Abstract
Antibody-drug conjugates (ADCs), combining the best features of monoclonal antibodies and small molecule drugs, are considered the “guided missiles” for cancer therapy, with eleven FDA-approved products. Yet the ADC modality still presents a huge challenge to drug developers, especially for targeting tumors with low abundance and/or heterogeneity of tumor-associated antigens. To improve therapeutic index and minimize drug product heterogeneity, a recent study by Procopio-Melino et al. [1] describes a revolutionary manufacturing process that produces superior quality next-generation site-specific cysteine (Cys)-based ADCs. It implements a unique Cys-capping technology for chemoselective-drug conjugation through a novel metabolic engineering approach in CHO cells. This innovative platform also provides a feasible path forward to integrate Cys-based site-specific conjugation with other site-specific conjugation methodologies for developing
multi-payload ADCs.
Keywords
Antibody drug conjugates, Cancer and the Immune System, Cancer immunotherapy, Cancer Therapy, Multi-payload ADCs
ADCs-Complex Biologics
With a burst of nine FDA approvals granted since 2017, ADCs are riding a significant market boom and gathering optimism as a promising therapeutic approach [2-8]. With >80 investigational candidates and around 150 active clinical trials [9], ADCs are among the fastest-growing drug classes in cancer therapeutics targeting both solid tumor indications and hematological malignancies. ADCs are complex molecules composed of three key components: a tumor-associated-antigen (TAA)-targeting monoclonal antibody, a cytotoxic drug payload, and a cleavable or non-cleavable linker. Conjugation technology is another important aspect of ADCs, by which payloads and linkers are covalently attached to the antibodies. Conventional drug conjugation methods are either through less precise interchain disulfide Cys or random lysine residue in the antibody components, resulting in a huge product heterogeneity with limited control over the conjugation locations. Next-generation site-specific conjugation technologies (Summarized in Table 1; reviewed by [10-12]) define precise and proper drug attachments, which determine drug potency and exposure, allow for an optimized and tunable structure-activity relationship, as well as better characterized and monitored homogeneous drug products.
|
Methodologies |
Conjugation site |
Merits & Demerits |
Reference |
|---|---|---|---|
|
Cys-based SSC |
Unpaired Cys residues |
Simple, fast, and versatile conjugation reactions, yet requiring redox treatments |
[30] |
|
Transglutaminase-mediated SSC |
glutamine residues through amide bonds |
Highly specific conjugation reactions, yet with more mutations needed and enzyme-sensitivity to hydrophobic solution |
[31,32] [33] |
|
Genetic code-expansion SSC |
Unnatural amino acids with biorthogonal chemical handles |
Highly specific conjugation reactions, yet with complex cell culture production conditions |
[34] |
|
Sortase-based SSC |
LPXTG tag at the C-terminus of a polypeptide with the N-terminal oligoglycine conjugation |
Highly specific conjugation reaction, yet with more mutations needed and enzyme-sensitivity to hydrophobic solution |
[35] |
|
