Abstract
Ruthenium readily forms coordinate-complexes and these complexes have their applications in diverse fields. A survey of literature shows that designing of new ligands that can be complexed with Ruthenium (Ru) in various oxidation states can lead to development of new materials with diverse applications. In 21st century the gravity of approach of material science together with computer science has shifted from, how to make a molecule to what molecule to make, in other words molecular design. And now these are also quantized. As we know that physics, chemistry, and biology were explored at atomic levels and finally mathematical data were fed to grow required software for aimed simulations. The aim of present study is to study halides and mixed halides of Ru(II), which more precisely can help in the development of new Ru(II) complexes of desired application and or can help in fine tuning the property of pre-existing Ru(II) complexes. Thus, this study of atomistic details of halides of Ru(II) compounds along with construction of molecular orbital diagram at a glance will provide an insight of physicochemical, biochemical, electrochemical, thermochemical, magnetic, spectrochemical, catalytic, photoactive, and materialistic details. And this will help to solve the difficulties of synthesis of various complexes yet not synthesized. We know that all chemical reaction of complex compounds will be affected firstly by availability of vacant orbital(s) on metal ion (Ru2+) and secondly by the easiness of donation of electron pair(s) by ligands as we have also studied cloud-expanding effect, nephelauxetic effect, and electrochemical series of these Ruthenium(II) halides. The substitution reaction of complex compounds will also be mechanized and new substitution products (compounds) can also be prepared as topological analysis have also been made, those yet cannot synthesize by ordinary methods in required time and required cost too. Hence the halides of Ru(II) studied firstly. With the help of results of these studies, we will able to study complexation of these halides with selective ligands to form selective complex compounds of Ru(II) yet not prepared or have difficulties in preparation.
Keywords
Halides amd mixedhalides of Ru(II), sd-hybridation, MO diagram, EHT, PM3
Introduction
The ability of Ru to exist in many oxidation states is an important property of this element which plays an important role in its applications [1]. Ru forms compounds in -2, 0, +2, +3, +4, +6, and +8 oxidation states. -2 and 0 oxidation states are unusual oxidation states (Scheme 1). Day-to-day expanding in the chemistry of Ruthenium and its complexes is due to their applications directly or indirectly pre-requisite for many of today’s problems related to anticancer drugs [2-8], polymers [9], materials science [6,8], and nanoscience [6,10-12] (Scheme 2). This is due to its versatile electron-transfer pathways [13-18]. Survey of literature shows that ruthenium and its compounds now used: in solar cells [19,20], as catalyst [2,3,7-23], as determination of calcitonin level in blood [6], in Eleecys folate RBC assay to estimate folate deficiency in RBC [6], as immunosuppressant [6], as antimicrobial agents [24], as antibiotic agents [25], as inhibitors to inhibit over production of nitric oxide in biological cell [2,3], as metallopharmaceuticals in treatment of various diseases including cancer [2,3], radio sensitizers complexes with ruthenium are used in radiation therapy, as anti-metastasis drugs [2-8]. Dragutan et al. in editorial special issue review wrote “The valorization of Ru complexes in photochemistry and agrochemicals will undoubtedly be forthcoming” [26]. Various complexes of Ru(II) with their various applications are described here. Poursharifi et al. have their focus on encapsulating Ruthenium-based carriers of nanosized structures or adduct with various macro biomolecules to generate efficacious anti-cancer therapies [6]. Ruthenium based complex compounds have also been used as drugs to activate or help to generate redox reactions [13-17]. As most of the Ruthenium complexes are less toxic than their Platinum counter parts, this offers development of Ruthenium-based anticancer drugs. (imidazole-H)[trans-RuCl4(dmso-S)(imidazole)] has entered onto phase-I clinical trial and showed anti-cancer activity against solid tumor metastasis [27]. Some bidentate N-donor or arene ligands, when complexed with Ru(II) have also been found to show significant anti-cancer activities [2-8]. Ruthenium(II) complex, “[Ru(tBu2byp)2-(2-appt)](PF6)2” has been shown to exhibit moderate cytotoxicity towards several established various human cancer cells [21]. Sun et al. [4] examined nucleotide binding properties and cytotoxicity of several ruthenium(II) complexes with quinonediimine as auxiliary ligand. Ruthenium(II) complexed with ligand 9,10-phenanthroquinonediimine