Keywords
HSV-2, Glycoprotein D, Mucosal Immunity, Molecular Adjuvant, CCL19, CCL28
Abbreviations
gD: glycoprotein D; DCs: Dendritic Cells; IgAASCs: IgA Ab-Secreting Cells; IRES: Internal Ribosome Entry Site; IZ: GCN4-based Isoleucine Zipper Trimerization Domain; s.c.: subcutaneous(ly); i.vag.: intravaginal(ly); MLN: Mesenteric Lymph Node; MLNL: Mesenteric Lymph Node Lymphocyte
Introduction
Herpes simplex virus type 2 (HSV-2) develops an annual incidence of 2.3 million people worldwide, which can lead to life-long latent infections, asymptomatic and reactivations [1,2], and with occasional symptomatic episodes causing ulcerative lesions in the genitalia and naus [3]. The most serious complications are the long-term neurological sequelae and death of newborns during pregnancy. Infection with HSV-2 often leads to fetal congenital malformations, developmental delay, stillbirth, and miscarriage, which causes a heavier disease economic burden and a wide range of social and psychological effects [4,5]. As the first line of defense for HSV-2 immune protection, the genital tract mucosa plays an important role. Although there are a large number of studies and clinical experiments in the past two decades, there is still a lack of safe and effective preventive vaccines. The main reason is the lack of establish long-term and effective memory immune protection at the mucosal portals [6,7].
Antigen (Ag) Components of HSV-2 Vaccine
Since the 1990s, DNA vaccines have been studied and tested for efficacy and safety as well as vaccines in clinical trial phase I to III [8,9]. Although the safety and application prospects of DNA vaccines are unquestionable, clinical trial of the HSV-2 vaccine has shown that its effectiveness in the total population is only 38% [10], and it is still ineffective for most populations [11,12]. Some studies have shown that HSV-2 antigen recombinant DNA or protein vaccines have the disadvantages of low immunogenicity and inability to establish long-term immune responses [13-15]. It is generally believed that the ideal HSV-2 vaccine should be able to eliminate viruses that enter the body without causing new infections, or eliminate latent viruses [15-18]. The target proteins of antibodies (Abs) and T cells are the Ag components most likely to become vaccine targets, and they are also the hotspots of vaccine research in recent years [19,20].
HSV-2 is enveloped double-stranded DNA viruses. The outer envelope is composed of 16 membrane proteins, out of which up to 12 glycoproteins participate in cell entry or are required for the spread of the virus in animals, albeit some are dispensable for replication in vitro [21]. Previous studies have shown that the ability of neutralizing virus induced by HSV- 2 gB is weak, and cannot increase immunity with increasing dose [22,23]. The gB protein not only induces the production of neutralizing antibodies, but also triggers cytotoxic T lymphocyte (CTL) reactions [24,25]. The gD/gC DNA vaccine, combined with aluminum hydroxide adjuvants, has been observed in animal trials of guinea pigs with significant protective effects on animals [26]. The gB, gC, gD, and gE DNA vaccines for HSV-2 have been evaluated in mouse, guinea pig and rabbit models [10]. However, many studies have shown that one or more of envelope glycoproteins used in combination cannot effectively prevent HSV-2 infection. For example, HSV-2 gD and gB DNA vaccine can have a certain preventive effect on HSV-1 strains, but only in HSV-1 and HSV- 2 serotype double-negative population to prevent 73% of people infected with HSV-2, and was invalid in protect men [ 12]. Until now, only gD has the ability to induce higher levels of neutralizing antibodies, indicating that it can be used as an ideal Ag to induce the production of neutralizing Abs.
Mucosal Immunity Associated Adjuvants
At present, the functions of adjuvants are often used to optimize the demand for HSV-2 vaccines. At present, in the research of vaccine adjuvants, cholera toxin is a good mucosal immune adjuvant, which can effectively induce mucosal immune response. However, the results showed that it can only help increase serum antigen-specific IgG and IgA antibodies after immunization. Both serum and vaginal lavage fluid have the ability to neutralize the virus in vitro, but they cannot protect against HSV-2 infection in the challenge experiment [10]. Therefore, the research on the production of high-titer antibodies in response to B cells to resist HSV-2 infection is controversial. Most research results speculate that the humoral immune response caused by B cells is a necessary but insufficient condition for protecting against HSV-2 infection [7,27]. There are also many studies still seeking proof that whether it is a necessary condition, so far, the role of humoral immunity in immune protection has not been adequately elucidated [27-29]. At present, studies on animal models have proved that T cell immunity and long-term memory are thus critical to the HSV-2 vaccine, and it is also necessary to find an ideal adjuvant to mobilize cellular immunity and mucosal immunity to prevent pathogens of intracellular infection.