Glycotransferases-based SSC |
Modified monosaccharides that enable bio-orthogonal chemical conjugation |
Highly specific conjugation reaction, yet with complex cell culturing and enzyme-sensitivity to hydrophobic solution |
[12,36] |
|
Formylglycine-generating enzyme-based SSC |
Engineered Cys residue in a specific peptide sequence such as CXPXR to produce an aldehyde tag for linker conjugation |
Highly specific conjugation reaction, yet with more mutations and specific cell line engineering needed. |
[37] |
|
Met-based SSC |
Engineered Met residues for selective conjugation |
Efficient conjugation, yet requiring more specific sites to be defined in antibodies. |
[38,39] |
|
ADP-ribosyl cyclases-based SSC |
Arabinosyl-ester conjugation through the catalytic glutamate 226 of CD38 fusion proteins |
Highly specific conjugation reaction, yet requiring a large fusion with CD38 protein. |
[40] |
|
Tyr-based SSC |
Tubulin tyrosine ligase attaches unnatural Tyr derivatives as small bioorthogonal handles to proteins containing a short tubulin-derived recognition sequence (Tub-tag). |
Highly specific conjugation reaction, yet with more mutations needed and enzyme-sensitivity to hydrophobic solution |
[41] |
|
Lys-based SSC |
Conjugation of 1,3-diketone and β-lactam linker-payloads to a reactive and catalytic Lys of anti-hapten antibody |
Highly specific conjugation reaction, yet requiring protein fusion with an antibody Fab. |
[42] |
|
SpyLigase-mediated SSC |
Conjugation of Ktag payloads to Spy-tag |
Highly specific conjugation reaction, yet requiring tag fusions. |
[43] |
|
Phosphopantetheinyl transferases (PPTases)-mediated SSC |
Serine-specific conjugation in a PPTase recognition sequence (11 amino acids) |
Highly specific conjugation reaction, yet with more mutations needed and enzyme-sensitivity to hydrophobic solution |
[44] |
|
Horseradish peroxidase (HRP)mediate Tyr SSC |
HRP catalyzed modification on surface-exposed Tyr residue for conjugation |
Highly specific conjugation reaction, yet requiring more specific Tyr sites to be defined in antibodies. |
[45] |
Manufacturing Challenges of Site-specific ADCs
However, manufacturing high-quality site-specific ADCs for clinical confirmation often encounters significant technical hurdles. For the most established Cys-based site-specific conjugation, it requires a complicated full reduction and re-oxidation conjugation process [13], due to a disulfide capping on the engineered unpaired Cys residues (Figure 1, [13-15]). This so-called Cys-capping modification is a type of post-translational modifications (PTMs) that are the disulfides typically with either Cys or glutathione (Figure 1). It is also detectable in endogenous membrane-bound or secreted proteins with solvent-exposed unpaired Cys residues [16-18]. This PTM on antibodies can have biological impacts which have been shown to inactivate [18] or enhance [16] antibody-antigen interaction, as well as affecting protein stability [19]. Since the Cys-cappings contain disulfide bonds, a strong reducing agent such as tris(2-carboxyethyl) phosphine (TCEP) is needed to remove this PTM to generate reactive thiol for drug conjugation. Hence the treatment also disrupts the antibody inter-chain disulfides and a reoxidation step is necessary to reform them after dialysis for removing byproducts and excess reagents. These extensive manipulations introduce disulfide scrambling, impact product quality, and limit the compatibility of the Cys-based site-specific conjugation method with other site-specific methodologies.
