Abstract
Background: The targeted inhibition of fatty acid synthase (FASN) by Orlistat, a potent FASN inhibitor, has been shown to block tumor proliferation and induce apoptosis in cultured tumor cells. Since Orlistat is insoluble, its solubility in blood circulation is limited. Cancer nanotherapeutics are rapidly progressing and are being implemented to solve several limitations of conventional drug delivery systems.
Aim: In this in vitro study, we aimed to overcome the limitation of orlistat changes by designing a nanoparticle and investigating its selective cytotoxic effects on cancer cells.
Method: To investigate the effects of encapsulated Orlistat on prostate cancer, we measured apoptosis and the distribution of cells via flow cytometry, confocal microscope, and Western blot.
Results: There is an improvement in the solubility of Orlistat with the effect of nanoparticles; it was observed that Orlistat decreased the expression of FASN and caused dose-dependent cytotoxicity with Caspase-3, Bax, and PARP activation in PC-3 cells, while it did not have a significant effect on normal prostate epithelial cells.
Conclusion: The present results indicate that the limitation of Orlistat can be overcome by assembling a nano-micellar formulation that can induce apoptotic death in the PC-3 cell line but normal prostate epithelial cells.
Keywords
Nano-encapsulation, Orlistat, Fatty acid synthase, Prostate cancer
Introduction
Prostate cancer (PCa) is the most widely recognized non-skin disease and the subsequent driving reason for cancer-related demise in men. PCa has a hereditary part, like different malignancies; however, it is muddled. Men are at a higher risk if there is a family background of sickness or African descent. Abnormal lipid metabolism, prompting expanded lipid synthesis, assumes an indispensable part in the pathogenesis of malignancies, incorporating prostate cancer [1-4]. In people, endogenous fatty acid synthesis in cells is typically negligible since normal cells preferentially use circulating free fatty acids from the eating regimen. Therefore, fatty acid synthase (FASN) is expressed at low or imperceptible levels. In contrast, the expression and activity of FASN are altogether expanded in many kinds of malignant growths, consequently making the potential for an attractive therapeutic target. The expression level of FASN is additionally associated with tumor progression, aggressiveness, and metastasis [1,2]. FASN has been displayed to have oncogenic activity. At the same time, a particular hindrance of FASN killed cancer cells with negligible secondary effects on normal cells. However, the mechanistic links between FASN and tumor cell death have not yet been characterized [3].
Targeted FASN inhibition by Orlistat, a potent FASN inhibitor, has been displayed to hinder cancer proliferation and induce apoptosis in cultured tumor cells and xenografts. Then again, FASN inhibition does not influence the survival of usually differentiated cells in vitro and shows no indications of toxicity in vivo [5,6].
Conventional cancer drugs are connected with massive restrictions for the most part. Rapid drug degradation and clearance, low bioavailability, low water dissolvability, and secondary effects in healthy cells through vague collection. In this manner, more up-to-date methodologies to target drugs to cancers are vital to improving the efficacy of chemotherapy. Regarding this, nanoparticles (NPs), such as disulfide cross-linked micelles, PEG-coated silica and gold NPs, cobalt oxide-chitosan based nanocomposites, and nanoporous polymer film, are of particular interest currently [7-15]. Adding additional chemical characteristics to many medications using polymer-based NPs is simple and advantageous. Utilizing polymer-based NPs has the advantage of lowering systemic toxicity because the body, at last, clears polymers under a certain size limit [16-19]. NPs, especially micelles, have expanded drug solubility in water, decreased nonspecific toxicity, improved permeability through sub-100nm membranes, and increased bioavailability [20].
Orlistat (ORL) is a powerful fatty acid synthase (FASN) inhibitor that irreversibly inhibits the thioesterase activity of this compound. It likewise diminishes tumor cell viability by inducing cancer cell apoptosis [21]. It was accounted for that treating cancer cells with Orlistat reduces DNA synthesis, arrests cell proliferation at the G1/S stage, followed by apoptotic cell death, and induces the activation of caspase -8 and -9 [22,23]. Orlistat's capacity to dissolve in a liquid media is constrained because it is a hydrophobic substance. This characteristic further restricts its bioavailability, which is now estimated to be less than 1%. Additionally, to some extent, the drug's systemic absorption and gastrointestinal excretion are connected to its limited bioavailability [24-26]. Paclitaxel, sold under the brand name Taxol, is a chemotherapy medication used to treat several types of cancers. It is one of several cytoskeletal drugs that target tubulin. Paclitaxel-treated cells have defects in mitotic spindle assembly, chromosome segregation, and cell division [27].
As a selective FASN inhibitor, Orlistat inhibits the thioesterase activity and inhibits cellular fatty acid synthesis in tumor cells, halting proliferation and inducing apoptosis. Since the cancer cells prefer to meet their need from de novo fatty acids synthesis, a particular hindrance of FASN, such as Orlistat, kills the cancer cells with negligible secondary effects on normal cells [3]. Orlistat also inhibits PC-3 prostate tumors in vitro and in vivo, demonstrating that FASN is an important oncology target that can be employed in treating cancers. All in all, this study aims to overcome the limitation of Orlistat mentioned above by self-assembled nanoformulation, target this new nanoparticle to the cancer cells, demonstrate its anti-cancer activity, compare it with classic anti-cancer drugs such as paclitaxel, and investigate their combination effects, so reaffirm the importance of self-assembled nanoformulation and Orlistat as a FASN inhibitor in tumor progression.