has highest DNA binding constant attributed due to its flat and large surface area, which provide a geometry that permits sizeable overlap with DNA base pairs. It has mild cytotoxicity with 50% inhibitory concentration of ~200 μM against carcinoma cell lines. The cell growth inhibition activity of complex [Cl(terpy)(tmephen)Ru]+ is due its binding affinity towards purines sites of nucleotides of DNA, and this depends upon chloride ligand. Binding of the trans-[Cl4(Im)2Ru]− to apotransferrin for its anti-tumor activity takes several hours, but its Ind-derivatives, trans-[Cl4(Ind)2Ru]− showed very fast action in few minutes. To kill the undesired cells in the body using N-heterocyclic ligands complexed with Ru(II) has also been reported in literatures. This photodynamic therapy (PDT) activity of agents depends on their ability to associate with biopolymers or cell membranes or DNA [28]. The radiophysical properties of 97Ru are nearly ideal for some type of radiodiagnostic imaging [8]. Ruthenium-oxo oxalato cluster Na7[Ru4(μ-O)4(C2O4)6] exhibited promising anti-HIV-1 activity over 98% inhibition [8]. The IC50 of this compound to reduce the catalytic activity of reverse transcriptase enzyme of human immunodeficiency virus-1 in nanomolar is 1.9 as reported by Sun et al. [4]. This activity of this compound is ten times more than the most used drug 3’-azido-3’-deoxythmidine-5’-triphosphate (AZT-TP) whose, IC50 in nanomolar is 68. Further, this compound was able to show only antiviral activity instead of killing the host cells [4,5]. Anti-microbial activities in both bacterial and fungal cells of “octahedral polypyridyl ruthenium(III) complexes” were also reported by Yang et al. [24]. It is worthwhile mentioned that “octahedral ruthenium(II) complexes” have remarkable applications in Bio-medical sciences [24,25]. Early metathesis catalysis formed in situ from transition-metal halides has their limitation due to their high compassion and lower easiness. Ruthenium based catalysts have remarkably high compassion and lower easiness. In 1960s first catalytic activity of Ru based complex has been reported in literature and this was due to the RuCl3(H2O)n metathesis. The catalysis of “areneruthenium(II) complexes” ranges from hydrogen transfer to ring-closing. García-Pe?a et al., have made solventless synthesis of Ru nanoparticles along with the use of polyphosphorhydrazone dendrons as coatings leads to Ru nanoparticles in zero oxidation state which are air stable, and proved to be very good nanomaterials for hydrogenation catalysis [9]. Santoro et al., have developed tris(1,10-phenantroline)ruthenium(II)(Ru(phen)3)+2 as a thermometer of nanosized doped with nanosized silica, applicable to monitor the temperature of membrane surfaces in real membrane process [11,12]. These workers also developed nanoparticles that have application for high photochemical and strong phosphorescence activity with respect to Ru(phen)3)+2. Millett and Durham have developed a new method to study intracomplex electron transfer that utilizes a photoactive tris(bispyridine)ruthenium group [Ru(II)] which was covalently attached to a protein (cytochrome c) [18]. Application of ruthenium based sensitizers having thiocyanate and other ligands have been reviewed by Qin and Peng for dye-sensitized solar cells (DSSCs), among them several sensitizers have 10% more efficiency with respect to available sensitizers [18-20]. The aim of present study is to study the atomic and sub-atomic i.e., electronic structure of ruthenium(II) halides by evaluating eigenvalues, eigenvector, and overlap matrix of molecular orbitals [29-32]. During the study, three objectives arose: (i) to draw the 3D structure of all the compounds and to optimize their geometry (ii) to evaluate the eigenvector, overlap matrix, and eigenvalues of the compounds and (iii) build up molecular orbital diagram of compounds that at a glance provides clear cut electronic picture of the molecule, which more precisely can explain or help to explain the various properties of the molecule. The basic picture of these halides help to achieve the goal for other complexes of Ru(II) those are vital for the society. A survey of literature also shows that designing of new ligands that can be complexed with ruthenium in different oxidation states can lead to development of new materials. For new materials of diverse applications there is and will be a continuous step-by-step study to discover new ligands and their new ruthenium complexes. Newly designed complex compounds can be synthesized from their halides or by substitution reactions from known complex compounds.
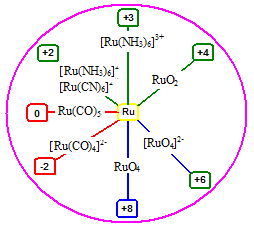
Scheme 1. Various oxidation states of Ru.
Scheme 2. Applications of Ru and its compounds.
Material and Methods
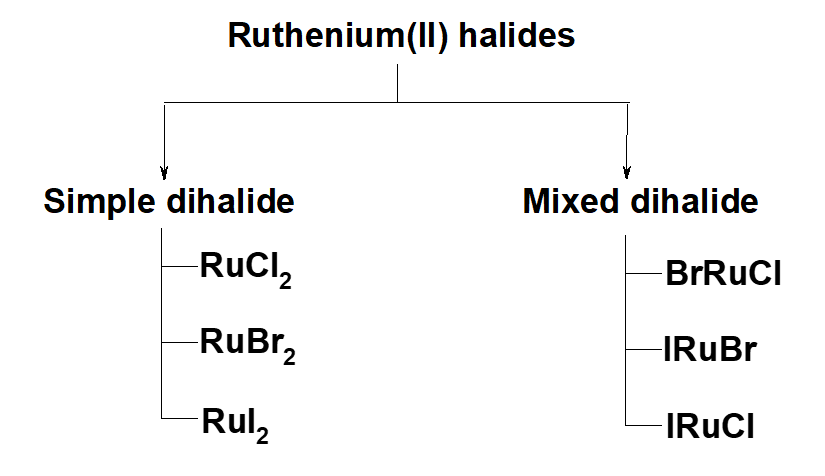
Scheme 3. Classification of ruthenium (II) halides.
