Abstract
A recent observational study by Jin et al., found male sex and pretreatment weight loss to be associated with worse progression free survival (PFS) and overall survival (OS) in non-small cell lung cancer (NSCLC) patients treated with immune checkpoint inhibition (ICI) [1]. Although sexual dimorphism in immunity is well established, recent studies have begun to elucidate the mechanisms by which sex-specific immunity contributes to diseases such as cancer, and autoimmunity [2-5]. Similarly, the complex interplay between gonadal hormones, sex-based gene expression, and metabolism, particularly immunometabolism and the subsequent ability to mount effective antitumor immunity is a burgeoning field of investigation [6,7]. Cancer cachexia, a multifactorial syndrome characterized by severe weight loss, muscle wasting, and systemic inflammation, further complicates the metabolic landscape of NSCLC, influencing treatment response and outcomes. Unlike obesity-associated cancers such as breast and colorectal cancer, where increased body mass index (BMI) is linked to higher cancer risk, NSCLC does not display this association [8]. Considering this distinction, we seek to frame the clinical findings of Jin et al., in NSCLC in the context of tripartite interactions between inflammation, metabolism, and the efficacy antitumor immunity potentiated by ICI.
Keywords
Immunotherapy, Sex-differences, Pretreatment weight loss, Cancer cachexia, Body weight, Sex hormones, Lung cancer
Obesity and Chronic Inflammation
The World Health Organization reported that in 2022 over 890 million adults (16% of the world’s population) were living with obesity, a number that has more than doubled since 1990. Increased body mass index (BMI), a measure of adiposity, is associated with increased cancer risk in multiple cancer types, including endometrial, colorectal, breast, and prostate cancers [8]. However, an inverse relationship between BMI and lung cancer risk has been observed, often attributable to residual confounding by smoking, as smoking is linked to lower body weight and reverse causality [9]. Despite this, body fat distribution, rather than overall obesity, has been shown to contribute to lung cancer development [10]. In 2014, 7.8% of all cancers (excluding non-melanoma skin cancers) were attributed to excess body weight among US adults aged 30 and older [11], with a significantly higher burden in women compared to men (10.9% vs. 4.8%) [12]. Contrary to associations with cancer incidence, studies have found that obesity is positively associated with improved immunotherapy efficacy and OS in non-small cell lung cancer (NSCLC) patients [1,13-17]. Nevertheless, we still have an incomplete understanding of the reciprocal interactions between inflammation and metabolism during obesity, and how these independent mechanisms interact to establish a tumor supportive microenvironment.
The obesity-associated secretome, consisting of over 50 adipokines, cytokines, and chemokines which regulate both immunity and metabolism [18] , establishes a state of chronic, subclinical inflammation and such “smoldering” inflammation is a hallmark of cancer development [19]. Interestingly, obesity-associated systemic inflammation appears to be a stronger predictor of cancer risk than BMI itself. In conjunction, both adipocyte-produced factors and subsequent chronic inflammation drive metabolic dysfunction. Notably, metabolic function itself may also independently predict obesity-related cancer risk. In the longitudinal Framingham Heart Study, metabolically unhealthy overweight individuals had a 210% greater risk of developing cancer, whereas metabolically healthy overweight individuals had only a 47% increased cancer risk both compared to normal weight subjects [20]. These studies implicate chronic inflammation and metabolic dysfunction in cancer susceptibility, and suggest that common immunologic features of both, namely immunosuppression and T cell exhaustion may play a role in response to immune checkpoint inhibition (ICI). Supporting this, ICI targeting the PD-1/PD-L1 axis has yielded positive outcomes in obese NSCLC patients [21-23], with significantly longer PFS (3.7 vs. 2.8 months; HR: 0.79; 95 % CI: 0.64-0.98; P= 0.04) and OS (15.4 vs. 13.5 months; HR: 0.73; 95 % CI: 0.57-0.95; P= 0.02) in patients with a high BMI [22]. This is referred to as the obesity paradox of cancer immunotherapy response. A more recent study of >12,000 advanced NSCLC patients who received ICI therapy (pembrolizumab, nivolumab, atezolizumab, ipilimumab), a U-shaped association was observed where higher BMI was linked with a lower risk of mortality that those with a lower BMI, supportive of this obesity paradox in NSCLC patients [24]. However, ICI treatment was not any better than standard chemotherapy in the overweight or obese patients [24]. Additional factors, such as visceral fat index, may further clarify the role of obesity as a predictive factor in ICI therapy [25]. Obesity-mediated inflammation increases adipocyte lipolysis and the subsequent release of soluble lipids. In conjunction, proinflammatory TNFα and IL-6, elevated glucose, insulin, fatty acids, and free lipids act locally to dysregulate metabolism in target tissues resulting in oxidative stress and contributing to cancer development and/or progression. Prior to tumor formation, obesity results in elevated leptin levels, with leptin-mediated activation of STAT3 contributing to T cell disfunction and PD-1 expression in humans, non-human primates and mice [26,27]. In the context of cancer initiation and progression, obesity and the associated leptin expression combine to promote tumor progression likely through overlapping mechanisms of immunosuppression, as well as metabolic and hormonal regulation [26,28]. Yet, obesity induced increases in T cell exhaustion/PD-1 expression prior to and during cancer progression may predispose individuals to respond to ICIs [26,29]. Therefore, it seems likely that obesity-dysregulated immunity resulting in and from chronic inflammation may be reverted via ICIs to restore effective antitumor immunity.
