Abstract
Background: Antiretroviral therapy (ART) for women with HIV is recommended for life, yet most studies measure retention in HIV care and treatment as a binary outcome rather than patterns of intermittent clinic attendance. Pregnancy and the post-partum period are critical times to study interruptions in care, as retention among these women is particularly challenging and can affect the outcomes of both the mother and her child.
Methods: The Kigali Antiretroviral and Breastfeeding Assessment for the Elimination of HIV (Kabeho) study was an observational prospective cohort of 608 pregnant and postpartum women with HIV followed for 2 years. Clinic visit attendance was used to construct an interruption variable defined as a missed visit followed by a return to care. In multivariate analyses, we examined interruption patterns as predictors of virologic failure and detectable viral load.
Results: During the study period, 48% of women had multiple visit interruptions and 29% had an interruption that lasted more than one month. Adjusting for age, ART regimen, time on ART, and CD4 count, the odds of virologic failure among women with multiple interruptions were almost 3 times higher than for women with one or no interruptions (aOR=2.72, 95%CI: 1.09, 6.77). The odds of virologic failure were nearly 3.5 times higher among women with an interruption lasting more than one month compared with women with shorter or no interruptions (aOR=3.47, 95%CI: 1.59, 7.66).
Conclusions: Interruptions in HIV care visits are common with significant increases in virologic failure among women with multiple and long-term interruptions. Measuring patterns of interruption, rather than a single binary measure captures the fluid nature of lifetime and health-seeking behavior.
Keywords
Treatment interruption, Postpartum, Virological failure, Retention in HIV care, Rwanda
Introduction
Initiating and retaining women with HIV on antiretroviral therapy (ART) during pregnancy and postpartum is critical to minimize the risk of transmission to their infants and for their own health [1]. In support of the prevention of mother-to-child transmission of HIV, the World Health Organization (WHO) published a series of regularly updated treatment guidelines and recommendations [2]. In 2013, the guidelines included the recommendation to start a 3-drug combination treatment immediately following a diagnosis of HIV regardless of CD4 count and to continue the treatment for life – an approach referred to as “Option B+” [3].
Initiating ART is an important first step, but maintaining treatment throughout breastfeeding can play a major role in decreasing the likelihood of mother-to-child transmission [4,5]. Likewise, continuing treatment for life is critical to reducing morbidity and mortality for women given the rapid rebound of the virus once treatment is stopped [6,7]. Achieving HIV viral suppression following the uptake of ART is a principal endpoint for improving individual health and reaching population-level HIV epidemic control [8,9]. However, continued treatment and adherence are challenging in resource-limited settings with retention rates in HIV care as low as 45% after 3 years [10].
Despite the long-term nature of ART, there is a dearth of information on specific interruption patterns over time, and how those patterns may affect viral load differently. Likewise, many studies examining retention in HIV care in Sub-Saharan Africa use binary outcomes at one point in time rather than patterns of attendance over a period of time which likely is a more accurate portrayal of the true nature of life-long engagement in care [11-15]. The viral load response to interruptions in treatment may vary by the timing, frequency, and duration of the interruptions. Pregnancy and the post-partum period are critical times to study interruptions, as retention among women during those periods tends to be more challenging than in other populations [16,17].
In 2013, the Kigali Antiretroviral and Breastfeeding Assessment for the Elimination of HIV (Kabeho) study was conducted by the Elizabeth Glaser Pediatric AIDS Foundation in collaboration with the Government of Rwanda to evaluate the effectiveness of Rwanda’s prevention of mother-to-child HIV transmission (PMTCT) program that included the Option B+ approach [18]. Using data from the Kabeho study, we describe patterns of interruptions in care and treatment over a 24-month period among pregnant and postpartum women and explore potential associations of interruptions and both virologic failure and detectable viral load.
