Abstract
The gut microbiota plays a crucial role in various physiological functions such as the production of essential compounds like short-chain fatty acids and vitamins, as well as in controlling inflammation, immune response, and maintaining intestinal barrier integrity. The imbalance in microbial composition, termed dysbiosis, is closely associated with both the pathogenesis of obesity and the development of obesity-related metabolic disorders such as metabolic syndrome and type 2 diabetes (T2D). Dysbiosis leads to inflammation, harmful metabolite production, reduced microbial diversity, and elevated proinflammatory markers, all contributing to obesity progression. Molecular links between gut bacteria-derived metabolites and adipokine imbalance in obesity suggest that there may be adaptive changes and modifications in signaling pathways. Although the interaction between gut microbiota and host health involves complex signaling pathways that are not fully understood, various interventions targeting gut microbiota and dysbiosis have the potential to develop alternative treatments for obesity and T2D. This mini-review aims to explore these interactions further to advance research in managing these conditions.
Keywords
Obesity, Dysbiosis, Type 2 diabetes, Signaling molecules, Insulin resistance
Introduction
Despite the increase in the average person’s lifespan, the rising obesity prevalence is considered an epidemic that threatens to reduce current and future generations’ life expectancy and quality of life [1]. Obesity is directly related to the risk of many diseases, including type 2 diabetes (T2D), cardiovascular disease and cancer. Inevitably, this will also pose a considerable challenge to future healthcare finances [2]. Obesity, which is a multifaceted disease affected by many factors such as genetics, environment, lifestyle, and habits, is also closely related to intestinal microbiota. Current data or hypotheses on mechanisms linking the gut microbiome to metabolic disorders involve changes in molecular signaling molecules released by bacteria and interacting with local tissue or distant organs such as the brain (gut-brain axis) [3]. The prevalence of obesity is increasing in all age groups due to the growing obesogenic environment, the availability and increased consumption of calorie-dense fast food and recent technological advances that significantly reduce daily physical activity [4]. Diet has a significant impact on the gut microbiome; it is known that high-fat diets, especially Western-style (rich in sugar and fat) diets or fibre-poor diets impair barrier function in mice, affecting the concentration of bacterial metabolites entering the circulation [5]. Besides direct effects on gut microbial diversity and composition, dietary components (nutrients, phytochemicals, antibiotics, etc.) can affect the host-microbiome interface, disrupting the protective functions of the gut barrier. This leads to dysbiosis and thus inflammatory processes, causing pathological and clinical effects on the host [5,6]. In the context of the obesity-gut microbiota relationship, this mini-review aims to explore these interactions further to advance research in managing these conditions.
Obesity and Related Signaling Pathways
Influenced by hereditary, physiological, and environmental factors, obesity usually occurs when the body's energy intake exceeds energy expenditure [7]. Although the basis of its pathogenesis is not yet fully understood, obesity is recognized as a heterogeneous disease regulated by multiple pathways. Various signaling pathways play active roles in the regulation of appetite, adipose tissue metabolism and function, glucose hemostasis and energy expenditure, and thus in the pathogenesis of obesity. The main signaling pathways involved in the pathogenesis of obesity include AMPK pathway, JAK/STAT pathway, MAPK pathway, PI3K/AKT pathway, TGF-β signaling pathway, and Wnt/β-catenin signaling pathway [8]. The complex pathophysiology of obesity involves multiple signaling pathways that affect energy metabolism in different tissues. The function of the PI3K/AKT pathway, which is crucial for metabolic homeostasis, in insulin-sensitive tissues is described in the context of health, obesity and obesity-related complications. In other words, insulin action occurs downstream along the PI3K/AKT signaling pathway in different tissues and organs [9]. Although the insulin-sensitive organs are liver, muscle and adipose tissue, the role and importance of this signaling pathway in other organs and systems cannot be ignored, given that insulin receptors are expressed everywhere including brain, pancreas, vasculature, and bones [8,9]. In this sense, obesity is characterized by excessive accumulation of adipose tissue that impairs major signaling pathways critical for metabolic homeostasis including the insulin PI3K/AKT pathway [10].
