedure: Only the continuous portion of the ossified PLL block at the apex is excised, avoiding excessive resection of the ossified blocks, thereby simplifying the surgical process. The progression of PLL ossification is influenced by multiple factors, with micromotion stress being a significant contributor to ossification growth. This surgical technique, through internal fixation and bone graft fusion, achieves stable fixation, thereby reducing the progression of ossification [24–27].
Reduced surgical steps: After complete lamina resection, kyphosis correction allows the spinal cord to move posteriorly, achieving indirect decompression. This results in a good clinical outcome while reducing the number of surgical steps.
Reduced surgical risk and blood loss: The use of the UBS and ultrasonic curette for bone cutting reduces the surgical risk and intraoperative blood loss [9].
Limitations of the study
Although the mid-term follow-up results for the 8 cases in this study showed excellent clinical outcomes, the small sample size warrants further long-term observation and improvement of the procedure and techniques.
Conclusion
In conclusion, the use of the UBS system for laminectomy, excision of ossified blocks, and combined kyphosis correction in multisegmented thoracic ossification of the PLL is a safe, effective, and relatively simple procedure, making it a feasible surgical approach.
Acknowledgements
Not applicable.
Declarations
Funding
This study was approved by the Medical Ethics Committee of Luohe Central Hospital (Ethics Approval No.: LH-KY-2020-001-021), and o Jointly Constructed Project of Henan Province Medical Science and Technology Key Research Program (Approval No.: LHGJ20230937) patient informed consent was waived for this retrospective study.
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors’ contributions
Shifeng Gu, Wei Cui and Yan He analyzed data and prepared tables, Yuwei Li, Xiu-Zhi Li, and Zimin Wang wrote the main manuscript text and prepared figures. Zimin Wang serves as the corresponding author of this manuscript. The corresponding author and the first authors contributed equally to this work. All authors read and approved the final manuscript.
Data availability
All data generated or analyzed in this study were included in this article.
Ethical approval
The study was approved by the Luoe Center Hospital of the Ethics Committee, under approval number. All procedures followed were in accordance with ethical standards and the Declaration of Helsinki.
References
2. Zhai SH, Hu PP, Liu XG. Intraoperative ultrasound assisted circumferential decompression for multilevel ossification of the posterior longitudinal ligament in thoracic vertebrae. Beijing da xue xue bao. Yi xue ban= Journal of Peking University. Health Sciences. 2022 Oct 1;54(5):1021–7.
3. Liu X, Zhai SH, Song QP, Wei F, Jiang L, Sun CG, et al. Long-Term Follow-Up of Multilevel Thoracic Ossification of the Posterior Longitudinal Ligament Following Circumferential Decompression via Posterior Approach: A Retrospective Study. Orthop Surg. 2022 Feb;14(2):298–305.
4. Liu F, Shen Y, Ding W, Yang D, Du W. Effectiveness of transarticular approach in treating thoracic spinal stenosis of calcified ligament. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Mar;25(3):311–5.
5. Zhai J, Guo S, Li J, Chen B, Zhao Y. Progression of Spinal Ligament Ossification in Patients with Thoracic Myelopathy. Orthop Surg. 2022 Sep;14(9):1958–63.
6. Morishita S, Yoshii T, Inose H, Hirai T, Yuasa M, Matsukura Y, et al. Comparison of perioperative complications in anterior decompression with fusion and posterior decompression with fusion for thoracic ossification of the posterior longitudinal ligament-a retrospective cohort study using a nationwide inpatient database. J Orthop Sci. 2022 May;27(3):600–5.
7. Sun CG, Chen ZQ, Li WS, et al. Posterior laminectomy, limited posterior longitudinal ligament ossification resection combined with kyphosis correction in the treatment of multisegmental thoracic ossification of the posterior longitudinal ligament. Chinese Journal of Orthopedics. 2019;39(4):193–200.
8. Nie H, Niu G, Zhou G, Liu T, Chen H, Li C. Clinical application of three-dimensional printing technique combined with thoracic pedicle screw track detector in thoracic pedicle screw placement. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021 May 15;35(5):586–92.
9. Li C, Li Z, Li L, Mei Y, Huang S. Angled Ultrasonic Bone Curette-Assisted Circumferential Decompression for Thoracic Myelopathy Caused by Severely Anterior Ossification. Orthop Surg. 2022 Sep;14(9):2369–79.
10. Eto T, Aizawa T, Kanno H, Hashimoto K, Itoi E, Ozawa H. Several pathologies cause delayed postoperative paralysis following posterior decompression and spinal fusion for thoracic myelopathy caused by ossification of the posterior longitudinal ligament. J Orthop Sci. 2022 May;27(3):725–33.