In our previous publication, we found that CCL19, as a molecular adjuvant related to mucosal immunity, can significantly improve mice systemic and mucosal immunization against HSV-2 gB antigen [30]. In our recent publication, our results have shown that the CCL28 is more potent than CCL19 in assisting HSV-2 gD to induce longlasting systemic immunity in protection against genital viral challenge [31]. Our recent studies also show that chemokines have received extensive attention as molecular adjuvants [30-33], but their detailed mechanism of functions is still poorly understood [28].
Chemokines are molecules that normally exist in animals with little side effects. They have the functions of promoting the activation, differentiation, proliferation, maturation and migration of immune cells [34]. As a bridge between natural immunity and adaptive immunity, they can mediate the directional migration and activation of immunocytes and enhance immunity in a balanced manner [28,35,36]. As molecular adjuvants, they can participate in inducing and changing the intensity and type of immune response [36].
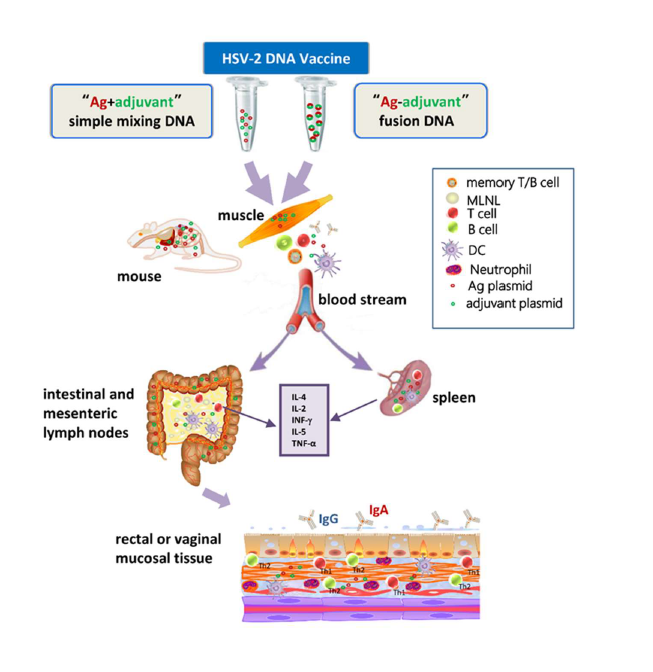
The chemokine CCL19 binds to the receptor molecule CCR7 on lymphocytes or macrophages, or CCL28 binds to CCR10/ CCR3, which can recruit lymphocytes and other innate immune-related immunocytes to migrate to lymphoid tissues for activation, and at the same time promote the migration of immunoglobulin An Ab-secreting cells (IgA ASCs) to the rectum and other mucosal sites [30,32,37]. According to this function, CCL19 and CCL28 can be used as a molecule adjuvant improves the cellular immunity and mucosal immunity of the vaccine and prevents pathogens such as HSV-2 and other pathogens from infecting through the genital tract mucous membranes. Our previous studies showed that CCL19 tends to migrate responsive T cells and DCs to the spleen and mesenteric lymph nodes (MLNs), but CCL28 tends to migrate responsive B cells to these two immune tissues, and balance enhances Th1/Th2 cellular immunity [32]; and our study has also clarified that CCL19 and CCL28 as effective mucosal immunity-related molecular adjuvants can enhance mouse HSV-2 Ag-specific immune responses and mucosal immune protection [30,31], but the cellular and molecular mechanisms based on the enhanced mucosal immune protection mediated by the chemokine/receptor axis need to be further studied. After intramuscular (i.m.) injection, DNA plasmids from Ags and adjuvants can be distributed throughout the mouse, such as liver, heart, spleen, lungs, kidneys, and other major organs [30,32] (Figure 1). Many studies have shown that upregulating the expression of CCR7 receptor can promote the migration and maturation of dendritic cells (DCs) and enhance the Ag-specific immune response [30,32,38-40]. The activation of DCs can provide a second signal for the complete activation of T cells, indirectly promote the proliferation of Ag-specific T cells, and promote the production of more antiviral cytokines (INF-γ) and Ig subclasses during immune response of Th1/Th2 cells in mucous membranes and immune tissues (Figure 1).