Materials and Methods
Unless otherwise stated, all substance reagents used in the experiment were of analytical quality or higher, obtained from commercial suppliers, and used without additional purification. Disulfide (PEG5k-CA8) cross-linked micelles (DCM), a nanocarrier, were obtained from the UC Davis Comprehensive Cancer Center. MTT [3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide], Orlistat (≥98%), and all other chemicals were purchased from Sigma-Aldrich (St. Luis). Paclitaxel was purchased from AK Scientific Inc. (Mountain View, CA). D-luciferin was purchased from Gold Biotechnology (St. Louis, MO). Cell culture media, fetal bovine serum (FBS), antibiotics, streptomycin, and penicillin (PS) were purchased from Invitrogen (Carlsbad, CA, USA). The American-Type Culture Collection purchased normal prostate epithelial cells (RWPE-1) and PC-3 prostate cancer cell lines (Manassas, VA, USA). Less than 40 passages were performed using cell lines. We consistently checked for mycoplasma contamination in cell lines.
Preparation and characterization of Orlistat (ORL) and paclitaxel (PTX) nanoformulation
The drying-out method (also called evaporation method) was used for ORL and PTX loading [17]. In this study, the DCM concentration was set at 20 mg/ml, and the nanoparticle solution was filtered with a 0.22 mm filter to sterilize the sample.
Dynamic light scattering (DLS) instruments (Nanotrac) were used to measure the micelles' size and size distribution. On a Philips CM-120 transmission electron microscope (TEM) working at an accelerating voltage of 80 kV, nano-ORL morphology was seen. A drop of an aqueous micellar solution was applied on a copper grid covered in carbon for roughly a minute before being wiped with filter paper. The grid was dyed with 0.1% (w/v) phosphotungstic acid after drying at room temperature. The sample was measured after being stored at room temperature.
In vitro apoptotic effect of nano-ORL, nano-PTX and their combination in PC-3 and RWPE-1 cells
By assessing cell viability, the methyl thiazolyl tetrazolium (MTT) assay is used to determine the cytotoxicity of Orlistat, paclitaxel, and their combination in PC-3 and RWPE-1 cells [27-29]. Both PC-3 cells (3x103 cells/well in 96 wells plated in regular RPMI with 10% FBS) and RWPE-1 cells (5x103 cells/well in 96 wells plated in keratinocytes - Medium II) were treated for 72 hours at 37°C with 5% CO2 with various concentrations of free drugs as well as nano-ORL, nano-PTX, and their combination.
Following a 48-hour treatment with various dosages of Orlistat and nano-ORL, FACS analysis based on PI staining evaluates the number of apoptotic cells that were induced in PC-3
In RPMI media supplemented with 10% FBS and 1% penicillin and streptomycin, PC-3 cells were cultured. We kept the cells at 37°C in a humid incubator with 5% CO2. Different quantities of Orlistat or nano-ORL were applied to cells plated in triplicate in 6-well plates with 2x105 cells in the standard medium for 48 hours. According to the manufacturer's instructions, apoptotic cells were identified using an Annexin V-FITC kit (Abcam). Both dead and live cells were trypsinized, collected, and fixed in 70% ice-cold ethanol after 48 h of incubation at 37oC with 5% CO2. We stored the samples in a -20oC freezer for 24 h before they were processed for FACS analysis. We washed the samples once in PBS and stained with 0.5 mL of PBS containing 0.5 μg/mL propidium iodide, 10 μg/mL RNAse A, and 0.1% TritonX-100. After 15 min of incubation at room temperature in the dark, cells were subjected to FACS analysis (BD FACS Canto), and we analyzed the generated data by FlowJo 8.8.6 software for quantifying live and dead cells. FlowJo 8.8.6 a software application with an integrated environment for viewing and analyzing flow cytometric data that helps unlock the data insights with accessible features for immunophenotyping, cell cycle, proliferation, kinetics studies, quantitative population comparison, and plate screening assays.
Effects of Orlistat and nano-ORL on cell cycle progression in PC-3
Cancer cells were exposed to 10 μM orlistat or nano-ORL and analyzed at different time points (0, 6, 24, 48, and 72 h). The distribution of cells at different cell cycle phases was followed by time-dependent flow cytometry. Adherent and detached cells were collected after trypsin detachment, washed in phosphate-buffered saline (PBS), and centrifuged at 1500 rpm. The cells were resuspended at 1×106 cells/ml in PBS and fixed in ice-cold 80% ethanol for at least 24 hours. Fixed cells were centrifuged at 300 x g, and each sample was resuspended in propidium iodide (PI) staining buffer (0.1% Triton X-100, 200 mg DNase-free RNase A, 20 mg of PI) in PBS for 30 min. After staining, samples were analyzed using BD FACS Canto software (Becton Dickinson; San Diego, CA, USA) and FlowJo 8.8.6.