The study material are ruthenium(II) halides. These halides have been classified in to two groups: simple ruthenium dihalides and mixed dihalides (Scheme 3). Three simple ruthenium dihalides exist. These are ruthenium(II) chloride, ruthenium(II) bromide and ruthenium(II) iodide, except ruthenium(II) fluoride which does not exist. Three ruthenium mixed dihalides are also possible. These are Br−Ru−Cl, I−Ru−Br, and I−Ru−Cl. The adopted methods for various calculations of ruthenium dihalides are based on Mulliken’s population analysis [23]. Mulliken defined (molecular orbital), nr,i (the contributions of electrons in each occupied MO) and nr-s,i (overlap population that explain bonding, antibonding and nonbonding nature of bond), as below:
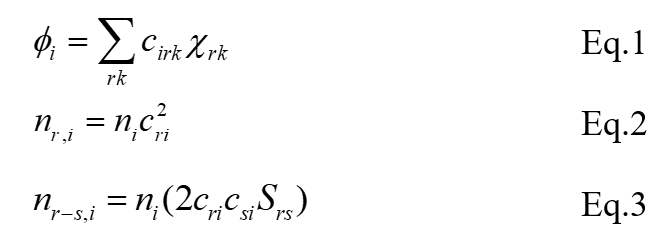
here ni is the number of electron in , i = 1-17, cri is the coefficient of atomic orbitals for Ru-atom, csi is the coefficient of AOs for other atom (Cl-2 or Cl-3) and Srs is the overlap integral between the two AOs (one of an atom Ru-1 or Cl-2 and one of another atom Cl-2 or Cl-3). In order to obtain minimum energy structure, we optimized the geometry of each halide by opting Extended Hückel Theory (EHT). All the calculations were performed on CAChe software [33,34]. From minimum energy structure we have extracted values of eigenvectors, overlap matrix, and eigenvalues. By submitting the values of eigenvector and overlap matrix in eqn.3, we have derived the values of overlap population and tabulated in Tables 1 and 2 for halides and mixed dihalides, respectively. And the summation of values of overlap population was obtained to describe bonding, nonbonding, and antibonding molecular orbitals have also been presented in these tables, respectively. After that using all above along with eigenvalues, molecular orbital diagram has been drawn (Figures 1-6). Finally topological data have been extracted to describe the feasibility of the breaking and making old and new bond formation, respectively. Before all these, we have also examined the nature and contribution of atomic orbitals and then their mixing (valence bond theory) or overlapping (molecular orbital theory). V.B. Theory and M.O. theory on their refinements gave same wave function for the molecule but they differ in their approximations only. Mulliken (1955) also correlated the bonded interaction of V.B.T. with positive overlap population and non-bonded repulsion of V.B.T. with negative population analysis [29-32].
|
MO No. |
RuCl2 |
RuBr2 |
RuI2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Σnr-s,i |
sign |
MOs |
Σnr-s,i |
sign |
MOs |
Σnr-s,i |
sign |
MOs |
|
|
f1 |
0.1924 |
+ |
BMO |
0.2091 |
+ |
BMO |
0.3042 |
+ |
BMO |
|
f2 |
0.1237 |
+ |
BMO |
0.1371 |
+ |
BMO |
0.1742 |
+ |
BMO |
|
f3 |
0.4502 |
+ |
BMO |
0.2487 |
+ |
BMO |
0.0936 |
+ |
BMO |
|
f4 |
0.2226 |
+ |
BMO |
0.1395 |
+ |
BMO |
0.0936 |
+ |
BMO |
|
f5 |
0.2228 |
+ |
BMO |
0.1395 |
+ |
BMO |
0.1248 |
+ |
BMO |
|
f6 |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
|
f7 |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
|
f8 |
0.2853 |
+ |
BMO |
0.3256 |
+ |
BMO |
0.3359 |
+ |
BMO |
|
f9 |
0.1182 |
+ |
BMO |
0.0941 |
+ |
BMO |
0.1849 |
+ |
BMO |
|
f10 |
0.1202 |
+ |
BMO |
0.0940 |
+ |
BMO |
0.0783 |
+ |
BMO |
|
f11 |
0.0735 |
+ |
BMO |
0.1433 |
+ |
BMO |
0.0783 |
+ |
BMO |
|
MOs is molecular orbitals, BMO is bonding molecular orbital, ABMO is antibonding molecular orbital and NBO is nonbonding molecular orbitals |
|||||||||
|
MO No. |
Ru(II)BrCl |
Ru(II)IBr |
Ru(II)ICl |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Σnr-s,i |
sign |
MOs |
Σnr-s,i |
sign |
MOs |
Σnr-s,i |
sign |
MOs |
|
|
f1 |
0.1604 |
+ |
BMO |
0.1358 |
+ |
BMO |
0.1585 |
+ |
BMO |
|
f2 |
0.1719 |
+ |
BMO |
0.2496 |
+ |
BMO |
0.2527 |
+ |
BMO |
|
f3 |
0.3051 |
+ |
BMO |
0.2372 |
+ |
BMO |
0.2523 |
+ |
BMO |
|
f4 |
0.1949 |
+ |
BMO |
0.1442 |
+ |
BMO |
0.1848 |
+ |
BMO |
|
f5 |
0.1949 |
+ |
BMO |
0.1471 |
+ |
BMO |
0.1848 |
+ |
BMO |
|
f6 |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
|
f7 |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
0.0000 |
0 |
NBO |
|
f8 |
0.2422 |
+ |
BMO |
0.1698 |
+ |
BMO |
0.1824 |
+ |
BMO |
|
f9 |
0.0504 |
+ |
BMO |
0.0064 |
+ |
BMO |
-0.0105 |
? |
ABMO |
|
f10 |
0.0495 |
+ |
BMO |
0.0063 |
+ |
BMO |
-0.0105 |
? |
ABMO |
|
f11 |
0.1626 |
+ |
BMO |
0.2292 |
+ |
BMO |
0.2270 |
+ |
BMO |
|
MOs is molecular orbitals, BMO is bonding molecular orbital, ABMO is antibonding molecular orbital and NBO is nonbonding molecular orbitals |
|||||||||
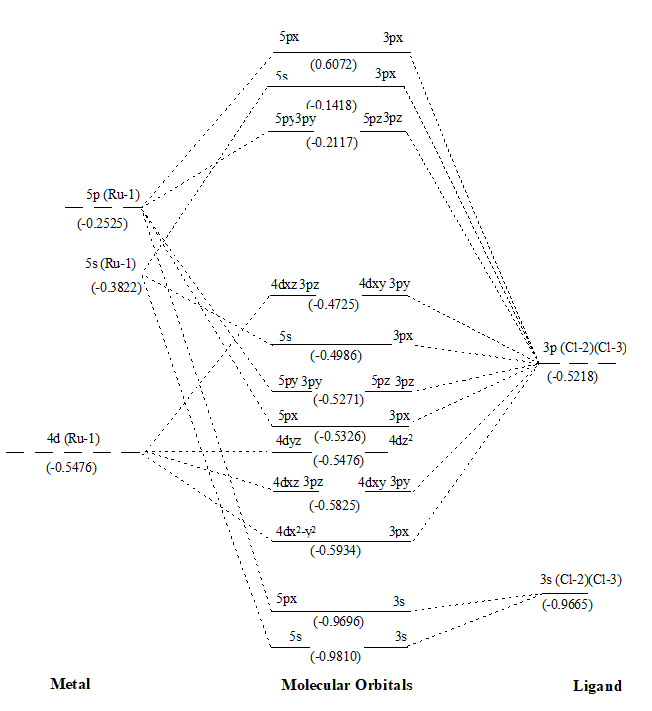
Figure 1. MO diagram of RuCl2 along with energy of AOs and MOs in eV as show in parenthesis.