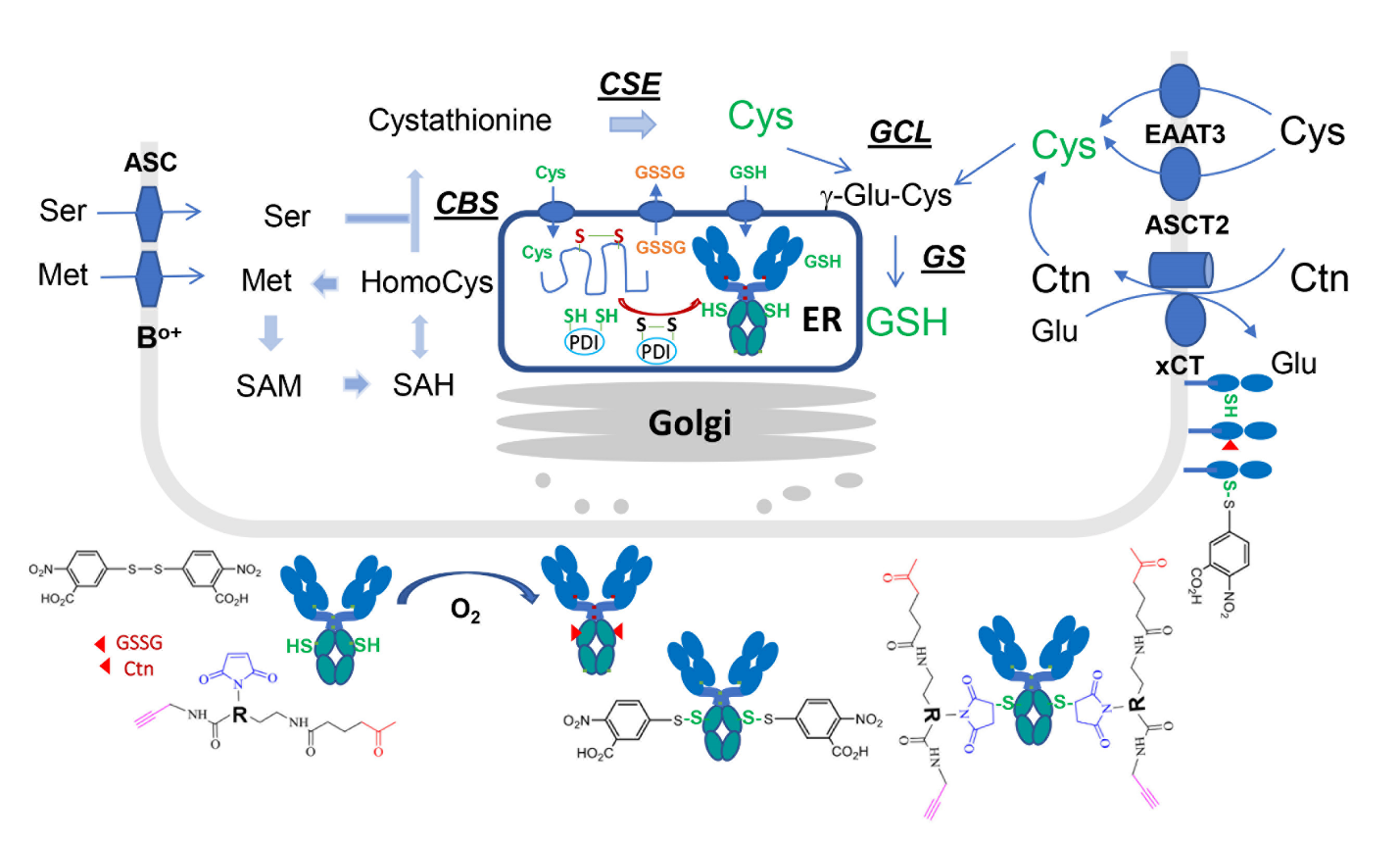
Figure 1. Cys biosynthesis metabolic pathways and Cys-capping cycle. Cys can be taken up either as Ctn through the Ctn-Glu antiporter system xCT or as Cys through the excitatory amino acid transporter (EAAT3) or the Ala-Ser-Cys-transporter 2 (ASCT2) [21]. Ctn is reduced to Cys in the cytosol. Cys can be de novo synthesized from Ser and Met which are transported by neutral amino acid transport system (Bo+ and ASC) [20]. Met is activated by ATP to form S-Adenosylmethionine (SAM) by Met adenosyltransferase. SAM mediated transmethylation reaction produces coproduct of S-adenosylhomocysteine (SAH) which is hydrolyzed to yield adenosine and homoCys. Remethylation of homoCys by Met synthase can regenerate Met. In the transsulfuration pathway, the enzyme cystathionine β-synthase (CBS) catalyzes the conversion from homoCys and serine into cystathionine, and cystathionine γ-lyase (CSE) catalyzes the further conversion into Cys. In the synthesis of glutathione (GSH), glutamate-Cys ligase (GCL) and glutathione synthase (GS) convert Cys into γ-glutamyl-Cys (γ-Glu-Cys) and further into GSH. For Cys-capping cycle, plasma membrane proteins or extracellular proteins (e.g., antibodies) form native disulfide bonds through protein disulfide isomerase (PDI) proteins in the lumen of ER. Although there are putative ER transporters for the import of Cys or GSH into ER lumen, the unpaired Cys residues in these proteins remain uncapped, due to less oxidized environment. Once the proteins reach into exocellular space, Ctn, GSSG, DTNB, or dual-functionalization linkers in the culture medium then forms different types of Cys-capping with the free thiol of the unpaired Cys residues.
Addressing Manufacturing Challenges of Cys-based Site-specific ADCs
Procopio-Melino et al. [1] tackle the aforementioned challenges by taking advantage of a surprising scientific observation that the Cys-capping PTM can be chemically engineered for improving the drug conjugation process. The same group previously reported that the Cys capping modification likely took place outside mammalian cells, not in the lumen of endoplasmic reticulum (ER) where protein disulfides normally occurred (Figure 1, [14]). This finding enables them to produce antibodies with a labile capping group on the engineered unpaired Cys residues during cell culturing. This chemically engineered labile capping group can be selectively removed with a mild reductant, without disrupting the interchain disulfides of the antibody. This strategy should avoid the full reduction-reoxidation process and keep the protein folding intact. During the study, they found that Ellman’s reagent, 5,5’-dithio-bis-(2-nitrobenzoic acid) (DTNB), which reacts readily with free thiols, generated Cys-TNB-disulfide that has a weak redox potential. By screening a number of reductants, they discovered that Tris (3-sulfonatophenyl) phosphine (TSPP) can selectively remove TNB-caps without attacking the interchain disulfides.