Hoechst 33342 staining
To obtain confocal microscopic images of PC-3-Fluc-eGFP cells, cells were stained with Hoechst 33342 for 48 hours. Cells in the logarithmic growth phase were removed and inoculated into the 6-well plate. After fixation, nano-ORL (final concentration of 0, 2.5, 5, 10, 20, and 40 μM) was added to the cells. Subsequently, the culture solution was replaced for 48 h incubation. The supernatant was discarded, and the cells were washed twice with PBS, treated with 1 ml of Hoechst 33242 fluorescent dyes, and incubated at 37°C for 15 min; the fluorescent dyes were discarded, and the cells were rewashed with PBS, followed by observation under an inverted confocal microscope and photography. The experiment was repeated three times.
An immunoblot study of FASN, PARP, Caspase 3, and Bax to corroborate the mechanism of orlistat-induced apoptosis in PC-3 cells
Following a 48-hour culture period, the expression of FASN, caspase-3, B-cell lymphoma-2-associated X protein (Bax), polyadenosine diphosphate ribose polymerase (PARP), and tubulin levels were assessed using immunoblot analysis in PC-3 cells coexisting with different concentrations of charged micellar NPs of treated Orlistat (2.5, 5, 10, and 20 M orlistat equivalent). As controls, the cells treated with DMSO and CDT served as the vehicle.
To obtain the total cell lysate after treatment, we collected the cells. We resuspended them in 100 µl of lysis buffer [50 mM Tris, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 5 mM EDTA, protease inhibitors (Roche, Pleasanton, CA)] and then lysed them by keeping them on ice for 30 minutes with intermittent mixing. Invitrogen's NuPAGE®Bis-Tris Mini Gels, Carlsbad, CA, were used to electrophoretically separate 30 g of total protein from cell lysates in a 4-12% gradient polyacrylamide gel. The membrane was then exposed to a mouse anti-PARP antibody (1:1000 dilution, Rockland, Limerick, PA) for 12 hours in TBS/0.05% Tween 20 and 5% powdered milk. The membrane was washed, and an anti-mouse HRP-conjugated antibody (1:10,000 dilution, Sigma Aldrich, St. Louis, MI) was then used to probe it. An ECL system was used to find the signal (Thermo Scientific, San Jose, CA). The membrane was disassembled, and FASN, caspase 3, and BAX were examined (anti-rabbit antibody, 1:1000 dilution, Cell Signaling Technology, Danvers, MA). Binding was visualized with IgG-rabbit horseradish peroxidase-conjugated antibody (1:1000 dilution, R&D Systems Biotech, Minneapolis, MN).
Statistical analysis
Data from the experiment were shown as means and standard deviations. We used the Student's t-test for statistical analysis, and the cutoff for statistically significant differences was set at p<0.05 or a 95% confidence interval.
Results and Discussion
Physicochemical characterizations of nano-ORLs
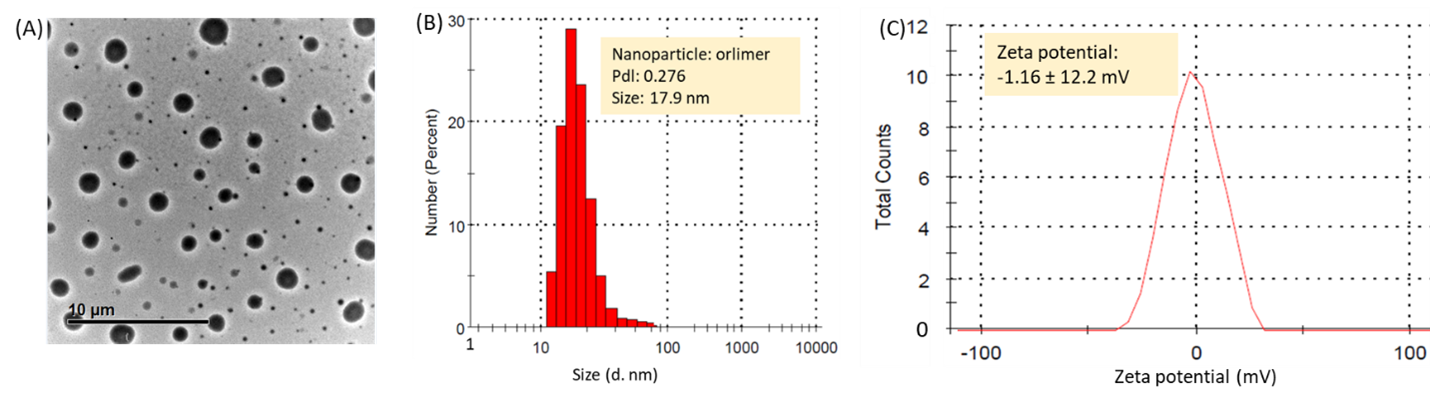
DCM, the PEG5k-CA8 telodendrimer can self-assemble to form core/shell micellar nanoparticles with a size of 15 nm in fluid media. ORL and PTX can be incorporated into polymeric micelles formed by physical entrapment by exploiting the hydrophobic interactions between these drugs and dendritic cholic acid clusters through the evaporation method. Micelle morphology was seen under transmission electron microscopy (TEM) after staining the air-dried micelles with phosphotungstic acid (0.1 wt%). As shown in Figure 1A, the nano-ORL (~18 nm) retained spherical shapes and size uniformity. Dynamic light scattering (DLS) measurements showed that the average nano-ORL diameters were 17.9 ± 6.5 nm (Figure 1B). NP diameters measured by DLS were consistent with those observed by TEM. The zeta potential of the nano-ORL was nearly neutral (-1.62 mV to 1.46 mV), as shown in Figure 1C. TEM and DLS measurements showed that the ORL-loaded micelles had a uniform size distribution (Figures 1A and 1B). Drug loading capacity and stability are critical to whether a micellar system can successfully act as a drug carrier [25]. Previous investigations have shown that solvent evaporation can effectively encapsulate various anti-tumor drugs into these micelles [20]. ??ORL is a potent FASN inhibitor, and it has been shown to display anti-tumor properties toward prostate cancer cells [21]. Due to its extremely low water solubility, ORL also has limited bioavailability, which is currently reported to be less than 1% [24, 30,31]. This study showed that ORL in an aqueous solution could be rapidly loaded into DCM. Higher cytotoxicity of drugs-loaded LPNs compared with free drugs indicates that new nanoformulation expands water solubility, improves membrane permeability, and increases ORL bioavailability in vitro.