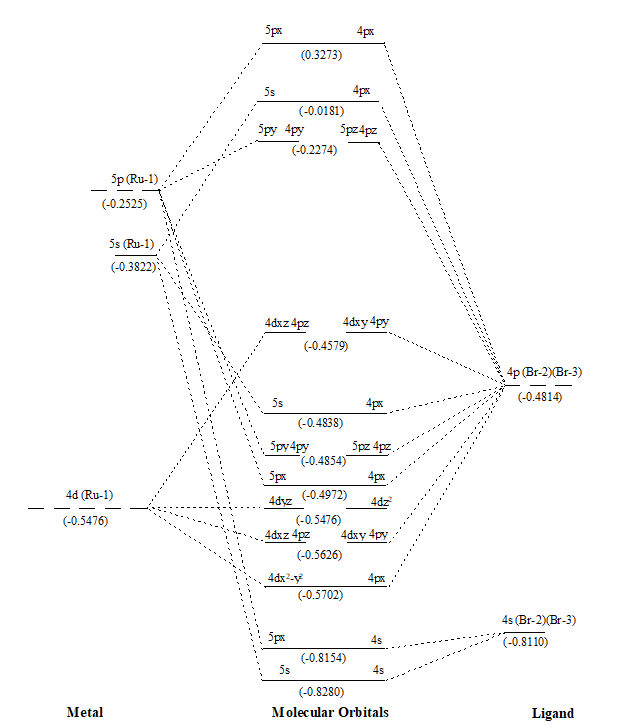
Figure 2. MO diagram of RuBr2 along with energy of AOs and MOs in eV as show in parenthesis.
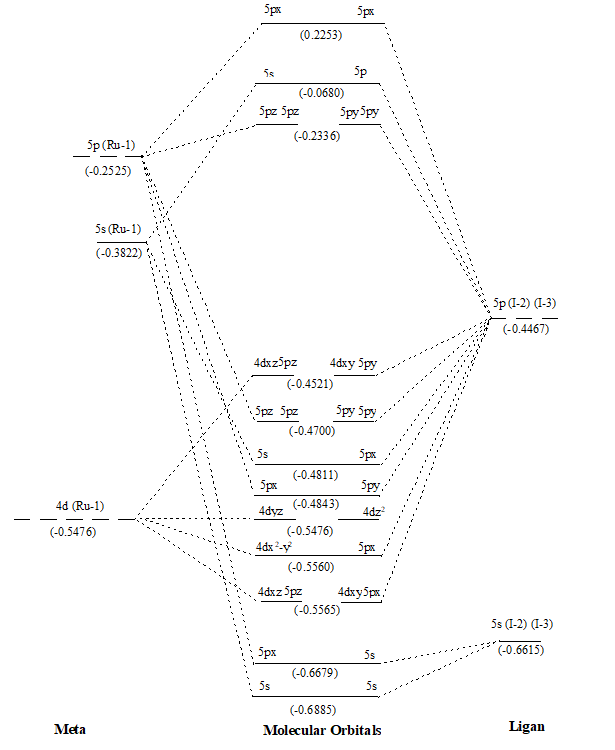
Figure 3. MO diagram of RuI2 along with energy of AOs and MOs in eV as show in parenthesis.
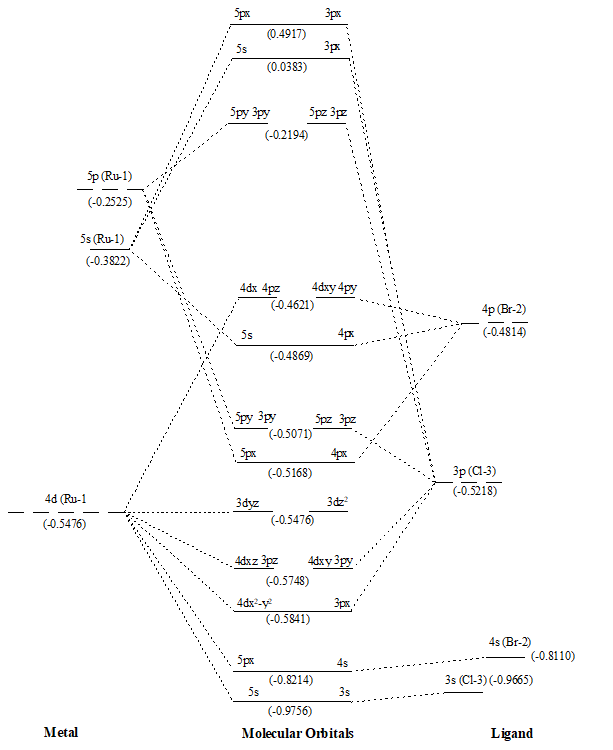
Figure 4. MO diagram of Ru(II)BrCl along with energy of AOs and MOs in eV as show in parenthesis.