Obesity and Gut Microbiota Relationship
The molecular mechanisms that connect the gut microbiota with obesity are still elusive. However, it has obvious effects on the whole metabolism, including energy extraction from food, intestinal barrier permeability, immune system functionality, and the production of certain metabolites that affect energy metabolism and signaling pathways [11]. Previous studies have suggested that gut microbiota also contributes to metabolic disorders in both rodents and humans [12]. Subsequent studies have confirmed that the gut microbiota is a complementary factor influencing host metabolism and has been suggested to play an important role not only in obesity but also in metabolic diseases such as insulin resistance (IR), cardiovascular disease, and T2D [13,14]. Finding that gut microbiota plays a direct role in promoting adipose inflammation, Caesar and colleagues reported that germ-free mice reduced macrophage accumulation in adipose tissue and improved glucose metabolism compared to their conventionally engineered counterparts [15]. Although the causal role of gut microbiota has generally been studied in animal models, cross-sectional studies have identified microbiota profiles associated with metabolic diseases in humans [16]. In this sense, recent studies support the role of dysbiotic alterations in metabolic diseases such as obesity [17] and T2D [18] and suggest that the gut microbiota may be a novel therapeutic target for the amelioration of T2D and related diseases [18]. On the other hand, diabetes, and obesity, which are well-known examples of impaired gut barrier function, not only exacerbate inflammation, but may also contribute to the link between gut microbiota and osteoporosis [19] and thus increased fracture risk [20]. Stephens and colleagues reported that the gut microbiota plays a role in the regulation of gut dysbiosis as well as fat storage, thus contributing to the development of obesity, IR, hyperglycemia, and hyperlipidemia [21]. Gut bacteria can maintain the energy balance of the human body due to their ability to share normally indigestible components of the mammalian diet. Furthermore, gut microbiota shares a symbiotic relationship with the host and benefits from each other. The gut flora also plays an important role in the development of the host immune system, assists in the breakdown and absorption of nutrients, protects against pathogenic infections, and maintains intestinal barrier function [22]. Whereas dysbiosis can change the functioning of the intestinal barrier and the gut-associated lymphoid tissues by allowing the passage of structural components of bacteria, such as lipopolysaccharides (LPS), which activate inflammatory pathways that may contribute to the development of IR [23]. Remarkably, concentrations of LPS leaking from the intestinal mucosa are higher in obese individuals [24]. Additionally, inflammatory signals from adipocytes could compromise the gut barrier integrity and function, facilitating the translocation of pathogens and their proinflammatory molecules [25]. Hence, the obesity-related chronic low-grade inflammatory state could be facilitated by microbiota alterations [26].