Methods
Study design and population
The Kabeho study was an observational prospective cohort of 608 women from 14 high-volume (>50 pregnant women with HIV per year) antenatal clinics in Kigali, Rwanda, enrolled between April 2013 and May 2014. All women had HIV and either in their third trimester of pregnancy or within two weeks post-delivery. Interviews were conducted at the enrollment visit to collect information on demographics, antenatal clinic (ANC) visit history, HIV and ART history, ART adherence, and ANC/PMTCT nutrition counseling. After delivery, eighteen subsequent study visits were conducted in conjunction with participants’ routinely scheduled monthly PMTCT facility visits. Two additional quarterly visits were conducted 21 and 24 months postpartum for a total of 20 study visits following enrollment.
In addition to patient information collected at the study visits, specimens were collected for HIV RNA-PCR testing for viral load, within two weeks of delivery, and at the 18 and 24-month visits. Testing specifications, procedures, and laboratory details have previously been described [18]. Of the 608 women enrolled, 567 came to at least one follow-up visit following enrollment. One woman exited the study as she relocated to a remote location following enrollment but then returned to the study at month 16. She was excluded from the analyses of interruption patterns resulting in a study population of 566 women. Analyses examining the relationship between interruptions and viral load were limited to the 420 women who had a viral load test done at their 24-month visit.
All study participants provided written informed consent, and all study personnel received training in protecting human subjects in research. The Kabeho study received ethical approvals from the Rwandan National Ethics Committee, the Rwanda National Health Research Committee, and the Institutional Review Board of George Washington University, and is registered at clinicaltrials.gov (NCT02295800).
Measures
Dependent variables: We examined HIV viral load results at the 24-month visit. Detectable viral load - defined as ≥ 20 copies/ml per the lower limit of detection for the Roche COBAS Ampliprep/COBAS Taqman assay - was categorized as a dichotomous variable (“detectable” and “undetectable”) [19]. We also included a similar dichotomous variable for virologic failure defined as ≥ 1000 copies/ml [20].
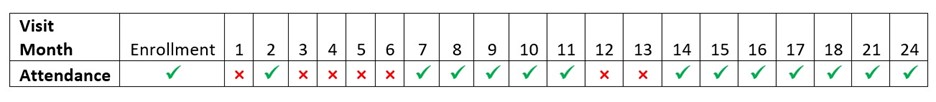
Primary Independent variables: The primary independent variable of interest was an interruption in care and treatment during the study period. An interruption was defined as one or more missed monthly study visits anytime between enrollment and their 24-month visit. Using data from visit records, we measured interruptions in care using two approaches introduced in previous studies [21]: The first was the number of interruptions (of any length) throughout the 2-year study period. The second was the length of interruptions (number of consecutive months missed) and since women experienced multiple interruptions, the length of the longest interruption was used. Figure 1 illustrates a hypothetical patient with 3 interruptions. The first interruption started after enrollment and lasted 1 month. The second interruption started at visit 3 and lasted 4 months. The third and final interruption started at visit 12 and lasted 2 months. The length of the longest interruption was 4 months (months 3-6). The number of interruptions and the longest interruption length were examined as continuous and categorical variables for a total of 4 separate interruption variables. The categorical variables were defined as less than 2 interruptions versus 2 or more interruptions, and less than 2 months versus 2 or more months, respectively [22,23].
Figure 1. Hypothetical situation to illustrate interruption patterns.
Additional covariates: We included several covariates in the multivariate models to control for potential confounders including age, length of time on ART, ART regimen, and CD4 count at enrollment. We used a bivariate variable for CD4 cell count with a cutoff at < 200 cells/mm3 as a proxy for advanced HIV disease (AHD) [24]. Length of time on ART was a continuous variable for the number of months the participant had been on ART prior to enrollment. Finally, we controlled for ARV regimen. The most common regimen at enrollment was the combination of Tenofovir, Lamivudine, and Efavirenz (TDF + 3TC + EFV). Women could change regimens at any time during the study period, so we created a dichotomous variable that included those who only took this TDF combination for the entire study period vs. those who took any other regimen at any time.