Figure 1. The micelle morphology (A) particle size (B), and zeta potential (C), of nano-ORL. The concentration of DCM was 20 mg/ml.
Evaluation of the cytotoxicity of Orlistat in PC-3
The cytotoxic effect of Orlistat (MTT assay): Most cancer drugs cause cell death and have cytostatic effects by preventing cell proliferation [5,21,32,33]. Therefore, using the MTT test to measure cell viability, we investigated the impact of free drugs (ORL, PTX), their nanoformulations (nano-ORL, nano-PTX), and the combination of nano-ORL and nano-PTX on cell proliferation and cell death. First, we examined cell viability in PC-3 and RWPE-1 cells 72 hours after treatment at various concentrations of ORL or nano-ORL (2.5–40 M) and PTX (2.5–20 nM) and nano-PTX. Similar quantities of DCM (telodendrimer equivalent) and DMSO (less than 5%) were used as a control to treat the cells.
Kridel and colleagues showed that Orlistat induces tumor cell death but has minimal effects on normal cells [21]. Qu and colleagues indicated that Higher cytotoxicity of drugs loaded LPNs compared with free drugs indicates that nano-systems can enhance the cytotoxicity and synergistic effects are achieved when combined with the traditional anti-cancer drug [33], which was in accordance with our findings since we showed that treating PC-3 cells with free and nanoformulation drugs significantly reduced cell viability dose-dependently. Still, the decline caused by nano-ORL was more pronounced at high concentrations compared to ORL. We also examined the effects of nano-drugs (nano-ORL and nano-PTX) on PC-3 cells and compared them to each drug alone. We treated PC-3 cells with 10 μM of nano-ORL and different doses (2.5–20 nM) of nano-PTX over the same period. The results demonstrated significant synergistic effects of the nano-drug combination compared to a single drug against PC-3 cells (Figure 2A), which supports these studies. Vehicle treatment alone has no discernible cytotoxic effects on the cells. We employed normal RWPE-1 prostate epithelial cells and treated them with nanodrugs and