Signaling Molecules Mediating the Interaction between Gut Microbiota and Metabolic Disorders
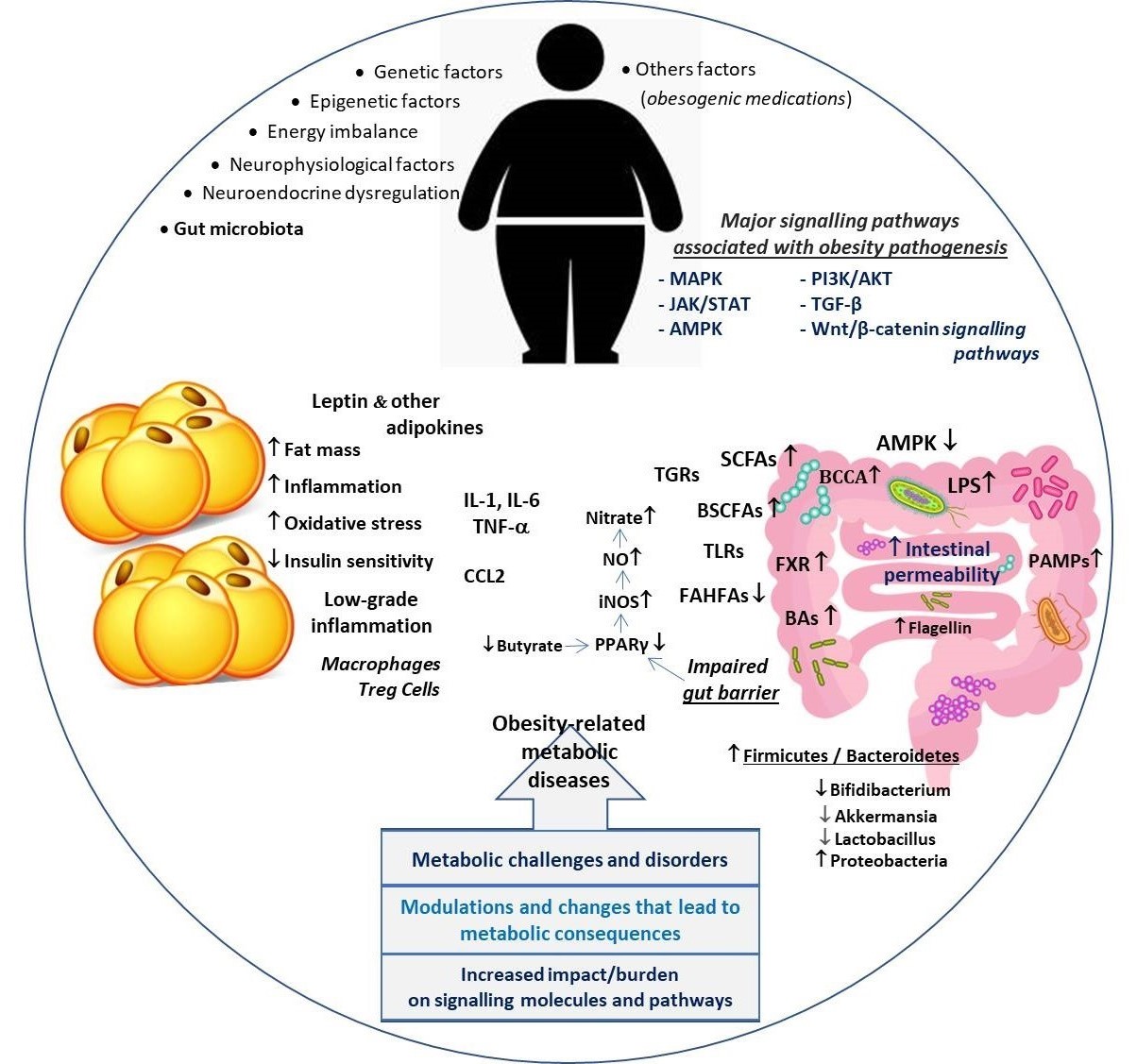
The gut microbiota contributes to the development of obesity by influencing the relevant signaling mechanisms in local and distant organs, particularly the liver, brain, and adipose tissue [27]. Notably, some metabolites produced by the bacteria of the microbiota, for example, short-chain fatty acids (SCFAs) may exert different effects on the host and can help or hinder host metabolism. SCFAs have been implicated in gastrointestinal and metabolic health, exert anti-inflammatory and immunomodulatory properties, and can influence central functioning [28]. SCFAs are ligands of G-protein-coupled receptor (GPR) 41 and 43, which are expressed in intestinal, skeletal muscle, liver, and pancreatic tissues. Interestingly, these receptors, also called free fatty acid 3 and 2 (FFA3 and FFA2) receptors, are also expressed in white adipose tissue and mediate SCFA-stimulated leptin secretion. This crosstalk suggests a distant contribution or effects of the gut microbiota to the host physiology [27,29]. SCFAs, particularly acetate, propionate, and butyrate, are mainly produced by anaerobic fermentation of gut microbes. SCFAs, which are the source of energy in the homeostasis of the intestinal environment, act as signaling molecules by binding to their GPRs [30]. Moreover, SCFAs have been shown to affect the host through multiple mechanisms, including regulation of GPRs, histone acetylation or methylation, facilitating the secretion of GLP-1, PYY and serotonin, as well as induction of vagus nerve signaling [31]. Studies in human and animal models describe disturbances of the gut microbial ecosystem associated with adiposity and hallmarks of metabolic disorders (Figure 1). Current evidence supports the potential role of the human gut microbiota in obesity [32]. The bacterial composition of the gut microbiota differs between obese and lean individuals; however, it seems likely that a Western-style diet high in fat and refined carbohydrates leads to increased gut bacteria linked to obesity [32,33]. On the other hand, there are also studies showing that different dietary fat profiles lead to different gut effects, and different metabolic outcomes independent of obesity [34]. Likewise, higher levels of circulating stimuli LPS and free fatty acids (FFA) caused by leak intestine increase M1 macrophages and by initiating inflammatory signaling pathways may lead to serious adipose tissue inflammation [35]. Interestingly, SCFA-mediated activation of GPR43 was found to suppress insulin signaling in adipocytes but not in liver and skeletal muscle, leading to inhibition of fat accumulation [36]. Besides the well-known SCFAs, lactate and succinate are also intermediates involved in the fermentation process of carbohydrates in the gut, and recent evidence suggests that succinate acts as a signaling agent for inflammation [37].