Statistical analysis
Frequencies and percentages were used to summarize all the categorical variables and medians with interquartile ranges (IQR) were used to describe the continuous variables. Both 24-month outcomes (detectable viral load and virologic failure) were summarized using frequencies and percentages. We used logistic regression models to examine the relationship between interruptions and viral load. For each outcome, we ran a separate model for each of the 4 interruption variables. We examined univariate relationships and then adjusted for potential confounders in multivariate models. All statistical analyses were conducted in Stata version 15.0.
Results
Of the 566 women in this study who had at least one follow-up visit, 121 (21%) attended all study visits. The remaining 79% of the cohort had at least one visit interruption. Table 1 shows the characteristics of the study population including metrics for the entire study population in the first column (“Total”) followed by the same variables by viral load outcome (detectable and virological failure) for the 420 women who had a viral load test conducted at the 24-month visit. The median age of the cohort was 29 years (IQR 25-34), with a median of 30 years among those with either detectable viral load or virological failure at 24 months. The median number of months on ART at the time of enrollment for all women was 16 months (IQR: 3-52) and was nearly twice as long for those with detectable viral load and virological failure (30 and 31 months, respectively) at their 24-month visit. Just over 5% of the women in the study had advanced HIV disease (AHD) (CD4 count< 200 cells/mm3) at enrollment. Six of the 92 (6.5%) women with detectable viral load and 5 of the 37 (13.5%) with virological failure at the 24-month visit had AHD at enrollment. Overall, just over half the cohort (53.7%) received the standard triple combination regimen of TDF + 3TC + EFV at every study visit they attended. Roughly 40% of those with either detectable viral load or virological failure received the standard regimen throughout the study period.
|
Variable |
Total |
Detectable VL |
Non-detectable VL (< 20 copies/ml) |
Virological failure |
Non-virological failure |
|
Characteristics at enrollment: |
n=566 |
n=92 |
n=328 |
n=37 |
n=383 |
|
Median (IQR) age |
29 (25-34) |
30 (25-36) |
29 (25-34) |
30 (25-35) |
29 (25-34) |
|
Median (IQR) months on ART |
16 (3-52) |
30 (4-77) |
10 (5-61) |
31 (4-89) |
11 (5-60) |
|
CD4 test results (cells/mm3) |
|||||
|
<200 |
31 (5.5) |
6 (6.5) |
15 (4.6) |
5 (13.5) |
16 (4.2) |
|
≥ 200 |
470 (83.0) |
77 (83.7) |
274 (83.5) |
29 (78.4) |
322 (84.1) |
|
missing |
65 (11.5) |
9 (9.8) |
39 (11.9) |
3 (8.1) |
45 (11.7) |
|
ART regimen |
|||||
|
TDF + 3TC + EFV |
304 (53.7) |
37 (40.2) |
182 (55.5) |
15 (40.5) |
204 (53.3) |
|
Other |
262 (46.3) |
55 (59.8) |
146 (44.5) |
22 (59.5) |
179 (46.7) |
|
Interruption variables: |
|||||
|
Number of interruptions |
|||||
|
< 2 |
293 (51.8) |
36 (39.1) |
174 (53.0) |
10 (27.0) |
200 (52.2) |
|
≥ 2 |
273 (48.2) |
56 (60.9) |
154 (47.0) |
27 (73.0) |
183 (47.8) |
|
Length of the longest interruption (months) |
|||||
|
< 2 |
403 (71.2) |
58 (63.0) |
253 (77.1) |
18 (48.6) |
293 (76.5) |
|
≥ 2 |
163 (28.8) |
34 (37.0) |
75 (22.9) |
19 (51.4) |
90 (23.5) |
Nearly half (48.2%) of all the women in the study population had multiple (more than 1) interruptions during the 2-year study period. Among women with detectable viral load or virological failure at 24 months, the percentages who experienced multiple interruptions were 61% and 73%, respectively. A similar pattern emerged when examining the length of interruptions. The percentage of women in the study population with an interruption lasting 2 or more months was nearly 29%. Thirty-seven percent of those who had detectable viral load and more than 51% of those with virological failure at 24 months experienced interruptions that lasted 2 months or more.