nanodrugs at concentrations similar to those used to treat cells PC-3 to investigate the effects of ORL and PTX on normal cells. The results indicated a meaningful impact when the greatest orlistat dosage was utilized for treatment. While 20 M orlistat treatment caused 5% to 10% apoptosis in RWPE-1 cells, it caused 40% to 60% apoptosis in PC-3 cells. This finding shows that Orlistat is exclusively able to kill cancer cells; in contrast, PTX did not have the same marked selective apoptotic effect on healthy prostate epithelial cells (Figure 2B). This noticeable difference might be due to the mechanism of action of the drugs.
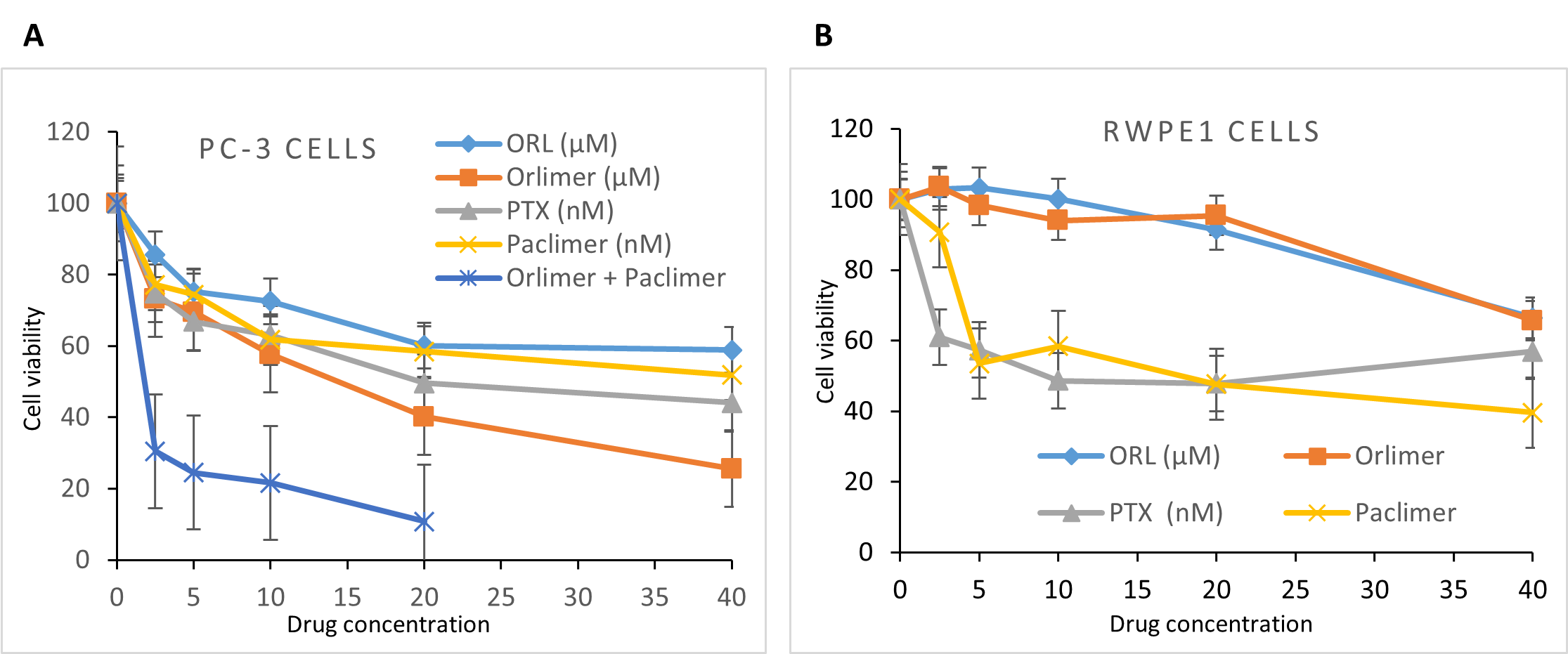
Figure 2. Cytotoxic effect of ORL, Nano-ORL, PTX, Nano-PTX, and nano-drug combination in PC-3 (A) and RWPE-1 (B) cells assessed by MTT assay. Cells treated with different concentrations of ORL and PTX (orlistat equivalent of 2.5-40 μM and PTX equivalent of 2.5-40 nM), and nano-drug concentration (10 μM fixed nano-ORL and combined with different dose (2.5 to 20 nM) of nano-PTX) at 72 h post-treatment.
The effect of Orlistat and nano-ORL on cell cycle progression in PC-3: When PC-3 cells were exposed to 10 μM orlistat, nano-ORL and their controls (DMSO and DCM) and analyzed at different time points (0, 6, 24, 48 and 72 h).
Choung and colleagues reported that Orlistat suppresses proliferation and causes cell cycle changes in PC3 cells. Orlistat resulted in significant G1 phase accumulations and S and G2/M phase reductions in PC3 cells in a dose-dependent manner. Their findings suggest that the block in G1/S progression is a common mechanism mediating the antiproliferative effects of Orlistat [34]. Our study showed that the cells underwent apoptosis, a reduced proliferation rate, and an increased percentage of cells in G0/G1 phases at 24 h for the nano-ORL only (Figure 3).
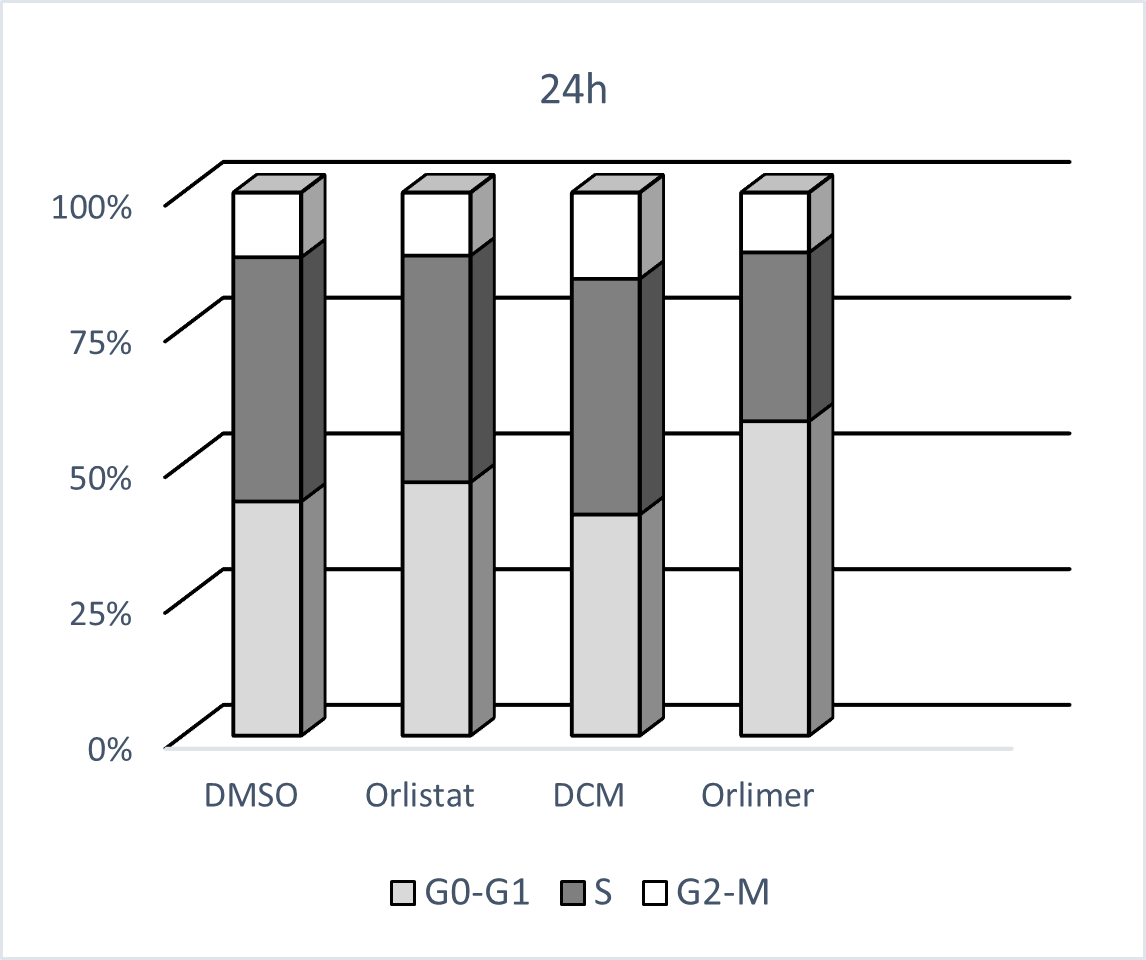
Figure 3. Nano-ORL blocked cell cycle progression in PC-3 cells. Cells were treated with 10 µM ORL, nano-ORL, and an equal amount of DMSO and DCM as their controls for 24 hours.