Figure 1. Primary factors involved in the relationship between obesity and dysbiosis and key signaling pathways in the pathogenesis of obesity-related metabolic disorders.
Another signaling molecule example is bile acids (BAs), which regulate metabolism and inflammation in a coordinated manner through nuclear farnesoid X receptor (FXR) and Takeda G protein-coupled receptor 5 (TGR5) receptors [38]. BAs have been shown to act as signaling molecules in the host, so a critical function of the gut microbiome is the processing of primary bile acids into secondary bile acids that directly affect farnesoid X receptor (FXR) signaling [39]. Bile acid-activated FXR plays an important role in the regulation of bile acid, lipid, and glucose homeostasis as well as in the regulation of inflammatory responses, barrier function, and prevention of bacterial translocation in the intestinal tract [40]. Indeed. BAs are not only digestive surfactants but also important cell signaling molecules that stimulate various signaling pathways to regulate several important biological processes. BAs also affect glucose, lipid, and energy homeostasis; both the concentration and composition of the pool of bile acids are altered in non-alcoholic fatty liver disease associated with obesity and intestinal dysbiosis [41,42].
The normal microbiota of the gastrointestinal tract induces anti-inflammatory effects that protect epithelial cells against pathogens through Toll-like receptors (TLRs) and other signaling pathways [43]. TLRs, important receptors in the innate immune mechanism of the intestinal epithelium, are mostly located in the cell membrane and intracellular endosomes [44]. Different types of TLRs activate molecular complex patterns in intracellular signaling pathways through their specific microbial ligands and induce the synthesis and release of inflammation-related factors such as TNF-a, IL-1 and IL-6 [44,45]. Caesar and colleagues suggested that gut microbiota may promote metabolic inflammation, hence obesity, through TLR signaling in diets rich in saturated lipids [46]. Remarkably, they reported that chemokine CCL2 contributes to microbiota-induced WAT inflammation in mice fed saturated fats. Their study results also showed that in contrast to those fed polyunsaturated lipids, saturated lipids cause increased TLR activation, WAT inflammation, and reduced insulin sensitivity.
Consequently, the trigger of adipose tissue inflammation can be initiated by different intrinsic signals, including adipocyte death, hypoxia and adipokine dysregulation, as well as substances derived from the gut microbiota [47]. Thus, given the interaction between adipose tissue and the gut, it is clear that molecular alterations and signaling mechanisms resulting from microbiota and dysbiotic changes play a critical role in the pathogenesis of metabolic diseases, particularly obesity. On the other hand, there has recently been a growing interest in the ‘role of organ interaction' in the course of metabolic and chronic diseases. In this sense, the interaction of adipose tissue with other tissues and organs has been emphasized in metabolic diseases such as metabolic syndrome [48], obesity-related aging [49], and T2D [50]. The gut microbiota, through various molecular interactions, may contribute to the onset of obesity and metabolic diseases by influencing in the host, insulin resistance, low-grade inflammation, and fat accumulation [51]. In other words, dysbiotic changes due to diet, alcohol, drugs, and other environmental factors can initiate and induce the progression of metabolic disorders.