The major obstacle to accomplishing this strategy of selective reduction was establishing a CHO manufacturing platform for generating TNB-capped materials for clinical development conjugation. At the research scale, fully TNB-capped antibodies in CHO cells can be produced through the exchange to Cys-free medium [14]. As shown in Figure 1, Cys is a semi-essential amino acid to animal cells. It can be synthesized from Met and Ser [20,21], yet not enough to support cell growth. Procopio-Melino et al. [1] found that Cys is actually essential to CHO cells as some of the enzymes in its metabolism cycle were deleted from the genome. They noticed that Cys was rapidly depleted during CHO cell expansion, likely for protein synthesis and glutathione synthesis [21]. There are several cystine transporters for active cystine uptake in support of essential cellular activities (Figure 1). It appears that Cys capping in the medium is a slow reaction. When cystine concentration in the medium is low and only enough for cell growth, nearly all secreted antibody proteins with unpaired Cys residues remain uncapped in the medium. When DTNB was titrated and added to the culture at various time points during cell culturing, it generated nearly fully TNB-capped antibodies. Procopio-Melino et al optimized the cystine feeding scenario based on a formula factoring cell growth and protein contents, and eventually established a process resulting in a high expression titer and nearly fully TNB-capped antibody. This process was tested in a 200L-bioreactor run and demonstrated to produce a high-quality ADC over that of the conventional Cys-TCEP process.
Cys-based site-specific conjugation efficiently exploits Cys’ low natural abundance and high nucleophilicity of deprotonated thiolate side chain [13,22]. The TNB-TSPP selective reduction approach makes the methodology compatible with other conjugation technologies by eliminating the full reduction and re-oxidation manipulation. For instance, one example is shown in Figure 2. The antibody component can be engineered with heavy chain (HC) K290C and light chain (LC) K183C [1] as well as additional HC mutation (N297Q) or LLQGA terminal fusion [23]. The antibody can be first produced by the DTNB capping process to produce TNB-capping at 290C(HC) and 183C(LC). Payloads such as microtubule inhibitors can be first conjugated to the positions of 297Q or LLQGA through transglutaminase [23], and then this ADC can be treated with TSPP to remove TNB capping. The exposed reactive thiol group can be conjugated with novel payloads such as potent inhibitors of nicotinamide phosphoribosyltransferase [24]. This improvement on Cys-based site-specific conjugation, which eliminates the treatment of strong reductant TCEP and a strong re-oxidation step, makes the technology much more compatible with other site-specific methods for multi-payload ADCs. Although no site-specific ADCs are found in the current eleven FDA-approved products (Adcetris®, Besponsa®, Blenrep® [initiating the process for market withdrawal], Enhertu®, Kadcyla®, Mylotarg®, Padcev®, Polivy®, Tivdak®, Trodelvy®, Zynlonta®), their TAA-targeting antibody scaffolds can presumably be further engineered for multi-payload conjugations to achieve better drug efficacy by utilizing the abovementioned site-specific conjugation technologies. The TNB-TSPP process can also be applied to produce immune-cell-targeting ADCs for inducing the infiltration of multiple immune cells into tumors [25,26].