CCL28 binds to the receptor molecules CCR3 or CCR10 and plays an important role in both innate immunity and adaptive immunity [37,41-43]. Its main function is to recruit lymphocytes to migrate to lymphatic tissues and promote the secretion of IgA antibodies in the large intestine and breast milk [32,44-46]. This function can use CCL28 as a molecular adjuvant to promote vaccine mucosal immunity [42,45]. Studies have shown that CCL28 can help more memory CD4+ T cells settle in the nasal mucosa, which helps to establish long-term immune memory [41,45]. Most studies have shown that CCL28 as a molecular adjuvant can enhance the systemic immune response and mucosal immune response in mice [32,42,47,48], however, before the publication of our article, the protective effect of this enhanced protection against HSV- 2 infection has not been elucidated.
T cell immunity plays a pivotal role in antiviral immunity, especially the role of helper T (Th) cells [33]. After receiving Ag stimulation signal, the initial T lymphocytes can differentiate into at least four different T cell subgroups: Th1, Th2, Th17 and regulatory T cells (Treg), each of which these Th subsets performs different biological functions [49,50]. However, its main function is to influence and restrict each other by secreting different cytokines in the body, thereby forming a network of T cell immune regulation, which plays an important role in the course of autoimmune diseases and inflammatory diseases [50,51]. CCL19 or CCL28, as an immune balance regulator, significantly improved the response level and immune protection of mouse Ag-specific systemic immunity [ 33,51], but the exogenous of CCL19 or CCL28 breaks the original immune balance in the body, and its detailed response cell subtypes in response to HSV-2 infection need to be further studied.
Ag-adjuvant Fusion DNA Vaccine
The most common strategy for producing HSV DNA vaccines is to use the envelope glycoprotein of the virus as the immunogen. Envelope glycoproteins gD and gB are often used to construct eukaryotic recombinant plasmids [30,52-54]. However, the clinical study found that glycoprotein DNA immunization produces slower rate of neutralizing antibodies lower level of neutral capacity, short half-life, and limited protection rate [24]. In addition to studying the traditional “Ag and adjuvant simple mixing” of DNA vaccines, we also used GCN4-based isoleucine zipper trimerization domain (IZ) linker to design the bivalent “Ag-adjuvant fusion” of DNA vaccines. Or two DNA structures are connected by an internal ribosome entry site (IRES), each of them uses the same open reading frame (ORF). For the fusion constructs, the translation product produces an embedded bifunctional protein molecule. The general method is to remove the stop code of the first gene (not required for the construction of IRES-linked), and connect its reading frame to the second gene without a start code (IRES-linked requires a second gene to have a start code) and can be read correctly.
Considering the tendency of each amino acid to form oligomers, the appropriate linking subprotein is conducive to the correct folding of the two proteins and the production of their own independent functions, so as to achieve the theoretical translation level of the equivalent expression. GCN4-based linkers allow us to develop stable naturallike polymers and facilitated the co-expression of Ag and chemokine [36,55]. There are many design strategies for the linking method, most of which choose neutral hydrophobic amino acids, such as polypeptide chains rich in glycine and serine, whose length is very important for the protein folding and stability, the main tips for these approach are [56-58]: (1) It is very likely that the function of one or two genes will be affected by configuration changes, and (2) if the cellular location of the two gene products is different, it will also affect the normal function of the gene, such as the unbalanced gene function [59].