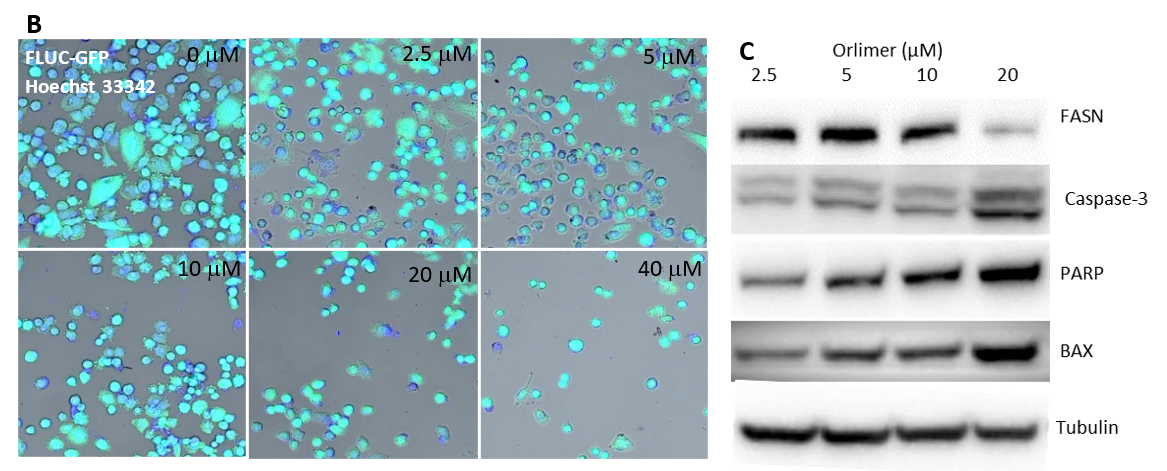
FACS analysis based on PI staining measures apoptotic cells, cell staining with Hoechst 33342, and immunoblot analysis of PC-3 cells treated with nano-ORL: Studies have shown that ORL inhibits apoptosis of a variety of tumor cells, thus promoting and inhibiting tumors and prostate cancer [21-23,35]. To further investigate the mechanism of ORL in inhibiting PC-3 cell proliferation, the effect of nano-ORL on apoptosis has been demonstrated. First, the apoptosis rates of PC-3 cells after treatment with different doses of nano-ORL were recorded by flow cytometry. Knowles and colleagues' study provided a new insight into the mechanisms underlying the connection between FAS and tumor cell proliferation since they demonstrated that Orlistat arrests the cell cycle at the G1/S transition [22]. Our results supported their study since we exhibited that nano-ORL could significantly promote apoptosis of PC-3 cells (Figure 4A). With the gradual increase in the amount of nano-ORL, the apoptosis rate also increased considerably. At the same time, Hoechst 33342 staining also showed consistent results: nano-ORL promoted apoptosis of PC-3 cells in a dose-dependent manner (Figure 4B). Then, to further investigate the pro-apoptotic mechanism of Nano-ORL, Western blot was used to detect changes in the expression of apoptosis-related proteins after treatment with Nano-ORL. FASN, caspase-3, Bax, and PARP expressions in PC-3 cells were seen after treatment with different concentrations of nano-ORL (2, 5, 5, 10, and 20 μM). Van de Sande and colleagues and Bandyopadhyay and colleagues demonstrated that direct inhibition of FAS results in apoptotic cell death, and expression of FASN was suppressed [1,5]. In consistence with their findings, we found that nano-ORL could significantly downregulate the expression of FASN and upregulate the expression of caspase-3, Bax, and PARP in a dose-dependent manner (Figure 4C). The higher the concentration of nano-ORL, the greater its effect.