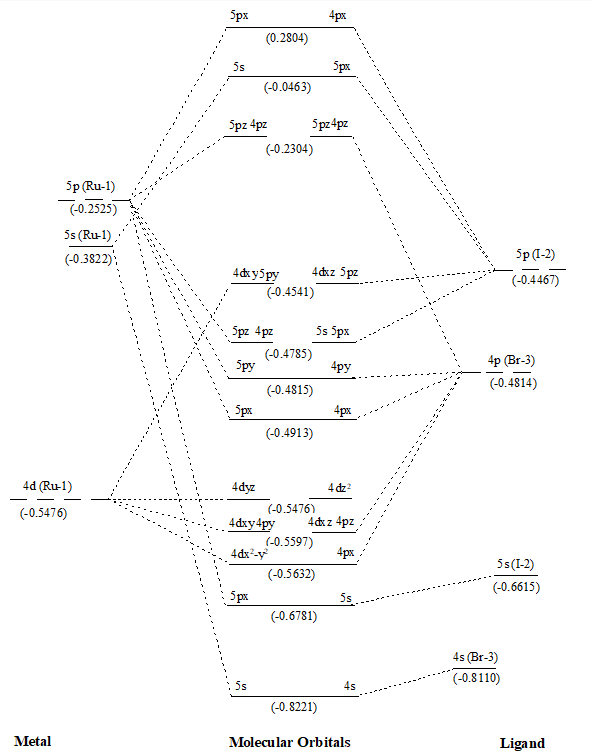
Figure 5. MO diagram of Ru(II)IBr along with energy of AOs and MOs in eV as show in parenthesis.
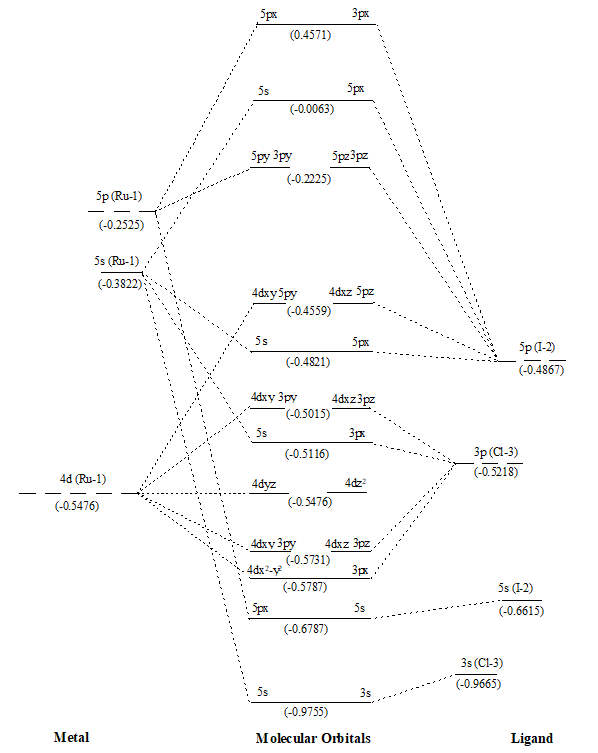
Figure 6. MO diagram of Ru(II)ICl along with energy of AOs and MOs in eV as show in parenthesis.
Result and Discussion
It was Landis, who discovered sdn-hybridization (n = 1 to 5) along with molecular shape and bond angles in his seminal publications [35-37]. Further, he also explained co-relationship between sdn-hybridization and its bond angle by plotting a graph between energy and bond angle. In order to explore atomic orbitals detail with respect to hybridization, we have examined the contribution of 4d, 5s, and 5p AOs of Ru-1 in RuX2. For this only occupied MO () were considered. And values of ci of each χ of 5s, 5px, 5py, 5pz, 4dx2-y2, 4dxy, 4xz of above eleven molecular orbitals were added and the results were tabulated in Table 3. Analysis of this table reflected that major contributions are from orbitals of 4d and 5s. Among 4d-orbitals the contribution is: 4dx2-y2 > 4dxz > 4dxy. The negligible contribution of 5s-orbutal has disclosed sd-hybridization (Schemes 4a and 5a).
|
AO |
Ru(II)Cl2 |
Ru(II)Br2 |
Ru(II)I2 |
Ru(II)BrCl |
Ru(II)IBr |
Ru(II)ICl |
|---|---|---|---|---|---|---|
|
Σ4dx2-y2 |
1.8387 |
1.9165 |
2.0004 |
2.4501 |
4.9002 |
9.8004 |
|
Σ4dxy |
1.0407 |
1.1820 |
1.2399 |
2.1893 |
4.3786 |
8.7572 |
|
Σ4dxz |
0.8364 |
0.9694 |
1.0145 |
2.1527 |
4.3054 |
8.2407 |
|
Σ5s |
0.6598 |
0.6635 |
0.6742 |
1.1012 |
2.2024 |
4.4047 |
|
Σ5px |
0.2471 |
0.2921 |
0.3385 |
0.7407 |
1.4814 |
2.8401 |
|
Σ5py |
0.1477 |
0.1494 |
0.1498 |
0.6454 |
1.2908 |
2.5816 |
|
Σ5pz |
0.1276 |
0.1254 |
0.1218 |
0.3866 |
0.7732 |
1.5464 |
Further, we know that nephelauxetic series for a given metal ion are: F- < H2O < NH3 < en < ox < SCN < Cl- - - < I- [38]. To explain cloud expending effect of these halides, we have made summation of values of nr-s,i and the results were also sketched and presented in Scheme 3b and 4b (ruthenium halides and ruthenium mixed dihalides). In case of normal ruthenium dihalides the sequence is RuI2 > RuBr2 > RuCl2, which is in accordance to nephelauxetic series i.e. the effective positive charge on Ru(II) is reduced greater by iodide and lesser by chloride (Scheme 4b). In mixed dihalides the sequence is I−Ru−Cl (ΔχI−Cl = 0.5) > I−Ru−Br (ΔχI−Br = 0.3) > Cl−Ru−Br (ΔχCl−Br =0.2). Here, the difference in electronegativity (Δχ) between X1 and X2 (X = Cl, Br, I) were used to explain the cloud-expending effect. Higher the value of Δχ greater will be the cloud-expending effect (Scheme 5b).