The GCN4-based linker (IZ) selected in our study is a peptide with a tendency to trimerization. It can stabilize the trimer protein or polypeptide, at least conducive to maintaining the multimeric composition of the fusion protein and enhancing the immunogenicity of exogenous protein [36,55]. This polypeptide chain helps to form two or more right-handed α-helical coiled-coil structures [60]. As a trimeric protein, coiled coil remains be widely used in the functional study of natural structures, and can be used to construct viral gene vaccines or subunit vaccines [61]. They have supercoiled α-helical structure, heptapeptide repeats and hydrophobic residues, which can be used to introduce some variant into de novo peptide sequence, and usually produce surprising results [61,62]. Some studies of HIV-1 DNA vaccines have shown that several GCN4-based linkers can help produce stable trimeric configuration of HIV-1 Env and CD40L connexin, while promoting the maturation of DCs and increasing the secretion of cytokines, as well as improving the Ab neutralization ability [63-66]. In view of previous studies, in order to make the fusion proteins expressing envelope glycoprotein and adjuvant form a more stable spatial structure, and perform their own independent biological functions, our research has expanded the IZ base sequence and added a hydrophobic, elastic and flexible peptide chain (G4S)2 before and after it, which can minimize the interference between the two proteins, can be translated and folded normally in the host cell, and can form protein polymer, and obtain satisfactory results after immunization [30,31]. Our recent study has proved that the “Ag-adjuvant” fusion DNA vaccine has genetic stability and repeatability, and can produce high-tipped antibodies faster, produce stronger neutralizing virus ability, and have better resistance to HSV-2 challenge; however, the bivalent DNA vaccines mediated by IRES have not shown the ideal immune protection [31]. Therefore, the DNA vaccine (simple mixed or fusion DNA vaccine) combining Ag and chemokine in this study provides a reference and basis for the development of effective preventive vaccines and clinical herpes immunotherapy methods.
Conclusion and Perspectives
CCL19 and CCL28 have potential application value as an HSV-2 vaccine adjuvant, and it is worth further verifying their adjuvant properties and mechanism of action in preclinical trials. Therefore, further study of the relationship between CCL19/CCR7 or CCL28/CCR3 (or CCR10) axis targeted activation of Th cells and HSV-2 immune protection will not only help reveal the mechanism of adjuvant action, but also help reveal the essential role in resistance to HSV-2 challenge. Several other chemokines also display important functions in mucosal immunity, such as CCL25, CCL17, CXCL13 and CXCL14 [46,67]. Our previous studies provide a broad theoretical and practical space for the design based on this immune principle.
Author Contributions Statement
Yan Yan wrote the manuscript. XW and LG edited the manuscript. All authors approved the final version.
Funding
This work was supported by the Open Research Fund Program of the State Key Laboratory of Virology of China (2019IOV005), the National Natural Science Foundation of China (81701550), the Top Talent Support Program for Yong and Middle-aged People of Wuxi Health Committee (BJ2020094), the Wuxi Key Medical Talents Program (ZDRC024).
References
2. Ayoub HH, Amara I, Awad SF, Omori R, Chemaitelly H, Abu- Raddad LJ. Analytic characterization of the herpes simplex virus type 2 epidemic in the United States, 1950-2050. InOpen Forum Infectious Diseases 2021 Apr 29., 8(7):ofab218.
3. Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. Journal of Infectious Diseases. 2008 Oct 15;198(8):1141-9.
4. Janbakhash A, Mansouri F, Vaziri S, Sayad B, Afsharian M, Abedanpor A. Seroepidemiology of herpes simplex virus type 2 (HSV2) in HIV infected patients in Kermanshah-Iran. Caspian Journal of Internal Medicine. 2012;3(4):546-9.
5. Mahiane SG, Legeai C, Taljaard D, Latouche A, Puren A, Peillon A, et al. Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS (London, England). 2009 Jan;23(3):377-83.
6. Johnston C, Koelle DM, Wald A. HSV-2: in pursuit of a vaccine. The Journal of Clinical Investigation. 2011 Dec 1;121(12):4600-9.
7. Zhu XP, Muhammad ZS, Wang JG, Lin W, Guo SK, Zhang W. HSV- 2 vaccine: current status and insight into factors for developing an efficient vaccine. Viruses. 2014 Feb;6(2):371-90.
8. Cattamanchi A, Posavad CM, Wald A, Baine Y, Moses J, Higgins TJ, et al. Phase I study of a herpes simplex virus type 2 (HSV-2) DNA vaccine administered to healthy, HSV-2-seronegative adults by a needle-free injection system. Clinical and Vaccine Immunology. 2008 Nov;15(11):1638-43.
9. Veselenak RL, Shlapobersky M, Pyles RB, Wei Q, Sullivan SM, Bourne N. A Vaxfectin®-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine. 2012 Nov 19;30(49):7046-51.
10. Zhu XP, Muhammad ZS, Wang JG, Lin W, Guo SK, Zhang W. HSV- 2 vaccine: current status and insight into factors for developing an efficient vaccine. Viruses. 2014 Feb;6(2):371-90.
11. Gilbert PB, Excler JL, Tomaras GD, Carpp LN, Haynes BF, Liao HX, et al. Antibody to HSV gD peptide induced by vaccination does not protect against HSV-2 infection in HSV-2 seronegative women. PLoS One. 2017 May 11;12(5):e0176428.