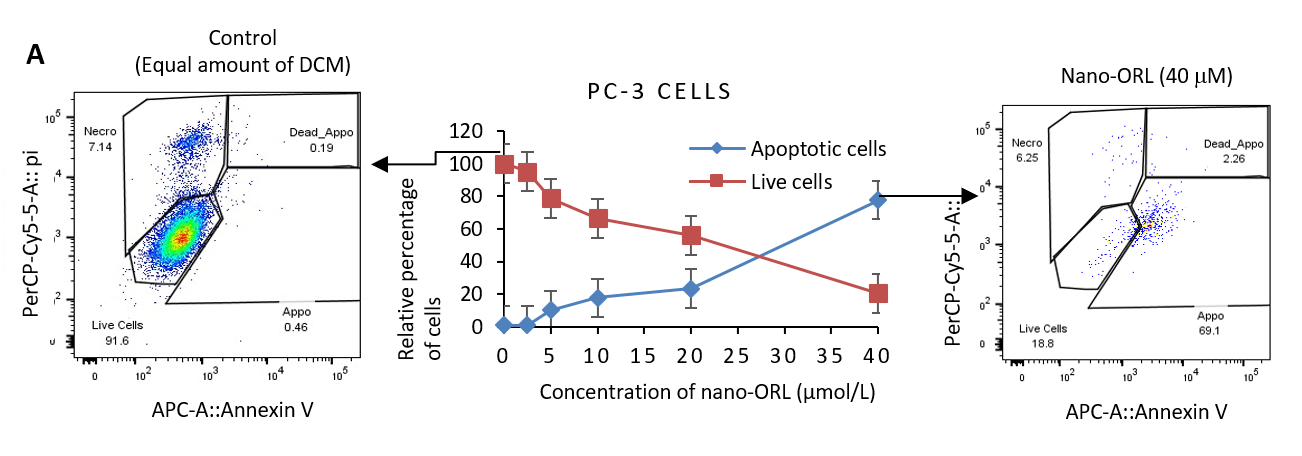
Figure 4. Cytotoxicity evaluation of nano-ORL in PC-3 cells treated with different concentrations. (A) PI staining based FACS analysis measures apoptotic cells, (B) confocal microscopic images of PC-3-Fluc-eGFP cells stained by Hoechst 33342 for 48 hours, and (C) immunoblot analysis for FASN, Caspase-3, BAX, and PARP expression.
In summary, the present results indicate that the limitations of Orlistat can be overcome by assembling a nanoparticle to create a nanodrug - nano-ORL-induced apoptotic death in the PC-3 cell line but normal prostate epithelial cells. Orlistat's selective apoptotic death effect likely relies on a mechanism associated with the downregulation of FASN due to upregulated signaling pathways involving caspase-3, BAX, and PARP. Nano-ORL works synergistically in combination with classic anti-cancer drugs such as paclitaxel. However, it might be desirable to develop a new FAS inhibitor like nano-ORL that is more specific in killing cancer cells and has little or no side effects on normal cells.
After all, an in vivo experiment needs to be conducted to investigate how improving the new nanoformulation's water solubility and membrane permeability helps target cancer cells. Further and thorough investigations of the reformulation are required to see if it has little or no side effects on normal cells. It also would be better if we performed XRD to demonstrate the nanoparticle's structure.
Acknowledgments
This study was conducted at the Comprehensive Cancer Center at UC Davis and supported by Dr. Kit Lam. Thanks to Dr. Lam for his generosity, supervision, and scientific support.
Authors' Contributions
BA designed the study, collated the data, conducted data analyses, produced the initial draft, and wrote the original manuscript draft. FA supported the writing of the original draft and contributed drawings. Reviewed and approved by both authors and submitted.
Conflict of Interest
The authors confirm no conflict of interest.
Ethics Statement
Ethical approval was not required for this article.
Funding
No funding was received for this article.
Disclosure
- Informed Consent: N/A
- Registry and Registration No: N/A
- Animal Studies: N/A
References
2. Murata S, Yanagisawa K, Fukunaga K, Oda T, Kobayashi A, Sasaki R, et al. Fatty acid synthase inhibitor cerulenin suppresses liver metastasis of colon cancer in mice. Cancer Science. 2010 Aug;101(8):1861-5.
3. Migita T, Ruiz S, Fornari A, Fiorentino M, Priolo C, Zadra G, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. Journal of the National Cancer Institute. 2009 Apr 1;101(7):519-32.
4. Alo PL, Visca P, Marci A, Mangoni A, Botti C, Di Tondo U. Expression of fatty acid synthase (FAS) as a predictor of recurrence in stage I breast carcinoma patients. Cancer: Interdisciplinary International Journal of the American Cancer Society. 1996 Feb 1;77(3):474-82.
5. Bandyopadhyay S, Zhan R, Wang Y, Pai SK, Hirota S, Hosobe S, et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Research. 2006 Jun 1;66(11):5934-40.
6. De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Research. 2003 Jul 1;63(13):3799-804.
7. Cho K, Wang XU, Nie S, Chen Z, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clinical Cancer Research. 2008 Mar 1;14(5):1310-6.
8. Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. InUrologic Oncology: Seminars and original investigations 2008 Jan 1;26(1):57-64.
9. Babu A, Templeton AK, Munshi A, Ramesh R. Nanoparticle-based drug delivery for therapy of lung cancer: progress and challenges. Journal of Nanomaterials. 2013 Jan 1;2013:863951.
10. Abu-Dief AM, Alsehli M, Awaad A. The bioreaction and immune responses of PEG-coated silica NPs and the role of the surface density coating after oral administration into mice. Applied Nanoscience. 2023 Jan 27;13: 5563-78.