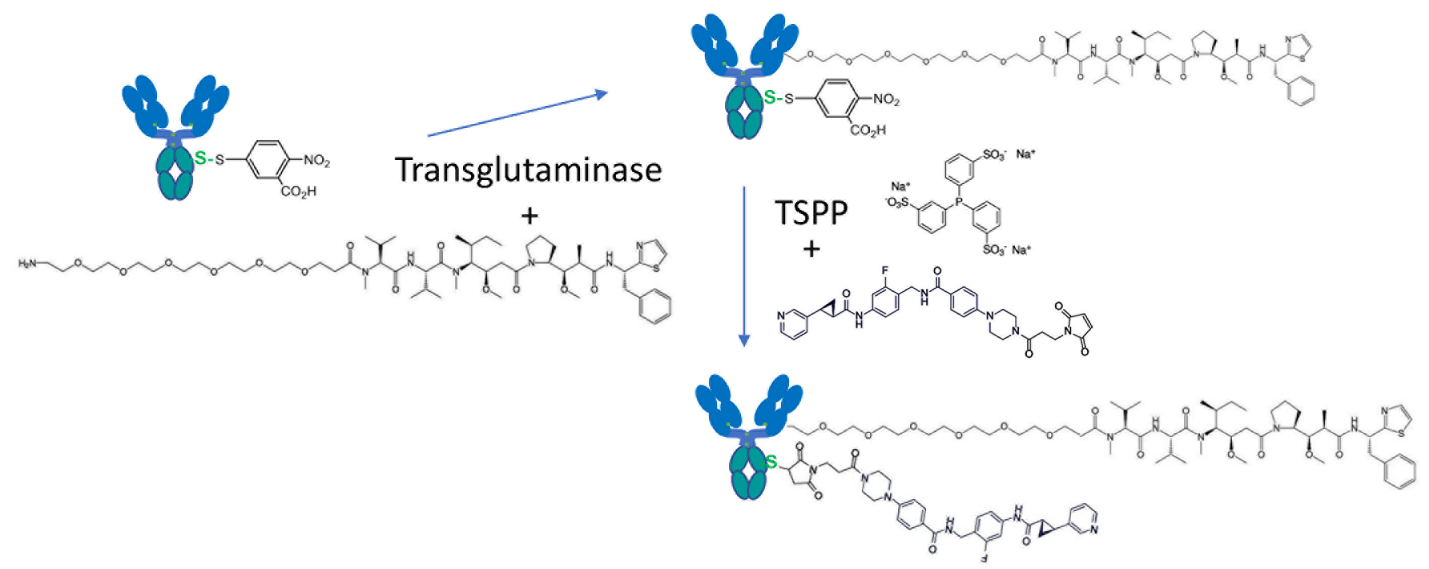
Figure 2. Bi-payload ADC with TNB-TSPP Cys-based site-specific conjugation. The antibody component engineered with HC K290C and LC K183C as well as HC (N297Q, native Q295, or LLQGA fusion) can be produced with TNB-capping, and first conjugated with transglutaminase-mediated amino-PEG6-C2-MMAD [23]. ADC5 linker-payload [24] is then conjugated after TSPP selective reduction to form bi-payload ADCs.
Perspective
Despite the recent regulatory successes and some remarkable scientific progress, ADC discovery and development still remains far from satisfactory and has many disappointing clinical results. As pointed out by several recent reviews [3,7,24], the improvements in ADC design have been focused on 1)exploiting new payload mechanisms; 2)increasing ADC penetration, uptake, and drug-antibody ratio (DAR, defined as conjugated drug molecules per antibody) for sufficient tumor delivery; 3) designing new linkers to improve payload solubility and pharmacokinetics; and 4) overcoming ADC resistance. The newly developed TNB-TSPP manufacturing process for Cys-based site-specific ADCs provides an efficient way to produce better characterized uniform drug products of new designs for low and heterogeneous TAA expression levels. It also opens up a new avenue for exploiting multi-mechanisms of action for effective cancer treatments by multi-payload ADCs. Besides combining multiple conjugation technologies, engineering a new kind of Cys-capping with new chemical handles enables biorthogonal chemistry for varied payloads. As shown in Figure 1, an antibody with dual functionalization linker Cys-cappings [27,28], e.g., maleimide derivatives containing orthogonal alkyne and ketone handles, can be produced for further conjugation with dual drug payloads. Approved ADCs with a homogeneous DAR8 hydrophilic linker-payloads like Enhertu® show encouraging efficacy in low TAA-expressing breast cancer and other indications [29], but a DAR of 8 is not suitable for many linker-payloads. Having drug payloads with different mechanisms of action within a single ADC molecule can be a way to overcome the drug-resistance and heterogeneity of cancer cells. New TNB-TSPP technology reported by Procopio-Melino et al. [1] originated from a small and unexpected observation on Cys-capping modification in mammalian cells. One would hope that breakthrough discoveries for ADCs and cancer therapeutics are coming out of scientists’ day-to-day research work.