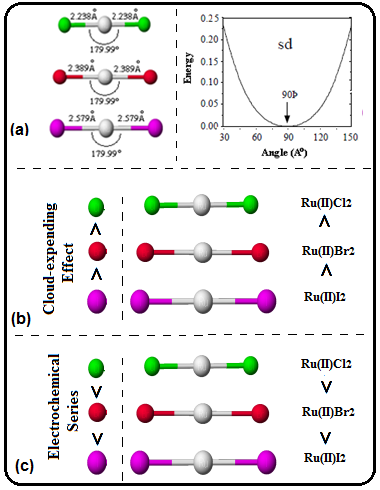
Scheme 4. (a) bond length; bond angle and plot between energy and bond angle for sd-hybridization, (b) cloud expending effect and (c) splitting effect of atoms in halides of Ruthenium(II).
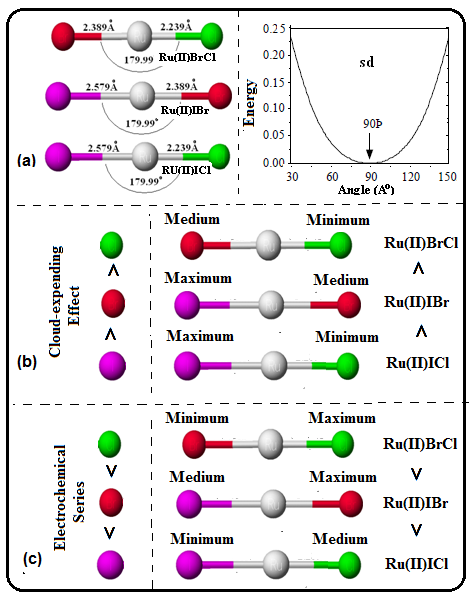
Scheme 5. (a) bond length; bond angle and plot between energy and bond angle for sd-hybridization, (b) cloud expending effect and (c) splitting effect of atoms in mixed halides of Ruthenium(II).
After that values of overlap population of eleven MOs of RuX2 and RuXX’ have also been added (∑nr-s,i) (Tables 1 and 2) to get a precise description of quantitative as well as qualitative nature of molecular orbitals and participating atomic orbitals. And the molecular orbital diagrams have also been constructed by using data of these tables along with eigenvalues. These values are also shown in parenthesis of the MO diagrams i.e. Figures 1 to 6. The AOs of respective atom involving in LCAOs are shown with their energy (in eV) and also the type of orbital combination that results in corresponding MOs (σBMOs, πBMOs, σABMOs, πABMOs, and NBOs). These diagrams at a glance provide very deep insight of the halides of Ru(II) in atomistic levels with LCAOs details. Table 1 also reflected that among the eleven molecular orbitalx, nine are BMO ( and
) and two are NBO (
and
) in simple ruthenium dihalides. In mixed dihalides as reflected by Table 2 that among the eleven molecular orbitalx, nine are BMO (
and
) and two NBO (
and
) in Ru(II)BrCl as well as in Ru(II)IBr. While in Ru(II)ICl, among the eleven molecular orbital, seven are BMO (
,
and
), two are NBO (
and
) and two are ABMO (
and
). A close look on Schemes 6 and 7, each of which explore magnitude of splitting of metal d-orbital in ruthenium(II) halide and mixed dihalides respectively, reflected the trends: RuCl2 > RuBr2> RuI2 and Ru(II)ICl > Ru(II)BrCl > Ru(II)IBr. These are also in accordance with electrochemical series [39].
|
Energy (eV) |
Ru++ |
RuCl2 |
RuBr2 |
RuI2 |
|---|---|---|---|---|
|
-0.4521 |
|
4dxz, 4dxy |
|
|
|
-0.4579 |
|
|
4dxy, 4dxz |
|
|
-0.4725 |
|
|
|
4dxy, 4dxz |
|
-0.5476 |
4dx2-y2, 4dz2, 4dxy, 4dyz, 4dxz |
4dyz, 4dz2 |
4dyz, 4dz2 |
4dyz, 4dz2 |
|
-0.5560 |
|
|
|
4dx2-y2 |
|
-0.5702 |
|
|
4dx2-y2 |
|
|
-0.5934 |
|
4dx2-y2 |
|
|
|
Energy (eV) |
Ru++ |
Ru(II)BrCl |
Ru(II)IBr |
Ru(II)ICl |
|---|---|---|---|---|
|
-0.4541 |
|
|
4dxy, 4dxz |
|
|
-0.4559 |
|
|
|
4dxy, 4dxz |
|
-0.4621 |
|
4dxz, 4dxy |
|
|
|
-0.5476 |
4dx2-y2, 4dz2, 4dxy, 4dyz, 4dxz |
4dyz, 4dz2 |
4dyz, 4dz2 |
4dyz, 4dz2 |
|
-0.5632 |
|
|
4dx2-y2 |
|
|
-0.5787 |
|
|
|
4dx2-y2 |
|
-0.5841 |
|
4dx2-y2 |
|
|
The topology of theses halides are also studied and presented in Table 4, and predicted that it is very difficult to replace Cl atom from Cl−Ru−Cl (∑∑nr-s,i = 1.8089) to form Br−Ru−Cl. But, it can be easily prepared from Br−Ru−Br (∑∑nr-s,i = 1.5309) by replacing one of its Br atoms by Cl atom through substitution reaction. Similarly, I−Ru−Br can be prepared easily by replacing one of the I−Ru−I (∑∑nr-s,i = 1.4678) by Br atom. And, I−Ru−Cl can be prepared easily by replacing one of the I−Ru−I (∑∑nr-s,i = 1.4678)by Cl atom than Br−Ru−Br. The study will be helpful in designing of new ligands that can form complexes with Ru in various oxidation states which can lead to development of new materials.