Table 2 shows odd ratios for detectable viral load and virologic failure at 24 months using the number of interruptions and length of the longest interruption as continuous and categorical variables. Univariate and multivariate estimates adjusted for age, the number of months previously on ART at enrollment, CD4 count, and ARV regimen are presented.
|
|
Detectable Viral Load |
Virological Failure |
||||||
|
|
Unadjusted |
Adjusted* |
Unadjusted |
Adjusted* |
||||
|
Continuous variables: |
OR (95%CI) |
p |
aOR (95%CI) |
p |
OR (95%CI) |
p |
aOR (95%CI) |
p |
|
Number of interruptions |
1.17 |
<0.05 |
1.06 |
0.58 |
1.33 |
0.01 |
1.28 |
0.08 |
|
Length of the longest interruption (months) |
1.21 |
<0.01 |
1.28 |
<0.01 |
1.34 |
<0.01 |
1.39 |
<0.01 |
|
Categorical variables: |
OR (95%CI) |
p |
aOR (95%CI) |
p |
OR (95%CI) |
p |
aOR (95%CI) |
p |
|
Number of interruptions |
||||||||
|
< 2 |
Reference |
Reference |
Reference |
Reference |
||||
|
≥ 2 |
1.76 |
0.02 |
1.41 |
0.22 |
2.95 |
0.01 |
2.72 |
0.03 |
|
Length of the longest interruption (months) |
||||||||
|
< 2 |
Reference |
Reference |
Reference |
Reference |
||||
|
≥ 2 |
1.98 |
0.01 |
2.11 |
0.01 |
3.44 |
<0.01 |
3.47 |
<0.01 |
|
*Adjusted for age, ART regimen, length of time on ART, and CD4 count |
||||||||
Effect of care interruption on virologic failure
Interruption of care was significantly associated with virologic failure. When examining the effect of interruptions on viral load using continuous variables, we found that each additional interruption during the study period resulted in a 33% increase in the odds of virologic failure in the univariate model (OR=1.33, 95%CI=1.08, 1.65). After adjusting for covariates as well as the length of the longest interruption, we observed a similar effect (aOR=1.28, 95%CI=0.97, 1.68), however, it was not statistically significant at the 0.05 α-level (p=0.08). In the multivariate model which also controlled for the number of interruptions, each additional month in the length of the longest interruption increased the odds of virologic failure by nearly 40% (aOR=1.39, 95%CI=1.15, 1.67).
The unadjusted odds of virologic failure for women who experienced multiple interruptions throughout the study period were nearly three times higher than the odds of virologic failure for women with a single or no interruptions (OR=2.95, 95%CI=1.39, 6.26). Results were similar after adjusting the longest length of interruptions as well as the covariates (aOR=2.72, 95%CI=1.09, 6.77). Likewise, adjusting for covariates and the number of interruptions, the odds of virologic failure among women who had an interruption of 2 months or longer were nearly 3.5 times higher than women with shorter interruptions (aOR=3.47, 95%CI=1.59, 7.66).
Detectable viral load
The series of regression models were repeated using detectable viral load as the outcome (rather than virologic failure) and resulted in similar patterns although while all the univariate models were statistically significant, only the results in the multivariate models examining the length of interruptions were significant. Specifically, after adjusting for covariates and the number of interruptions, each one-month increase in the length of the longest interruption increased the odds of having detectable viral load at 24 months (aOR=1.28, 95%CI=1.09, 1.50). Similarly, the odds of detectable viral load among women who had an interruption of at least 2 months were more than twice as high as those who had shorter interruptions (aOR=2.11, 95%CI=1.18, 3.75).