Concurrent with T cell dysregulation, adipocyte-derived leptins, TNFα, IL-6, and CCL2 promote myeloid cell proliferation, recruitment, and inflammatory (“M1”) macrophage polarization during obesity. Inflammatory macrophages express high level of iNOS, have increased phagocytic ability, and secrete a number of pro-inflammatory cytokines and chemokines which contribute to chronic inflammation [30]. In obese animals, inflammatory macrophages are recruited to dead or dying adipocytes forming crown-like structures (CLS), histologic hallmarks of proinflammatory processes in adipose tissues. Crown-like structures produce pro-inflammatory cytokines and acute-phase proteins and are associated with both cancer incidence and insulin resistance [31,32]. Interestingly, estrogen receptor beta (ERβ) knockout mice had increased CLS, with macrophage recruitment and inflammatory polarization driven by upregulation of HIF-1α and osteopontin. Delivery of an ERβ agonist (LY3201) reduced CLS numbers, HIF-1α and osteopontin, in part, through the upregulation of prolyl hydroxylase 2 to prevent HIF-1α-mediated macrophage activation [33].
Yet, chronic inflammation invariably drives reactive immunosuppression to mitigate tissue damage and as such, adipose tissues also contain varying proportions of anti-inflammatory, wound healing (“M2”) macrophages. These wound healing macrophages have reduced inflammatory functions resulting in decreased immunosurveillance and establish tissue microenvironments capable of supporting tumor growth and metastatic dissemination [34-36]. Macrophages exposed to byproducts of extracellular matrix remodeling in adipose tissues adopt a phenotype similar to that of tumor-associated macrophages (TAMs) which support cancer initiation and progression [37]. Over time, polarization of macrophages and/or monocyte precursors may promote the establishment of the pre-metastatic niche supporting tumor initiation and subsequent tumor progression. Interestingly, administration of leptin, with or without anti-PD-1 ICI, enhanced antitumor immunity and reduced tumor volumes in both obese and lean mice associated with repolarization of TAMs towards a pro-inflammatory phenotype [38]. In addition, TAMs in humans and mice express PD-1 which inhibits their ability to phagocytose tumor cells, and similar to T cells, leptin signaling upregulates PD-1 expression on macrophages via STAT1 [27,39]. Collectively, macrophage polarization by chronic obesity-derived inflammation may be reversed via ICI therapy resulting in increased responsiveness observed experimentally and clinically.
Cachexia-induced Metabolic Disruption and Tumor Immunity
As illustrated by Jin et al., cachexia and pretreatment weight loss are detrimental to patients receiving immunotherapy [1]. Cachexia, the involuntary loss of >5 percent of someone’s weight, is a complex metabolic syndrome resulting in systemic inflammation. Cachexia results in the overexpression of pro-inflammatory cytokines, namely IL-1, IL-6, TNFα, and IFNγ which drive the body to a catabolic state promoting the breaking down of fat and muscle [40]. In previous studies on ICI efficacy focused on BMI, underweight patients were either excluded from the analysis or grouped into the “low BMI” category. However, it is now evident that just as being overweight can impact ICI efficacy, being underweight can also play a significant role by altering the immune landscape.