References
2. Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2021;26(19).
3. Carter PJ, Rajpal A. Designing antibodies as therapeutics. Cell. 2022;185(15):2789-805.
4. Zhong X, D'Antona AM. Recent Advances in the Molecular Design and Applications of Multispecific Biotherapeutics. Antibodies (Basel). 2021;10(2).
5. Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-Drug Conjugates: The Last Decade. Pharmaceuticals (Basel). 2020;13(9).
6. Gauzy-Lazo L, Sassoon I, Brun MP. Advances in Antibody-Drug Conjugate Design: Current Clinical Landscape and Future Innovations. SLAS Discov. 2020;25(8):843-68.
7. Beck A, Goetsch L, Dumontet C, Corvaia N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315-37.
8. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18(6):327-44.
9. Dean AQ, Luo S, Twomey JD, Zhang B. Targeting cancer with antibody-drug conjugates: Promises and challenges. MAbs. 2021;13(1):1951427.
10. Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6(1):34-45.
11. Chudasama V, Maruani A, Caddick S. Recent advances in the construction of antibody-drug conjugates. Nat Chem. 2016;8(2):114-9.
12. Walsh SJ, Bargh JD, Dannheim FM, Hanby AR, Seki H, Counsell AJ, et al. Site-selective modification strategies in antibody-drug conjugates. Chem Soc Rev. 2021;50(2):1305-53.
13. Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925-32.
14. Zhong X, He T, Prashad AS, Wang W, Cohen J, Ferguson D, et al. Mechanistic understanding of the cysteine capping modifications of antibodies enables selective chemical engineering in live mammalian cells. J Biotechnol. 2017;248:48-58.
15. Chen XN, Nguyen M, Jacobson F, Ouyang J. Charge-based analysis of antibodies with engineered cysteines: from multiple peaks to a single main peak. MAbs. 2009;1(6):563-71.
16. Omorodion O, Wilson IA. Structural and Biochemical Characterization of Cysteinylation in Broadly Neutralizing Antibodies to HIV-1. J Mol Biol. 2021;433(24):167303.
17. Banks DD, Gadgil HS, Pipes GD, Bondarenko PV, Hobbs V, Scavezze JL, et al. Removal of cysteinylation from an unpaired sulfhydryl in the variable region of a recombinant monoclonal IgG1 antibody improves homogeneity, stability, and biological activity. J Pharm Sci. 2008;97(2):775-90.
18. McSherry T, McSherry J, Ozaeta P, Longenecker K, Ramsay C, Fishpaugh J, et al. Cysteinylation of a monoclonal antibody leads to its inactivation. MAbs. 2016;8(4):718-25.
19. Buchanan A, Clementel V, Woods R, Harn N, Bowen MA, Mo W, et al. Engineering a therapeutic IgG molecule to address cysteinylation, aggregation and enhance thermal stability and expression. MAbs. 2013;5(2):255-62.
20. Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539-77.
21. Bonifacio VDB, Pereira SA, Serpa J, Vicente JB. Cysteine metabolic circuitries: druggable targets in cancer. Br J Cancer. 2021;124(5):862-79.
22. Marino SM, Gladyshev VN. Analysis and functional prediction of reactive cysteine residues. J Biol Chem. 2012;287(7):4419-25.
23. Strop P, Delaria K, Foletti D, Witt JM, Hasa-Moreno A, Poulsen K, et al. Site-specific conjugation improves therapeutic index of antibody drug conjugates with high drug loading. Nat Biotechnol. 2015;33(7):694-6.
24. Tumey LN. An Overview of the Current ADC Discovery Landscape. Methods Mol Biol. 2020;2078:1-22.
25. He L, Wang L, Wang Z, Li T, Chen H, Zhang Y, et al. Immune Modulating Antibody-Drug Conjugate (IM-ADC) for Cancer Immunotherapy. J Med Chem. 2021;64(21):15716-26.
26. Yu X, Long Y, Chen B, Tong Y, Shan M, Jia X, et al. PD-L1/TLR7 dual-targeting nanobody-drug conjugate mediates potent tumor regression via elevating tumor immunogenicity in a host-expressed PD-L1 bias-dependent way. J Immunother Cancer. 2022;10(10).