Discussion
Our analyses demonstrated that postpartum women enrolled in the Kabeho study and followed for 24 months frequently missed visits but then returned to care at some point afterward. This finding is consistent with similar studies including one by Mills et al. who found that more than 11% of the cohort studied returned to care following an interruption of at least 12 months [25]. In Rwanda, Nsanzimana et al. suggest that re-engagement after a 3-month interruption may be as high as 40% [26]. Traditional methods of measuring retention fail to capture this more fluid nature of engagement in care that we observed. Further, by measuring retention at one point in time, women may be classified as “not retained” even though they were simply in the middle of an “interruption” and eventually returned to care. Better identification of how women engage, interrupt, and re-engage in care may lead to more effective interventions and keep women continuously in care receiving their treatment.
Regardless of how we classified interruptions, there was a clear relationship between missed visits and virologic failure. Our study suggests that multiple interruptions are associated with virologic failure regardless of the length of those interruptions. The same is true for women who experience an interruption longer than one month, regardless of the number of interruptions they experience. It is important to note that retention in the Kabeho study may be higher than in a non-study environment as the protocol included procedures for study nurses to take following missed visits. These included efforts to contact women who were late for their visits beyond what would otherwise happen as part of routine care. Therefore, the interruptions we observed may be an underestimate of the true nature of interruptions outside of a study environment where women may have more and longer interruptions. In this study, missing only 2 consecutive visits more than tripled the likelihood of virologic failure. Our study period was only two years, therefore the number of interruptions we observed is likely to be just a fraction of what one would expect in a lifetime, and therefore the impact on viral load could be even worse than what we observed.
The different measurements of interruptions used in our study yielded similar results. PMTCT and ART programs vary dramatically in terms of staff availability, staff skills, and data systems. It therefore may be difficult to anticipate which type of interruption measurement is most feasible. Given the lack of a gold standard and the similar results found across the different measurements used in this study, using the most practical method of measurement given the resources and systems available may still be valuable in identifying women at risk of virologic failure. It may be easier for one clinic, for example, to flag any women who miss multiple visits as a risk. Another clinic may have systems in place that make it easier to monitor the length of an interruption. This study did not attempt to evaluate or compare the different methods of measurement.
Our study did have some limitations. Our results may be confounded by the fact that those with more interruptions (and longer interruptions) are more likely to experience an interruption just before the 24-month visit in which viral load was tested. In other words, it is possible that the virologic failure we observed was due to stopping ART just before being tested, as opposed to the frequency or length of interruptions throughout the study period. To test this potential confounding factor, we ran the same regression models adjusting for the time between the last interruption and the viral load test at 24 months. This resulted in similar associations suggesting that the timing of interruptions relative to the viral load test date did not confound our results.
Furthermore, we did not explore reasons for missed visits. To develop interventions aimed at reducing treatment interruptions, it would be helpful to understand the factors that contribute to the patterns most likely to lead to viral failure. Gill et al. examined attitudes and norms related to treatment in the Kabeho study and found potential barriers to continuity of treatment such as stigma, drug side effects, lack of social support, and lack of understanding or non-belief in ART benefits [27]. These barriers were also described in a systematic review of 34 studies to explore health system, individual, and contextual factors associated with retention among pregnant and postpartum women with HIV by Hodgson et al. [16]. Results from both studies are worth exploring as potential opportunities for interventions aimed at improving retention among women with frequent interruptions in care.
Despite these limitations, our analyses add to the existing research focused on the retention of postpartum women in PMTCT care in resource-limited settings. By looking at patterns of interruption rather than an overall outcome at one point in time, we were able to better capture the fluid nature of lifetime health-seeking behavior. We demonstrated that most women have interruptions in care and that multiple and long interruptions are associated with both virologic failure and detectable viral load. PMTCT programs could benefit from developing interventions targeted at women at risk of missing frequent visits.