Obviously, among the possible causes of obesity and obesity-related metabolic derangements such as IR, adipose organ dysfunction and altered adipose metabolic processes play a major role [52]. Visceral fat depot and adipocyte size are also associated with IR. It has been suggested that abdominal obesity may contribute significantly to the secretion of cytokines that alter insulin signaling through increased inflammation and ultimately to IR [53]. Obesity-mediated systemic inflammation and related mediators have been implicated in the onset and progression of many obesity-related diseases. Similarly, the bidirectional relationship between gut microbiota and microbe-derived components may regulate physiological and molecular signals that sustain lipid metabolism [53,54]. Obesity induced by a high-fat diet in mice promotes dysbiosis, causing a shift toward bacterial-derived metabolites that contribute to the onset and progression of gastrointestinal disorders [55]. Abnormal lipid accumulation and metabolism directly affect the diversity and abundance of gut microbiota, leading to an imbalance of the gut microbiota and further chronic metabolic diseases. For example, colonization of the intestinal tract by certain microorganisms of the Firmicutes family such as Faecalibacterium, Lachnospiraceae, Ruminococcaceae, and Anaerophilum can lead to obesity. However, some probiotic species are beneficial against obesity, including Ligilactobacillus salivarius, Lacticaseibacillus paracasei, Lactobacillus gasseri, Limosilactobacillus reuteri, Bifidobacterium lactis, and they support body weight reduction [56].
Obesity-induced adipose tissue dysfunction leads to dysregulated adipokine production that has both local and systemic effects on inflammatory cells. Thus, adipocyte-derived pro-inflammatory adipokines such as leptin and resistin lead to the activation of signaling pathways (e.g. JNK, JAK2/STAT3, p38 MAPK) [57]. In particular, leptin is associated with intracellular signaling pathways as well as inflammatory molecules, including IL-6, TNF-α, NO, eicosanoid and cyclooxygenase 2 [58,59]. Additionally, similar signaling pathways such as AMPK, JAK/STAT, and PI3K pathways are also involved in inflammation and immunity, therefore, in the pathogenesis of obesity and related metabolic disorders [60]. Notably AMPK signaling pathway plays an important role in the regulation of gut microbiota-mediated metabolism. In this context, Song and colleagues reported that inulin, a kind of prebiotic, alleviates glucose and lipid metabolism disorders by partially restoring leptin-related pathways mediated by gut microbiota [61]. Indeed, increasing evidence suggests that adipose tissue and various adipokines are related in β cell function and viability, suggesting play role in adipose tissue dysfunction in the development of T2D [62,63]. The release of pro-inflammatory cytokines disrupts glucose metabolism and insulin signaling. T2D patients display elevated levels of TNF-α, which is strongly associated with altered glucose tolerance, enhanced IR, and islet dysfunction [53]. A recent study showed that administration of an insulin receptor antagonist leading to systemic IR to mice significantly altered the gut microbiome and severely worsened barrier permeability as a result of loss of Paneth cell antimicrobial function. The results of the study by Gueddouri and colleagues [64] also revealed that insulin signaling is an indispensable gatekeeper of intestinal barrier integrity, acting as a protection against microbial imbalance and acute infections caused by enteropathogens. The current consensus suggests that inflammation, which is activated early during fat expansion and becomes chronic with the progression of obesity, drives the immune system towards a persistently pro-inflammatory phenotype. Moreover, circulating miRNAs secreted by adipose tissue also mediate multi-organ involvement in obesity and related complications. In other words, obesity-induced characteristic adipose tissue changes such as inflammation and hypoxia cause dysregulation of circulating miRNA profiles. For example, miR-17 activates the PI3K/AKT signaling pathway [65]. Ultimately, inflammation, oxidative stress, and IR, as well as the interaction between host adipose tissue and gut microbiota, directly or indirectly contribute to the pathogenesis of obesity and related metabolic diseases [66]. As seen in Table 1, the increased production of certain metabolites associated with gut dysbiosis may be linked to the progression of obesity and T2D, changes in signaling mechanisms as well as the extent of organ interactions.