27. Yamazaki CM, Yamaguchi A, Anami Y, Xiong W, Otani Y, Lee J, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun. 2021;12(1):3528.
28. Kumar A, Kinneer K, Masterson L, Ezeadi E, Howard P, Wu H, et al. Synthesis of a heterotrifunctional linker for the site-specific preparation of antibody-drug conjugates with two distinct warheads. Bioorg Med Chem Lett. 2018;28(23-24):3617-21.
29. Siddiqui T, Rani P, Ashraf T, Ellahi A. Enhertu (Fam-trastuzumab-deruxtecan-nxki) - Revolutionizing treatment paradigm for HER2-Low breast cancer. Ann Med Surg (Lond). 2022;82:104665.
30. Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, et al. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res. 2010;16(19):4769-78.
31. Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20(2):161-7.
32. Lhospice F, Bregeon D, Belmant C, Dennler P, Chiotellis A, Fischer E, et al. Site-Specific Conjugation of Monomethyl Auristatin E to Anti-CD30 Antibodies Improves Their Pharmacokinetics and Therapeutic Index in Rodent Models. Mol Pharm. 2015;12(6):1863-71.
33. Jeger S, Zimmermann K, Blanc A, Grunberg J, Honer M, Hunziker P, et al. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angewandte Chemie. 2010;49(51):9995-7.
34. Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109(40):16101-6.
35. Beerli RR, Hell T, Merkel AS, Grawunder U. Sortase Enzyme-Mediated Generation of Site-Specifically Conjugated Antibody Drug Conjugates with High In Vitro and In Vivo Potency. PLoS One. 2015;10(7):e0131177.
36. van Geel R, Wijdeven MA, Heesbeen R, Verkade JM, Wasiel AA, van Berkel SS, et al. Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs Provides Homogeneous and Highly Efficacious Antibody-Drug Conjugates. Bioconjug Chem. 2015;26(11):2233-42.
37. Rabuka D, Rush JS, deHart GW, Wu P, Bertozzi CR. Site-specific chemical protein conjugation using genetically encoded aldehyde tags. Nat Protoc. 2012;7(6):1052-67.
38. Lin S, Yang X, Jia S, Weeks AM, Hornsby M, Lee PS, et al. Redox-based reagents for chemoselective methionine bioconjugation. Science. 2017;355(6325):597-602.
39. Elledge SK, Tran HL, Christian AH, Steri V, Hann B, Toste FD, et al. Systematic identification of engineered methionines and oxaziridines for efficient, stable, and site-specific antibody bioconjugation. Proc Natl Acad Sci U S A. 2020;117(11):5733-40.
40. Dai Z, Zhang XN, Nasertorabi F, Cheng Q, Li J, Katz BB, et al. Synthesis of site-specific antibody-drug conjugates by ADP-ribosyl cyclases. Sci Adv. 2020;6(23):eaba6752.
41. Schumacher D, Helma J, Mann FA, Pichler G, Natale F, Krause E, et al. Versatile and Efficient Site-Specific Protein Functionalization by Tubulin Tyrosine Ligase. Angewandte Chemie. 2015;54(46):13787-91.
42. Nanna AR, Li X, Walseng E, Pedzisa L, Goydel RS, Hymel D, et al. Harnessing a catalytic lysine residue for the one-step preparation of homogeneous antibody-drug conjugates. Nature Commun. 2017;8(1):1112.
43. Siegmund V, Piater B, Zakeri B, Eichhorn T, Fischer F, Deutsch C, et al. Spontaneous Isopeptide Bond Formation as a Powerful Tool for Engineering Site-Specific Antibody-Drug Conjugates. Sci Rep. 2016;6:39291.
44. Grunewald J, Klock HE, Cellitti SE, Bursulaya B, McMullan D, Jones DH, et al. Efficient Preparation of Site-Specific Antibody-Drug Conjugates Using Phosphopantetheinyl Transferases. Bioconjug Chem. 2015;26(12):2554-62.
45. Sato S, Matsumura M, Kadonosono T, Abe S, Ueno T, Ueda H, et al. Site-Selective Protein Chemical Modification of Exposed Tyrosine Residues Using Tyrosine Click Reaction. Bioconjug Chem. 2020;31(5):1417-24.