Declarations
Ethics approval and consent to participate
All study participants provided written informed consent, and all study personnel received training in protecting human subjects in research. The Kabeho study received ethical approvals from the Rwandan National Ethics Committee, the Rwanda National Health Research Committee, and the Institutional Review Board of George Washington University, and is registered at clinicaltrials.gov (NCT02295800).
Conflicts of interest
The authors declare no conflicts of interest.
Funding
The Kabeho study was funded by the United States Agency for International Development Cooperative Agreement No. AID-OAA-A-12-00024.
Author contributions
EN carried out analyses and drafted and finalized the manuscript. All authors reviewed and approved the final manuscript. In addition, KA significantly contributed to the conception and design of the manuscript. RN contributed significantly to the statistical analyses used. EB and PM conceptualized the Kabeho study as they were two of the principal investigators. DN was the data manager for the study, leading data compilation and cleaning.
Acknowledgments
The authors would like to thank the Kabeho Study participants and the clinic staff for their participation and support. The authors also thank and recognize the incredible work of the Kabeho Study support team including the investigators, study nurses, and those who contributed to the collection and cleaning of data. Finally, the authors thank Laura Guay and Lynne Mofenson for their multiple reviews and suggestions, and Michelle Gill (all from EGPAF) for her advice and support.
References
2. WHO | Scaling up antiretroviral therapy in resource-limited settings: Treatment guidelines for a public health approach [Internet]. WHO. [cited 2019 Sep 16]. Available from: http://www.who.int/3by5/publications/documents/arv_guidelines/en/
3. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013.
4. Mugwaneza P, Lyambabaje A, Umubyeyi A, Humuza J, Tsague L, Mwanyumba F, et al. Impact of maternal ART on mother-to-child transmission (MTCT) of HIV at six weeks postpartum in Rwanda. BMC Public Health. 2018;18:1248.
5. Tolossa T, Mulisa D, Fetensa G, Fekadu G. Magnitude and factors associated with lost to follow-up among women under option B+ PMTCT program at East Wollega public health facilities, western Ethiopia. International Journal of Africa Nursing Sciences. 2020;13:100212.
6. Li JZ, Etemad B, Ahmed H, Aga E, Bosch RJ, Mellors JW, et al. The Size of the Expressed HIV Reservoir Predicts Timing of Viral Rebound after Treatment Interruption. AIDS. 2016;30:343-53.
7. Lodi S, Meyer L, Kelleher AD, Rosinska M, Ghosn J, Sannes M, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med. 2012;172:1252-5.
8. Crawford TN, Thornton A. Retention in Continuous Care and Sustained Viral Suppression: Examining the Association among Individuals Living with HIV. Journal of the International Association of Providers of AIDS Care (JIAPAC). 2017;16:42-7.
9. Redd AD, Mukonda E, Hu N-C, Philips TK, Zerbe A, Lesosky M, et al. ART Adherence, Resistance, and Long-term HIV Viral Suppression in Postpartum Women. Open Forum Infect Dis. 2020;7:ofaa346.
10. Mee P, Rice B, Lemsalu L, Hargreaves J, Sambu V, Harklerode R, et al. Changes in patterns of retention in HIV care and antiretroviral treatment in Tanzania between 2008 and 2016: an analysis of routinely collected national programme data. J Glob Health. 9:010424.
11. Phillips TK, Clouse K, Zerbe A, Orrell C, Abrams EJ, Myer L. Linkage to care, mobility and retention of HIV?positive postpartum women in antiretroviral therapy services in South Africa. J Int AIDS Soc. 2018;21:e25114.
12. Babatunde O, Ojo OJ, Atoyebi OA, Ekpo DS, Ogundana AO, Olaniyan TO, et al. Seven year review of retention in HIV care and treatment in federal medical centre Ido-Ekiti. Pan African Medical Journal [Internet]. 2015 [cited 2023 Sep 7];22.