In pre-cachectic mice with colon cancer, IL-6 was found to reduce ketogenesis, impairing hepatic metabolism. In combination with the calorie deficient state, hypoketonemia increased the synthesis of glucocorticoids leading to suppression of anti-tumor immunity along with failure of immunotherapy [41]. Cachexia and resulting protein catabolism reduces circulating amino acids concentration, namely serine and arginine [42,43]. In mice, L-arginine was found to be essential in regulating multiple metabolic pathways in T cells, promoting T cell proliferation and differentiation, and most importantly, improving both survival and tumoricidal activity [44]. While the exact mechanism remains unknown, it was hypothesized that L-arginine promoted a central memory like T cell phenotype [44]. Serine was found to be essential in T effector cell proliferation, mainly by contributing to one-carbon intermediates and purine for cellular metabolism, and its deficiency in mice impaired their ability to effectively mount an immune response against bacterial pathogens [45]. Thus, cachexia and pretreatment weight loss may deprive the immune system of metabolic components required to mount an effective antitumor immune response to respond to ICI-mediated disinhibition.
In patients with pancreatic ductal adenocarcinoma and cachexia, the upregulation of IL-20 induces tissue fibrosis and correlates with poor prognosis [46]. However, several studies treating cancer patients experiencing cachexia with cytokine inhibitors have shown limited weight recovery and no significant improvement in survival [47]. Concomitantly, immune cell subsets have been associated with cancer-associated cachexia. Myeloid-derived suppressor cells in a mouse model of 4T1-induced breast cancer accelerates the development of cachexia via greater energy expenditure and chronic inflammation [48]. Moreover, a recent pilot study revealed an association between increased regulatory T cells and reduced lean mass index across patients with heterogeneous types of cancers [49]. This suggests the possibility of other immune cells and factors playing coordinating roles in the immunometabolic response to cachexia.
By contrast, some immune cells can alleviate and even prevent weight loss in cancer-induced cachexia models. In a mouse model of colon cancer, delivery of exogenous IL-4 inhibited muscle loss via increased myogenesis and was associated with increased CD8+ T cell and macrophage infiltration into tumors [50]. Microglial cells, specialized macrophages in the brain, were shown to have a protective effect during pancreatic cancer associated cachexia in mice, and microglial depletion via CFS-1R antagonist resulted in increased anorexia, fatigue, and muscle catabolism [51]. The efflux of macrophages from adipose tissues observed in mice with hepatocellular carcinoma was shown to mitigate fat loss and delay tumor development [52]. Furthermore, CD4+CD44+ T cells help reduce muscle degradation in mice with Lewis lung carcinoma and cachexia, though further research is needed to investigate its specific antitumor effects [53].
Nearly 50% of lung cancer cases demonstrate cachexia, with rates increasing as the disease advances, primarily driven by sarcopenia (muscle wasting) rather than loss of adipose tissue [54]. A retrospective study of more than 8,000 patients from the Japanese Lung Cancer Registry found that males and those with comorbid lung conditions such as emphysema were more likely to be cachectic, and cachectic patients had worse outcomes from chemotherapy and radiotherapy [55]. When examining the impact of sarcopenia on anti-PD-1 efficacy, non-sarcopenic NSCLC patients had a 40% overall response rate versus only 9.1% in sarcopenic patients, and a higher 1-year PFS rate (38.1% vs. 10.1%) [56]. Similarly, in a prospective study of 83 NSCLC patients treated with PD-1 or PD-L1 inhibitors, those with cachexia at treatment initiation had worse OS and increased frequency of disease progression [57]. These clinical studies align with those reported in Jin et al. [1].
In lung cancer, tumor loss-of-function mutations in STK11/LKB1, a key regulator of the energy sensor AMP-activated protein kinase, have been shown to induce cachexia in preclinical models and was correlated with pretreatment weight loss in NSCLC patients [58]. Silencing of STK11/LKB1 in preclinical lung and colorectal cancer models was associated with an altered immune tumor microenvironment (TME) and increased cachexia-associated cytokines. Additionally, STK11/LKB1 mutations are linked to reduced T cell activity, lower PD-L1 tumor expression and resistance to ICI [59]. It is clear that these mutations play a critical role in promoting metabolic and inflammatory conditions that contribute to cancer cachexia in NSCLC patients, and targeting metabolic pathways in STK11 mutant tumors is an active area of research to help mitigate cachexia.