|
Metabolites/molecules |
Obesity or obesity-induced T2D
|
Roles in pathogenesis
|
Ref. |
|
Lipopolysaccharides
|
Increase
|
Metabolic endotoxemia, inflammation
|
[24,67]
|
|
Endotoxins
|
Decrease
|
Boost of mucosal barrier function |
[68] |
|
Short-chain fatty acids (SFCAS: acetate, propionate, butyrate) |
Decrease
|
Anti-inflammatory, glucose-stimulated insulin secretion, GLP-1 & Peptide YY ↑ |
[28,68,69] |
|
Linoleic acid |
Decrease |
Adipose T Pro-inflammatory cytokines↓ |
[70] |
|
Polyamines |
Increase |
Inflammation |
[69] |
|
Hydrogen sulphide |
Increase |
Pro-inflammatory and toxic effects |
[71] |
|
BCAAs (branched-chain amino acids: isoleucine, leucine, valine)
|
Increase |
Insulin resistance
|
[72] |
|
BCFAs (branched-chain fatty acids: -mono, -di/-poly methyl C chain)
|
Increase
|
Inflammation and dyslipidemia |
[69,73]
|
|
The other microbial virulence factors on pathogenesis: Cyclooxygenase2, Ethanolamine, IL-1β, IL-6, TNF-α, Nitric oxide |
[46,66]
|
||
Conclusion and Future Perspective
Today, abnormalities in the gut microbiota are linked to many diseases such as obesity, T2D, hepatic steatosis, inflammatory bowel diseases, and various types of cancer. The gut microbiota also induces obesity as well as obesity-related metabolic diseases through various mechanisms, including control of energy homeostasis, LPS-induced inflammation, bile acids, and regulation of fat accumulation. Therefore, there is a growing interest in elucidating bacterial signaling pathways in obesity patients that may influence host physiology and trigger diseases such as T2D. As supported by this study, there are receptors in host cells that can activate or inhibit signaling pathways and can be beneficial or harmful to the health of the host. As indicated by this study, there are receptors on host cells that can activate or inhibit signaling pathways and can be beneficial or harmful to the health of the host.
For further research, microbiota and dysbiotic alterations are recognized as a new tool that cannot be ignored in the prevention, diagnosis and treatment of obesity and obesity-related metabolic diseases. Given the physiological importance of the gut-adipose tissue axis, a better understanding of the molecular links between adipokines and gut bacteria-derived metabolites could lead to the discovery of novel and complementary clinical biomarkers in the context of obesity. However, since eating habits are an important factor, intestinal dysbiosis and metabolic disorders need to be addressed in a broader perspective with further and high-quality studies.
On the other hand, thanks to advances in different fields of medicine and omics analysis, the treatment of obesity, like many other diseases, is slowly moving towards personalized medicine. Therefore, microbiota modulation, the use of personalized pre- or probiotics or fecal transplantation could be alternative treatments for metabolic diseases associated with obesity. Ultimately, advanced, and well-controlled experimental clinical studies are needed to fully understand the mechanisms by which bacterial metabolites modulate adipokine production and metabolism and to develop tailored alternative treatment options.
List of Abbreviations
AMPK pathway: AMP-activated protein kinase pathway; JAK/STAT pathway: The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway; MAPK pathway: Mitogen-activated protein kinase pathway; PI3K/AKT pathway: Phosphatidylinositol 3-kinase pathway; TGF-β signaling pathway: Transforming growth factor beta (TGFB) signaling pathway; GLP: Glucagon-like peptide; PYY: Gut hormone peptide YY; IL-1: Interleukin-1; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor alpha
Declarations
Conflict of interest
Authors FA and EVM declare that they have no conflict of interest.
Funding
This research received no external funding.
References
2. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:254-66.
3. Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747-56.
4. Meldrum DR, Morris MA, Gambone JC. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017;107:833-39.
5. Malesza IJ, Malesza M, Walkowiak J, Mussin N, Walkowiak D, et al. High-fat, Western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells 2021;10(11):3164.
6. Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 2012;7(10):e47713.
7. Caballero B. Humans against Obesity: Who Will Win? Adv Nutr. 2019;10(Suppl 1):S4-S9.
8. Wen X, Zhang B, Wu B, Xiao H, Li Z, Li R, et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Sig Transduct Target Ther 2022;7:298.
9. Savova MS, Mihaylova LV, Tews D, Wabitsch M, Georgiev MI. Targeting PI3K/AKT signaling pathway in obesity. Biomed Pharmacother. 2023;159:114244.
10. Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018;14(11):1483-96.
11. Ballini A, Scacco S, Boccellino M, Santacroce L, Arrigoni R. Microbiota and obesity: Where are we now? Biology (Basel) 2020;9(12):415.
12. DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc. 2008;83(4):460-9.
13. Harris K, Kassis A, Major G, Chou CJ. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J Obes. 2012;2012:879151.
14. Cunningham AL, Stephens JW, Harris DA. A review on gut microbiota: a central factor in the pathophysiology of obesity. Lipids Health Dis. 2021;20(1):65.