12. Cohen J. Painful failure of promising genital herpes vaccine [J]. Science, 2010, 330(6002):304.
13. Haarr L, Nilsen A, Knappskog PM, Langeland N. Stability of glycoprotein gene sequences of herpes simplex virus type 2 from primary to recurrent human infection, and diversity of the sequences among patients attending an STD clinic. BMC Infectious Diseases. 2014 Dec; 14(63):1471-2334.
14. Marshak JO, Dong L, Koelle DM. The murine intravaginal HSV-2 challenge model for investigation of DNA vaccines. InHerpes Simplex Virus 2014 (pp. 305-327). Humana Press, New York, NY.
15. Görander S, Harandi AM, Lindqvist M, Bergström T, Liljeqvist JÅ. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. Journal of Virology. 2012 Jul 15;86(14):7544-53.
16. Eo SK, Lee S, Kumaraguru U, Rouse BT. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine. 2001 Sep 14;19(32):4685-93.
17. Hoshino Y, Dalai SK, Wang K, Pesnicak L, Lau TY, Knipe DM, et al. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. Journal of Virology. 2005 Jan 1;79(1):410-8.
18. Skoberne M, Cardin R, Lee A, Kazimirova A, Zielinski V, Garvie D, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. Journal of Virology. 2013 Apr 1;87(7):3930-42.
19. Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, et al. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathogens. 2011 Sep 29;7(9):e1002277.
20. Gill N, Rosenthal KL, Ashkar AA. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. Journal of Virology. 2005 Apr 1;79(7):4470-8.
21. Lamers SL, Newman RM, Laeyendecker O, Tobian AA, Colgrove RC, Ray SC, et al. Global diversity within and between human herpesvirus 1 and 2 glycoproteins. Journal of Virology. 2015 Aug 15;89(16):8206-18.
22. Krawczyk A, Krauss J, Eis-Hübinger AM, Däumer MP, Schwarzenbacher R, Dittmer U, et al. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. Journal of Virology. 2011 Feb 15;85(4):1793-803.
23. Liu K, Jiang D, Zhang L, Yao Z, Chen Z, Yu S, et al. Identification of B-and T-cell epitopes from glycoprotein B of herpes simplex virus 2 and evaluation of their immunogenicity and protection efficacy. Vaccine. 2012 Apr 19;30(19):3034-41.
24. Caselli E, Boni M, Di Luca D, Salvatori D, Vita A, Cassai E. A combined bovine herpesvirus 1 gB-gD DNA vaccine induces immune response in mice. Comparative Immunology, Microbiology and Infectious Diseases. 2005 Mar 1;28(2):155-66.
25. Bright H, Perez DL, Christy C, Cockle P, Eyles JE, Hammond D, et al. The efficacy of HSV-2 vaccines based on gD and gB is enhanced by the addition of ICP27. Vaccine. 2012 Dec 14;30(52):7529-35.
26. Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, et al. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. Journal of Virology. 2014 Feb 15;88(4):2000-10.
27. Lee AJ, Ashkar AA. Herpes simplex virus-2 in the genital mucosa: insights into the mucosal host response and vaccine development. Current Opinion in Infectious Diseases. 2012 Feb 1;25(1):92-9.
28. Deruaz M, Luster AD. Chemokine‐mediated immune responses in the female genital tract mucosa. Immunology and Cell Biology. 2015 Apr;93(4):347-54
29. Awasthi S, Shaw C, Friedman H. Improving immunogenicity and efficacy of vaccines for genital herpes containing herpes simplex virus glycoprotein D. Expert Review of Vaccines. 2014 Dec 1;13(12):1475-88.
30. Yan Y, Hu K, Deng X, Guan X, Luo S, Tong L, et al. Immunization with HSV-2 gB-CCL19 fusion constructs protects mice against lethal vaginal challenge. The Journal of Immunology. 2015 Jul 1;195(1):329-38.
31. Yan Y, Hu K, Fu M, Deng X, Luo S, Tong L, et al. CCL19 and CCL28 Assist Herpes Simplex Virus 2 Glycoprotein D To Induce Protective Systemic Immunity against Genital Viral Challenge. Msphere. 2021 Apr 28;6(2):e00058-21.