13. McNairy ML, Lamb MR, Carter RJ, Fayorsey R, Tene G, Mutabazi V, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda and Tanzania. J Acquir Immune Defic Syndr. 2013;62:e70-81.
14. Cassidy T, Grimsrud A, Keene C, Lebelo K, Hayes H, Orrell C, et al. Twenty-four-month outcomes from a cluster-randomized controlled trial of extending antiretroviral therapy refills in ART adherence clubs. Journal of the International AIDS Society. 2020;23:e25649.
15. Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056.
16. Hodgson I, Plummer ML, Konopka SN, Colvin CJ, Jonas E, Albertini J, et al. A Systematic Review of Individual and Contextual Factors Affecting ART Initiation, Adherence, and Retention for HIV-Infected Pregnant and Postpartum Women. PLoS One. 2014 Nov 5;9(11):e111421.
17. Akama E, Nimz A, Blat C, Moghadassi M, Oyaro P, Maloba M, et al. Retention and viral suppression of newly diagnosed and known HIV positive pregnant women on Option B+ in Western Kenya. AIDS Care. 2019;31:333-9.
18. Gill MM, Hoffman HJ, Bobrow EA, Mugwaneza P, Ndatimana D, Ndayisaba GF, et al. Detectable Viral Load in Late Pregnancy among Women in the Rwanda Option B+ PMTCT Program: Enrollment Results from the Kabeho Study. PLoS ONE. 2016;11:e0168671.
19. Swenson LC, Cobb B, Geretti AM, Harrigan PR, Poljak M, Seguin-Devaux C, et al. Comparative Performances of HIV-1 RNA Load Assays at Low Viral Load Levels: Results of an International Collaboration. J Clin Microbiol. 2014;52:517-23.
20. Telele NF, Kalu AW, Marrone G, Gebre-Selassie S, Fekade D, Tegbaru B, et al. Baseline predictors of antiretroviral treatment failure and lost to follow up in a multicenter countrywide HIV-1 cohort study in Ethiopia. PLOS ONE. 2018;13:e0200505.
21. Boeke CE, Nabitaka V, Rowan A, Guerra K, Kabbale A, Asire B, et al. Assessing linkage to and retention in care among HIV patients in Uganda and identifying opportunities for health systems strengthening: a descriptive study. BMC Infect Dis. 2018 Mar 23;18(1):138.
22. Teklu AM, Yirdaw KD. Patients who restart antiretroviral medication after interruption remain at high risk of unfavorable outcomes in Ethiopia. BMC Health Services Research. 2017;17:247.
23. Kranzer K, Lewis JJ, Ford N, Zeinecker J, Orrell C, Lawn SD, et al. Treatment interruption in a primary care antiretroviral therapy programme in South Africa: cohort analysis of trends and risk factors. J Acquir Immune Defic Syndr. 2010;55:e17-23.
24. Ndlovu Z, Burton R, Stewart R, Bygrave H, Roberts T, Fajardo E, et al. Framework for the implementation of advanced HIV disease diagnostics in sub-Saharan Africa: programmatic perspectives. The Lancet HIV. 2020;7:e514-20.
25. Mills EJ, Funk A, Kanters S, Kawuma E, Cooper C, Mukasa B, et al. Long-Term Health Care Interruptions Among HIV-Positive Patients in Uganda. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;63:e23.
26. Nsanzimana S, Binagwaho A, Kanters S, Mills EJ. Churning in and out of HIV care. The Lancet HIV. 2014;1:e58-9.
27. Gill MM, Umutoni A, Hoffman HJ, Ndatimana D, Ndayisaba GF, Kibitenga S, et al. Understanding Antiretroviral Treatment Adherence Among HIV-Positive Women at Four Postpartum Time Intervals: Qualitative Results from the Kabeho Study in Rwanda. AIDS Patient Care and STDs. 2017;31:153-66.