15. Caesar R, Reigstad CS, Bäckhed HK, Reinhardt C, Ketonen M, Lundén GÖ, et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut. 2012;61(12):1701-7.
16. Basic M, Dardevet D, Abuja PM, Bolsega S, Bornes S, Caesar R, et al. Approaches to discern if microbiome associations reflect causation in metabolic and immune disorders. Gut Microbes. 2022;14(1):2107386.
17. Geng J, Ni Q, Sun W, Li L, Feng X. The links between gut microbiota and obesity and obesity related diseases. Biomed Pharmacother. 2022;147:112678.
18. Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712.
19. Chen Y, Wang X, Zhang C, Liu Z, Li C, Ren Z. Gut microbiota and bone diseases: a growing partnership. Front Microbiol. 2022;13:877776.
20. Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32(1):145-56.
21. Stephens RW, Arhire L, Covasa M. Gut microbiota: From microorganisms to metabolic organ influencing obesity. Obesity (Silver Spring). 2018;26(5):801-9.
22. Islam MR, Arthur S, Haynes J, Butts MR, Nepal N, Sundaram U. The role of gut microbiota and metabolites in obesity-associated chronic gastrointestinal disorders. Nutrients 2022;14(3):624.
23. Gomes AC, Hoffmann C, Mota JF. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes. 2018;9(4):308-25
24. Trøseid M, Nestvold TK, Rudi K, Thoresen H, Nielsen EW, Lappegård KT. Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care. 2013;36(11):3627-32.
25. Fei N., Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880-84.
26. Petersen C, Bell R, Klag KA, Lee SH, Soto R, Ghazaryan A, et al. T cell-mediated regulation of the microbiota protects against obesity. Science. 2019;365:eaat9351.
27. Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein & Cell. 2018;9(5):397-403.
28. Machate DJ, Figueiredo PS, Marcelino G, Guimarães RCA, Hiane PA, Bogo D, et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int J Mol Sci. 2020;21(11):4093.
29. Breton J, Galmiche M, Déchelotte P. Dysbiotic Gut Bacteria in Obesity: An overview of the metabolic mechanisms and therapeutic perspectives of next-generation probiotics. Microorganisms 2022;10(2):452.
30. He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356.
31. Koh A, de Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332-45.
32. Sanz Y, Olivares M, Moya-Pérez Á, Agostoni C. Understanding the role of gut microbiome in metabolic disease risk. Pediatr Res. 2015;77(1-2):236-44.
33. Davis CD. The gut microbiome and its role in obesity. Nutr Today. 2016;51(4):167-74.
34. Lam YY, Ha CWY, Hoffmann JMA, Oscarsson J, Dinudom A, Mather TJ, et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity (Silver Spring). 2015;23(7):1429-39.
35. Tang C, Kong L, Shan M, Lu Z, Lu Y. Protective and ameliorating effects of probiotics against diet-induced obesity: A review. Food Res Int. 2021;147:110490.
36. Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
37. Mills E, O'Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24(5):313-20.
38. Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292(26):11055-69.
39. Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17(2):225-35.
40. Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5(2):135-44.
41. Vincent RP, Omar S, Ghozlan S, Taylor DR, Cross G, Sherwood RA, et al. Higher circulating bile acid concentrations in obese patients with type 2 diabetes. Ann Clin Biochem. 2013;50(Pt 4):360-4.
42. Chavez-Talavera O, et al. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. 2017;152(7):1679-94 e3.
43. Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015:489821.
44. El-Zayat SR, Sibaii H, Mannaa FA. Toll-like receptors activation, signaling, and targeting: an overview. Bull Natl Res Cent. 2019;43:187.
45. Duan T, Du Y, Xing C, Wang HY, Wang RF. Toll-like receptor signalling and its role in cell-mediated immunity. Front Immunol. 2022;13:812774.
46. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22(4):658-68.
47. Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633-43.
48. Armutcu F, Akyol S, Vural H. Metabolic syndrome is an important cornerstone in the health-disease line and pathological organ interaction. J Cell Signal. 2020;1(3):70-5.
49. Armutcu F, Ozen OA. inter-organ crosstalk and the effect on the aging process in obesity. Curr Aging Sci. 2023;16(2):97-111.