|
Molecule |
∑∑nr-s,i |
Bond |
Length (Å) |
Bond |
Length (Å) |
Bond Angle(°) |
|
Cl−Ru−Cl |
1.8089 |
Cl−Ru |
2.238 |
Ru−Cl |
2.238 |
179.99° |
|
Br−Ru−Br |
1.5309 |
Br−Ru |
2.389 |
Ru−Br |
2.389 |
179.99° |
|
I−Ru−I |
1.4678 |
I−Ru |
2.579 |
Ru−I |
2.579 |
179.99° |
|
Br−Ru−Cl |
1.5319 |
Br−Ru |
2.389 |
Ru−Cl |
2.239 |
179.99° |
|
I−Ru−Br |
1.3256 |
I−Ru |
2.579 |
Ru−Br |
2.389 |
179.99° |
|
I−Ru−Cl |
1.4215 |
I−Ru |
2.579 |
Ru−I |
2.239 |
179.99° |
Conclusions
- The study well support the Landis concepts [35-37] of sdn-hybridation (here n = 1) as bond angle and contributions of s-orbital and d-orbital of Ru(II) is maximum with negligible contribution of p-orbitals.
- These halides also support the cloud-expanding effect with experimental data and also follow the nephelauxetic effect [38].
- The results are also in agreement with spectrochemical series in connection with splitting effects [39].
- This study of atomistic details of halides of Ru(II) compounds and construction of molecular orbital diagram that at a glance will provide an insight of physicochemical, biochemical, electrochemical, thermochemical, magnetic, spectrochemical, catalytic, photoactive, and materialistic details. And this will help to solve the difficulties of synthesis of various complexes yet not synthesized.
- Topological data as presented in Table 4, will describe the feasibility of substitution reactions of halogens with other ligands to prepare various complexes yet not synthesized.
Acknowledgements
Authors are very thankful to Principal and Head of Department of Maharani Lal Kunwari Post Graduate College, Tulsipur Road, Balrampur-271201(U.P.) for laboratory facilities.
The paper is dedicated to my teacher; mentor, guru father and eminent chemist – respected Prof. P.P. Singh (DSc., F.N.A.Sc.) also known as Doctor Sahab, Ex. Principal Maharani Lal Kunwari Post Graduate College, Tulsipur Road, Balrampur-271201 (U.P.), India.
References
2. Clarke MJ. Ruthenium metallopharmaceuticals. Coordination Chemistry Reviews. 2002 Oct 1;232(1-2):69-93.
3. Clark MJ. Ruthenium metallopharmaceuticals. Coordination Chemistry Reviews. 2003 Jan;236(1-2):209-33.
4. Sun RW, Ma DL, Wong EL, Che CM. Some uses of transition metal complexes as anti-cancer and anti-HIV agents. Dalton Transactions. 2007(43):4884-92.
5. Hill CL, Weeks MS, Schinazi RF. Anti-HIV-1 activity, toxicity, and stability studies of representative structural families of polyoxometalates. Journal of Medicinal Chemistry. 1990 Oct;33(10):2767-72.
6. Poursharifi M, Wlodarczyk MT, Mieszawska AJ. Nano-based systems and biomacromolecules as carriers for metallodrugs in anticancer therapy. Inorganics. 2018 Dec 20;7(1):2.
7. Keeppler BK (Ed.) Metal Complexes in Cancer Chemotherapy. Weinheim, Germany: VCH; 1993. pp187-202.
8. Srivastava SC, Mausner LF, Clarke MJ. Radioruthenium-labeled compounds for diagnostic tumor imaging. In: Ruthenium and Other Non-Platinum Metal Complexes in Cancer Chemotherapy. Berlin Heidelberg: Springer; 1989. pp. 111-149.
9. García-Peña NG, Caminade AM, Ouali A, Redón R, Turrin CO. Solventless synthesis of Ru (0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis. RSC advances. 2016;6(69):64557-67.
10. Hudson R, Chazelle V, Bateman M, Roy R, Li CJ, Moores A. Sustainable synthesis of magnetic ruthenium-coated iron nanoparticles and application in the catalytic transfer hydrogenation of ketones. ACS Sustainable Chemistry & Engineering. 2015 May 4;3(5):814-20.
11. Santoro S, Sebastian V, Moro AJ, Portugal CA, Lima JC, Coelhoso IM, et al. Development of fluorescent thermoresponsive nanoparticles for temperature monitoring on membrane surfaces. Journal of Colloid and Interface Science. 2017 Jan 15;486:144-52.