32. Hu K, Luo S, Tong L, Huang X, Jin W, Huang W, et al. CCL19 and CCL28 augment mucosal and systemic immune responses to HIV-1 gp140 by mobilizing responsive immunocytes into secondary lymph nodes and mucosal tissue. The Journal of Immunology. 2013 Aug 15;191(4):1935-47.
33. Yan Y, Chen R, Wang X, Hu K, Huang L, Lu M, et al. CCL19 and CCR7 expression, signaling pathways, and adjuvant functions in viral infection and prevention. Frontiers in Cell and Developmental Biology. 2019 Oct 1;7:212.
34. Schaerli P, Moser B. Chemokines. Immunologic Research. 2005 Feb;31(1):57-74.
35. Zou Y, Song G, Ding L, Chen T, Wang HW, Yan WM, et al. Involvement of CXCR3-associated chemokines in MHV-3 induced fulminant hepatic failure. Virologica Sinica. 2009 Dec 1;24(6):537-44.
36. Aldon Y, Kratochvil S, Shattock RJ, McKay PF. Chemokineadjuvanted plasmid DNA induces homing of antigen-specific and non–antigen-specific B and T cells to the intestinal and genital mucosae. The Journal of Immunology. 2020 Feb 15;204(4):903-13.
37. Mora JR, Von Andrian UH. Differentiation and homing of IgAsecreting cells. Mucosal Immunology. 2008 Mar;1(2):96-109.
38. Yanagawa Y, Onoé K. CCL19 induces rapid dendritic extension of murine dendritic cells. Blood, The Journal of the American Society of Hematology. 2002 Sep 15;100(6):1948-56.
39. Hartoonian C, Sepehrizadeh Z, Yazdi MT, Jang YS, Langroudi L, Kalvanagh PA, et al. Enhancement of immune responses by codelivery of CCL19/MIP-3beta chemokine plasmid with HCV core DNA/ protein immunization. Hepatitis Monthly. 2014 Mar;14(3):e14611
40. Song JH, Kim JI, Kwon HJ, Shim DH, Parajuli N, Cuburu N, et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. The Journal of Immunology. 2009 Jun 1;182(11):6851-60.
41. Danilova E, Skrindo I, Gran E, Hales BJ, Smith WA, Jahnsen J, et al. A role for CCL28–CCR3 in T-cell homing to the human upper airway mucosa. Mucosal immunology. 2015 Jan;8(1):107-14.
42. Cha HR, Ko HJ, Kim ED, Chang SY, Seo SU, Cuburu N, et al. Mucosa-associated epithelial chemokine/CCL28 expression in the uterus attracts CCR10+ IgA plasma cells following mucosal vaccination via estrogen control. The Journal of Immunology. 2011 Sep 15;187(6):3044-52.
43. Liu J, Ren Z, Wang H, Zhao Y, Wilker PR, Yu Z, et al. Influenza virus-like particles composed of conserved influenza proteins and GPI-anchored CCL28/GM-CSF fusion proteins enhance protective immunity against homologous and heterologous viruses. International Immunopharmacology. 2018 Oct 1;63:119-28.
44. Wang P, Qi X, Xu G, Liu J, Guo J, Li X, et al. CCL28 promotes locomotor recovery after spinal cord injury via recruiting regulatory T cells. Aging (Albany NY). 2019 Sep 30;11(18):7402-15.
45. Hu S, Yang K, Yang J, Li M, Xiong N. Critical roles of chemokine receptor CCR10 in regulating memory IgA responses in intestines. Proceedings of the National Academy of Sciences. 2011 Nov 8;108(45):E1035-44.
46. Hernández-Ruiz M, Zlotnik A. Mucosal chemokines. Journal of Interferon & Cytokine Research. 2017 Feb 1;37(2):62-70.
47. Castelletti E, Lo Caputo S, Kuhn L, Borelli M, Gajardo J, Sinkala M, et al. The mucosae-associated epithelial chemokine (MEC/CCL28) modulates immunity in HIV infection. PloS One. 2007 Oct 3;2(10):e969.
48. Rainone V, Dubois G, Temchura V, Überla K, Clivio A, Nebuloni M, et al. CCL28 induces mucosal homing of HIV-1-specific IgA-secreting plasma cells in mice immunized with HIV-1 virus-like particles. PLoS One. 2011 Oct 31;6(10):e26979.
49. Levine AG, Mendoza A, Hemmers S, Moltedo B, Niec RE, Schizas M, et al. Stability and function of regulatory T cells expressing the transcription factor T-bet. Nature. 2017 Jun;546(7658):421-5.
50. Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015 Jul 1;74(1):5-17.
51. Yan Y, Zhao W, Liu W, Li Y, Wang X, Xun J, et al. CCL19 enhances CD8+ T-cell responses and accelerates HBV clearance. Journal of Gastroenterology. 2021 Aug;56(8):769-85.
52. Meseda CA, Elkins KL, Merchlinsky MJ, Weir JP. Prime-boost immunization with DNA and modified vaccinia virus Ankara vectors expressing herpes simplex virus–2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. The Journal of Infectious Diseases. 2002 Oct 15;186(8):1065-73.
53. Toka FN, Gierynska M, Rouse BT. Codelivery of CCR7 ligands as molecular adjuvants enhances the protective immune response against herpes simplex virus type 1. Journal of Virology. 2003 Dec 1;77(23):12742-52.
54. Egan K, Hook LM, LaTourette P, Desmond A, Awasthi S, Friedman HM. Vaccines to prevent genital herpes. Translational Research. 2020 Jun 1;220:138-52.
55. Ringe RP, Ozorowski G, Yasmeen A, Cupo A, Cruz Portillo VM, Pugach P, et al. Improving the expression and purification of soluble, recombinant native-like HIV-1 envelope glycoprotein trimers by targeted sequence changes. Journal of Virology. 2017 Apr 5;91(12):e00264-17.
56. Braxton CL, Puckett SH, Mizel SB, Lyles DS. Protection against lethal vaccinia virus challenge by using an attenuated matrix protein mutant vesicular stomatitis virus vaccine vector expressing poxvirus antigens. Journal of Virology. 2010 Apr 1;84(7):3552-61.
57. Bernhardt D, Müller M, Reichert AS, Osiewacz HD. Simultaneous impairment of mitochondrial fission and fusion reduces mitophagy and shortens replicative lifespan. Scientific Reports. 2015 Jan 20;5(1):1-9.
58. Namkoong H, Song MY, Seo YB, Choi DH, Kim SW, Im SJ, et al. Enhancement of antigen-specific CD8 T cell responses by co-delivery of Fc-fused CXCL11. Vaccine. 2014 Feb 26;32(10):1205-12.
59. Mason JM, Arndt KM. Coiled coil domains: stability, specificity, and biological implications. ChemBioChem. 2004 Feb 6;5(2):170-6.
60. Grigoryan G, Keating AE. Structural specificity in coiled-coil interactions. Current Opinion in Structural Biology. 2008 Aug 1;18(4):477-83.
61. Harbury PB, Kim PS, Alber T. Crystal structure of an isoleucinezipper trimer. Nature. 1994 Sep;371(6492):80-3.
62. Suzuki K, Hiroaki H, Kohda D, Tanaka T. An isoleucine zipper peptide forms a native-like triple stranded coiled coil in solution.Protein Engineering. 1998 Nov 1;11(11):1051-5.
63. Melchers M, Matthews K, de Vries RP, Eggink D, van Montfort T, Bontjer I, et al. A stabilized HIV-1 envelope glycoprotein trimer fused to CD40 ligand targets and activates dendritic cells. Retrovirology. 2011 Dec;8(1):1-5.
64. Morris AE, Remmele RL, Klinke R, Macduff BM, Fanslow WC, Armitage RJ. Incorporation of an isoleucine zipper motif enhances the biological activity of soluble CD40L (CD154). Journal of Biological Chemistry. 1999 Jan 1;274(1):418-23.
65. Luo K, Zhang H, Zavala F, Biragyn A, Espinosa DA, Markham RB. Fusion of antigen to a dendritic cell targeting chemokine combined with adjuvant yields a malaria DNA vaccine with enhanced protective capabilities. PloS One. 2014 Mar 5;9(3):e90413.
66. Melchers M, Bontjer I, Tong T, Chung NP, Klasse PJ, Eggink D, et al. Targeting HIV-1 envelope glycoprotein trimers to B cells by using APRIL improves antibody responses. Journal of Virology. 2012 Mar 1;86(5):2488-500.
67. Furukawa M, Ito S, Suzuki S, Fuchimoto D, Onishi A, Niimi K, et al. Organogenesis of Ileal Peyer’s Patches Is Initiated Prenatally and Accelerated Postnatally With Comprehensive Proliferation of B Cells in Pigs. Frontiers in Immunology. 2020 Dec 4;11:3176.