11. Aizawa T, Hashimoto K, Kanno H, Handa K, Takahashi K, Onoki T, et al. Retrospective comparison of the surgical results for patients with thoracic myelopathy caused by ossification of the posterior longitudinal ligament: Posterior decompression with instrumented spinal fusion versus modified anterior decompression through a posterior approach. J Orthop Sci. 2022 Mar;27(2):323–9.
12. Yang P, Ge R, Chen ZQ, Wen BT. Treatment of Thoracic Ossification of Posterior Longitudinal Ligament with One-Stage 360 Degree Circumferential Decompression Assisted by Piezosurgery. J Invest Surg. 2022 Feb;35(2):249–56.
13. Min JH, Jang JS, Lee SH. Clinical results of ossification of the posterior longitudinal ligament (OPLL) of the thoracic spine treated by anterior decompression. J Spinal Disord Tech. 2008 Apr;21(2):116–9.
14. Kumar V, Neradi D, Salaria AK, Dagar A, Singh Dhatt S, Jindal K. Role of ultrasonic bone scalpel in spine surgery: a review article. SN Compr Clin Med. 2020 Oct;2(10):1883–9.
15. Özgen U, Başak AT, Hekimoğlu M, Akçakaya MO, Öktenoğlu T, Özer AF, et al. Safety and efficacy of ultrasonic bone scalpel compared with a high-speed drill in spinal surgery: our experience in sixty cases. Int Orthop. 2025 May;49(5):1199–210.
16. Shikalgar T, Patel P, Nene A, Bhaladhare S, Puri S, Gaddiker M. Ultrasonic Bone Scalpel and Its Role In Spine Surgeries: An Article Review. J Clin Orthop. 2022 Jan;7(1):20–7.
17. Frenkel MD. Ultrasonic spine surgery – Don’t get scammed. FrenkelMD.com. 2024. Available from: https://frenkelmd.com/2024/08/23/ultrasonic-spine-surgery-dont-get-scammed/.
18. Goldstein JA. Ultrasonic bone scalpel. SpineSurgeryDoctor.com. 2025. Available from: https://www.spinesurgerydoctor.com/treatments/ultrasonic-bone-scalpel.
19. Uei H, Tokuhashi Y, Oshima M, Maseda M, Nakahashi M, Nakayama E. Efficacy of posterior decompression and fixation based on ossification-kyphosis angle criteria for multilevel ossification of the posterior longitudinal ligament in the thoracic spine. J Neurosurg Spine. 2018 Aug;29(2):150–6.
20. Chen C, Kallakuri S, Vedpathak A, Chimakurthy C, Cavanaugh JM, Clymer JW, et al. The effects of ultrasonic and electrosurgery devices on nerve physiology. Br J Neurosurg. 2012 Dec;26(6):856–63.
21. Chen C, Kallakuri S, Cavanaugh JM, Broughton D, Clymer JW. Acute and subacute effects of the ultrasonic blade and electrosurgery on nerve physiology. Br J Neurosurg. 2015;29(4):569–73.
22. Chen C, Cavanaugh JM, Kallakuri S, Tanimoto K, Broughton D, Clymer JW, et al. Acute Effects of Ultrasonic Shears and Monopolar Electrosurgery on Sciatic Nerve Electrophysiology. J Adv Med Med Res. 2016;14(12):1–8.
23. Bertke BD, Scoggins PJ, Welling AL, Widenhouse TV, Chen C, Kallakuri S, et al. Ex vivo and in vivo evaluation of an ultrasonic device for precise dissection, coagulation, and transection. Open Access Surgery. 2014 Dec 18:1–7.
24. Wang ZC, Li SZ, Qu XF, Sun YL, Yin CQ, Wang YL, et al. Transdural circumferential decompression for thoracic spinal stenosis caused by beak-type ossification of the posterior longitudinal ligament: a technical note. Br J Neurosurg. 2023 Oct;37(5):1371–4.
25. Yoshihara H, Horowitz E, Nadarajah V. Prevalence and characteristics of thoracic ossification of the posterior longitudinal ligament in 3299 Black patients: a cross-sectional study of a prospectively registered database. BMJ Open. 2022 Aug 26;12(8):e059238.
26. Ando K, Nakashima H, Machino M, Ito S, Segi N, Tomita H, et al. Postoperative progression of ligamentum flavum ossification after posterior instrumented surgery for thoracic posterior longitudinal ligament ossification: long-term outcomes during a minimum 10-year follow-up. J Neurosurg Spine. 2021 Dec 24;36(6):986–96.
27. Sun K, Zhang S, Yang B, Sun X, Shi J. The Effect of Laminectomy with Instrumented Fusion Carried into the Thoracic Spine on the Sagittal Imbalance in Patients with Multilevel Ossification of the Posterior Longitudinal Ligament. Orthop Surg. 2021 Dec;13(8):2280–8.