50. Xourafa G, Korbmacher M, Roden M. Inter-organ crosstalk during development and progression of type 2 diabetes mellitus. Nat Rev Endocrinol. 2024;20(1):27-49.
51. Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42.
52. Hocking S, Samocha-Bonet D, Milner KL, Greenfield JR, Chisholm DJ. Adiposity and insulin resistance in humans: the role of the different tissue and cellular lipid depots. Endocr Rev. 2013;34(4):463-500.
53. Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611-6.
54. Basnet TB, Gc S, Basnet R, Fatima S, Safdar M, Sehar B, et al. Interaction between gut microbiota metabolites and dietary components in lipid metabolism and metabolic diseases. Access Microbiol. 2023;5(6):acmi000403.
55. Pirozzi C, Coretti L, Opallo N, Bove M, Annunziata C, Comella F, et al. Palmitoylethanolamide counteracts high-fat diet-induced gut dysfunction by reprogramming microbiota composition and affecting tryptophan metabolism. Front Nutr. 2023;10:1143004.
56. Zawada A, Rychter AM, Ratajczak AE, Lisiecka-Masian A, Dobrowolska A, Krela-Kaźmierczak I. Does gut-microbiome interaction protect against obesity and obesity-associated metabolic disorders? Microorganisms. 2020;9(1):18.
57. Kirichenko TV, Markina YV, Bogatyreva AI, Tolstik TV, Varaeva YR, Starodubova AV. The role of adipokines in inflammatory mechanisms of obesity. Int J Mol Sci. 2022;23(23):14982.
58. Naylor C, Petri WA Jr. Leptin regulation of immune responses. Trends Mol Med. 2016;22(2):88-98.
59. La Cava A. Leptin in inflammation and autoimmunity. Cytokine. 2017;98:51-58.
60. Ray A, Bonorden MJL, Pandit R, Nkhata KJ, Bishayee A. Infections and immunity: associations with obesity and related metabolic disorders. J Pathol Transl Med. 2023;57(1):28-42.
61. Song X, Zhong L, Lyu N, Liu F, Li B, Hao Y, et al. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genomics Proteomics Bioinformatics. 2019;17(1):64-75.
62. Patra D, Banerjee D, Ramprasad P, Roy S, Pal D, Dasgupta S. Recent insights of obesity-induced gut and adipose tissue dysbiosis in type 2 diabetes. Front Mol Biosci. 2023;10:1224982.
63. Sikalidis AK, Maykish A. The gut microbiome and type 2 diabetes mellitus: discussing a complex relationship. Biomedicines 2020;8(1):8.
64. Gueddouri D, Caüzac M, Fauveau V, Benhamed F, Charifi W, Beaudoin L, et al. Insulin resistance per se drives early and reversible dysbiosis-mediated gut barrier impairment and bactericidal dysfunction. Mol Metab. 2022;57:101438.
65. Liu X, Sun H, Zheng L, Zhang J, Su H, Li B, et al. Adipose-derived miRNAs as potential biomarkers for predicting adulthood obesity and its complications: A systematic review and bioinformatic analysis. Obes Rev. 2024;8:e13748.
66. Turpin T, Thouvenot K, Gonthier MP. Adipokines and bacterial metabolites: A pivotal molecular bridge linking obesity and gut microbiota dysbiosis to target. Biomolecules. 2023;13(12):1692.
67. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761-72.
68. Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev. 2011;12(4):272-81.
69. Canfora EE, Hermes GDA, Müller M, Bastings J, Vaughan EE, van Den Berg MA, et al. Fiber mixture-specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes. 2022;14(1):2009297.
70. Vallianou NG, Stratigou T, Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract. 2018;146:111-18.
71. Palmas V, Pisanu S, Madau V, Casula E, Deledda A, Cusano R, et al. Gut microbiota markers associated with obesity and overweight in Italian adults. Sci Rep. 2021;11(1):5532.
72. Kim MH, Yun KE, Kim J, Park E, Chang Y, Ryu S, et al. Gut microbiota and metabolic health among overweight and obese individuals. Sci Rep. 2020;10(1):19417.
73. Choi BS, Daniel N, Houde VP, Ouellette A, Marcotte B, Varin TV, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12(1):3377.