12. Krause RA. Synthesis of ruthenium (II) complexes of aromatic chelating heterocycles: Towards the design of luminescent compounds. InCoordination Compounds: Synthesis and Medical Application 2005 Mar 2 (pp. 1-52). Berlin, Heidelberg: Springer Berlin Heidelberg.
13. Casimiro DR, Richards JH, Winkler JR, Gray HB. Electron transfer in ruthenium-modified cytochromes c.. sigma.-tunneling pathways through aromatic residues. The Journal of Physical Chemistry. 1993 Dec;97(50):13073-7.
14. Rillema DP, Allen G, Meyer TJ, Conrad D. Redox properties of ruthenium (II) tris chelate complexes containing the ligands 2, 2'-bipyrazine, 2, 2'-bipyridine, and 2, 2'-bipyrimidine. Inorganic Chemistry. 1983 May;22(11):1617-22.
15. Prier CK, Rankic DA, MacMillan DW. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chemical Reviews. 2013 Jul 10;113(7):5322-63.
16. Tfouni E. Photochemical reactions of ammineruthenium (II) complexes. Coordination Chemistry Reviews. 2000 Jan 1;196(1):281-305.
17. Wolfgang S, Strekas TC, Gafney HD, Krause RA, Krause K. Spectral and photophysical properties of ruthenium (II) 2-(phenylazo) pyridine complexes. Inorganic Chemistry. 1984 Aug;23(17):2650-5.
18. Millett F, Durham B. Design of photoactive ruthenium complexes to study interprotein electron transfer. Biochemistry. 2002 Sep 24;41(38):11315-24.
19. Qin Y, Peng Q. Ruthenium sensitizers and their applications in dye-sensitized solar cells. International Journal of Photoenergy. 2012 Jan 1;2012.
20. Nazeeruddin MK, De Angelis F, Fantacci S, Selloni A, Viscardi G, Liska P, et al. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. Journal of the American Chemical Society. 2005 Dec 7;127(48):16835-47.
21. Bruneau C, Dixneuf PH, editors. Ruthenium in Catalysis. Heidelberg: Springer; 2014.
22. Wang L, Duan L, Tong L, Sun L. Visible light-driven water oxidation catalyzed by mononuclear ruthenium complexes. Journal of Catalysis. 2013 Oct 1;306:129-32.
23. Laine TM, Kärkäs MD, Liao RZ, Åkermark T, Lee BL, Karlsson EA, et al. Efficient photochemical water oxidation by a dinuclear molecular ruthenium complex. Chemical Communications. 2015;51(10):1862-5.
24. Yang Y, Liao G, Fu C. Recent advances on octahedral polypyridyl ruthenium (II) complexes as antimicrobial agents. Polymers. 2018 Jun 10;10(6):650.
25. Donnici CL, Nogueira LJ, Araujo MH, Oliveira SR, Magalhães TF, Lopes MT, et al. In vitro studies of the activity of dithiocarbamate organoruthenium complexes against clinically relevant fungal pathogens. Molecules. 2014 Apr 24;19(4):5402-20.
26. Dragutan I, Dragutan V, Demonceau A. Editorial of special issue ruthenium complex: the expanding chemistry of the ruthenium complexes. Molecules. 2015 Sep 18;20(9):17244-74.
27. Berners-Price SJ, Sadler PJ. Phosphines and metal phosphine complexes: relationship of chemistry to anticancer and other biological activity. In Bioinorganic Chemistry 2005 Mar 3 (pp. 27-102). Berlin, Heidelberg: Springer Berlin Heidelberg.
28. Mesmaeker AK, Lecomte JP, Kelly JM. Photoreactions of metal complexes with DNA, especially those involving a primary photo-electron transfer. Electron Transfer II. 2005 May 28:25-76.
29. Mulliken RS. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. I. The Journal of Chemical Physics. 1955;23:1833.
30. Mulliken RS. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. II. Overlap Populations, Bond Order, and Covalent Bond Energies. The Journal of Chemical Physics. 1955;23:1841.
31. Mulliken RS. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. III. Efffects of Hybridization on Overlap and Gross AO Populations. The Journal of Chemical Physics. 1955;23:2338.
32. Mulliken RS. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. The Journal of Chemical Physics. 1955;23:2343.
33. Shukla A, Mishra KK, Soni AK. sd-Hybridization, Population Analysis and MOT In Ru2+. I. Simple Ruthenium Dihalides. IOSR Journal of Engineering (IOSRJEN). 2019;9(12):28-37.
34. Shukla A, Mishra KK, Soni AK. sd-Hybridization, Population Analysis and MOT in Ru2+. II. Ruthenium mixeddihalides. IOSR Journal of Engineering (IOSRJEN). 2019;9(12):38-47.
35. Landis CR, Lipkowitz KB, Boyd DB. Reviews in Computational Chemistry. Vol. 6, VCH, 1995, Chapter 2.
36. Landis CR, Cleveland T, Firman TK. Making sense of the shapes of simple metal hydrides. Journal of the American Chemical Society. 1995 Feb;117(6):1859-60.
37. Landis CR, Firman TK, Root DM, Cleveland T. A valence bond perspective on the molecular shapes of simple metal alkyls and hydrides. Journal of the American Chemical Society. 1998 Mar 4;120(8):1842-54.
38. Tchougréeff AL, Dronskowski R. Nephelauxetic effect revisited. International Journal of Quantum Chemistry. 2009;109(11):2606-21.
39. Shimura Y. A quantitative scale of the spectrochemical series for the mixed ligand complexes of d6 metals. Bulletin of the Chemical Society of Japan. 1988 Mar;61(3):693-8.