11. Abu-Dief AM, Alsehli M, Awaad A. A higher dose of PEGylated gold nanoparticles reduces the accelerated blood clearance phenomenon effect and induces spleen B lymphocytes in albino mice. Histochemistry and Cell Biology. 2022 Jun;157(6):641-56.
12. Bashal AH, Khalil KD, Abu-Dief AM, El-Atawy MA. Cobalt oxide-chitosan based nanocomposites: Synthesis, characterization and their potential pharmaceutical applications. International Journal of Biological Macromolecules. 2023 Dec 31;253:126856.
13. Yuan W, Lu Z, Li CM. Charged drug delivery by ultrafast exponentially grown weak polyelectrolyte multilayers: amphoteric properties, ultrahigh loading capacity and pH-responsiveness. Journal of Materials Chemistry. 2012;22(18):9351-7.
14. Yuan W, Lu Z, Wang H, Li CM. Stimuli‐Free Reversible and Controllable Loading and Release of Proteins under Physiological Conditions by Exponentially Growing Nanoporous Multilayered Structure. Advanced Functional Materials. 2012 May 9;22(9):1932-9.
15. Yuan W, Li CM. Exponentially growing layer-by-layer assembly to fabricate pH-responsive hierarchical nanoporous polymeric film and its superior controlled release performance. Chemical Communications. 2010;46(48):9161-3.
16. Wiradharma N, Tong YW, Yang YY. Design and evaluation of peptide amphiphiles with different hydrophobic blocks for simultaneous delivery of drugs and genes. Macromolecular Rapid Communications. 2010 Jul 1;31(13):1212-7.
17. Devulapally R, Paulmurugan R. Polymer nanoparticles for drug and small silencing RNA delivery to treat cancers of different phenotypes. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2014 Jan;6(1):40-60.
18. Devulapally R, Sekar NM, Sekar TV, Foygel K, Massoud TF, Willmann JK, et al. Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano. 2015 Mar 24;9(3):2290-302.
19. Wang TY, Choe JW, Pu K, Devulapally R, Bachawal S, Machtaler S, et al. Ultrasound-guided delivery of microRNA loaded nanoparticles into cancer. Journal of Controlled Release. 2015 Apr 10;203:99-108.
20. Li Y, Xiao K, Luo J, Lee J, Pan S, Lam KS. A novel size-tunable nanocarrier system for targeted anticancer drug delivery. Journal of Controlled Release. 2010 Jun 15;144(3):314-23.
21. Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Research. 2004 Mar 15;64(6):2070-5.
22. Knowles LM, Axelrod F, Browne CD, Smith JW. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. Journal of Biological Chemistry. 2004 Jul 16;279(29):30540-5.
23. Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Research. 2007 Feb 1;67(3):1262-9.
24. McNeely W, Benfield P. Orlistat. Drugs 1998;56(2):241-249.
25. Charmot D. Non-systemic drugs: a critical review. Current Pharmaceutical Design. 2012 Apr 1;18(10):1434-45.
26. Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: drug loading and release. Current Pharmaceutical Design. 2006 Dec 1;12(36):4685-701.
27. Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004 Mar;23(11):2016-27.
28. Alahmadi M, Mohamed WS, Zhukov A, Salaheldeen M, Alsaedi WH, Alhashmialameer D, et al. One-step hydrothermal synthesis of flower-like MoS2/VS2 nanocomposite for biomedical applications. Inorganic Chemistry Communications. 2023 Nov 1;157:111336.
29. Saddik MS, Elsayed MM, Abdelkader MS, El-Mokhtar MA, Abdel-Aleem JA, Abu-Dief AM, et al. Novel green biosynthesis of 5-fluorouracil chromium nanoparticles using harpullia pendula extract for treatment of colorectal cancer. Pharmaceutics. 2021 Feb 6;13(2):226.
30. Abu‐Dief AM, Abdel‐Rahman LH, Abd‐El Sayed MA, Zikry MM, Nafady A. Green Synthesis of AgNPs () ultilizing delonix regia extract as anticancer and antimicrobial agents. Chemistry Select. 2020 Nov 13;5(42):13263-8.
31. Paulmurugan R, Bhethanabotla R, Mishra K, Devulapally R, Foygel K, Sekar TV, et al. Folate receptor-targeted polymeric micellar nanocarriers for delivery of orlistat as a repurposed drug against triple-negative breast cancer. Molecular Cancer Therapeutics. 2016 Feb 1;15(2):221-31.
32. Drew BS, Dixon AF, Dixon JB. Obesity management: update on orlistat. Vascular Health and Risk Management. 2007 Dec 1;3(6):817-21.
33. Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000 Mar 1;16(3):202-8.
34. Qu Z, Ren Y, Shen H, Wang H, Shi L, Tong D. Combination therapy of metastatic castration-recurrent prostate cancer: hyaluronic acid decorated, cabazitaxel-prodrug and orlistat co-loaded nano-system. Drug Design, Development and Therapy. 2021 Aug 20:3605-16.
35. Chuang HY, Lee YP, Lin WC, Lin YH, Hwang JJ. Fatty acid inhibition sensitizes androgen-dependent and-independent prostate cancer to radiotherapy via FASN/NF-κB pathway. Scientific Reports. 2019 Sep 16;9(1):13284.
36. Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharmaceutical Research. 2007 Jan;24:1-6.