Another promising approach for cachexia management is targeting growth factor differentiation factor 15 (GDF-15), a stress-induced cytokine and known mediator of anorexia and weight loss. GDF-15 was identified in a proteomic profiling analysis of plasma samples from NSCLC patients with and without cancer cachexia. GDF-15 was significantly elevated in plasma from cachectic NSCLC patients, both early- and late-stage disease [60]. NSCLCs from those with cachexia also had a distinct transcriptomic profile with higher expression of pro-inflammatory signaling markers along with epithelial–mesenchymal transitional pathways [60]. The humanized monoclonal antibody to GDF-15, ponsegromab, was shown to inhibit serum GDF-15 in patients with cancer-associated cachexia leading to improved weight gain, appetite, and physical activity [61]. In the follow up phase 2 trial (NCT05546476) of 187 patients (40% with NSCLC), ponsegromab induced a significant dose-dependent weight gain along with improvements in appetite and physical activity, and decreased cachexia symptoms at higher doses [62,63]. These studies provide support for further development of GDF-15-targeting therapies for the treatment of cancer-associated cachexia.
Sex differences in cancer cachexia is an emerging area of research, with strong evidence that biological sex plays a significant role in how cancer cachexia develops and progresses. However, few studies have investigated the molecular mechanisms of sexual dimorphism in cancer cachexia [64]. In a recent study, Activin A, a protein involved in muscle breakdown, was identified as a driver of cachexia in a lung cancer murine model [65]. Interestingly, treatment with a decoy ligand targeting activin signaling, combined with an appetite-stimulating drug, was more effective in female mice, with benefits dependent on ovarian function. A separate study identified the myogenic microRNA miR-486 as a key marker of sex-specific differences in cancer-induced skeletal muscle defects and is regulated by the ERα signaling pathway [66]. These recent studies are beginning to shed light on the role of sex hormones in modulating cachexia outcomes, and it will be interesting to evaluate sex differences in response to the emerging therapeutic approaches discussed above.
Sex and Immunotherapy
It is increasingly appreciated that sex-linked genes and hormones play a pronounced role in differences in NSCLC presentation, molecular characteristics, and response to treatment between women and men [67-69]. There is a growing concern in the increasing incidence and mortality of NSCLC in women worldwide, especially in non-smoking and younger women [70-72]. The estrogen pathway is implicated in playing a role in lung cancer tumorigenesis, presentation, and treatment response [73]. Additionally, estrogens can alter the tumor immune microenvironment [73]. This has led to researchers questioning how biologic sex impacts immunotherapy response.
Sexual dimorphism in immunity has long been recognized to affect disease, with females generally possessing stronger innate and adaptive immune responses [74]. This is associated with increased response to vaccination and faster clearance of pathogens but also increased susceptibility to autoimmunity with females representing ~80% of all individuals with autoimmune disease [74,75]. By contrast, males exhibit higher mortality across all ages following infection and cancer. There are roughly 50 genes regulating immunity encoded on the X chromosome (e.g. TLR7, CXCR3, FOXP3, OGT) affecting cell differentiation, activation, pathogen/damage sensing, trafficking, proliferation and metabolism and incomplete epigenetic silencing of one of two X chromosomes in females via the long non-coding RNA Xist may result in deleterious overexpression [3,5,76,77].
The data regarding sex differences in ICI efficacy in NSCLC are mixed. A meta-analysis of 20 randomized control trials including 11,351 patients with solid tumors (mainly NSCLC and melanoma) found that males derive significantly improved response and survival compared to females when ICI therapies are administered as monotherapy compared to standard chemotherapy [78]. Interestingly, a follow up meta-analysis by these investigators found that among patients with NSCLC, women treated with combination chemotherapy and ICI had statistically superior OS compared to men, suggesting that alternate sex-specific therapeutic strategies may be required to effectively leverage antitumor immunity [79]. Subsequent retrospective and meta-analyses did not find sex differences [80,81]. In the study by Jin et al., females had a significantly lower risk of death compared to males, which was limited to first-line ICI monotherapy [1]. Of note, the previous studies did not use patient level data and did not directly compare males to females. Since female NSCLC patients generally have improved outcomes in NSCLC compared to males [82], these findings reported in Jin et al. may reflect the effect of a more favorable prognosis for females regardless of treatment, despite the relative benefit males may gain from ICI monotherapy compared to chemotherapy. In addition, immune-related adverse drug reactions to immunotherapy are more commonly reported in female patients [83], an observation also found in Jin et al. Presence of immune-related adverse events is associated with improved ICI efficacy and survival.
Cancer cells harbor oncogenic alterations that result in tumor-associated antigen expression by MHC class I and II molecules with subsequent immune recognition. Increased tumor mutational burden (TMB) and expression of neoantigens is associated with beneficial cancer prognosis. In a study of 151 NSCLC patients, high TMB was associated with increased PFS and OS in combination or mono-immunotherapy treated patients compared to those with low TMB [84]. At the same time, immune pressure drives selection cancer of cells/tumors with diminished expression of driver mutations. Likely due to more robust immunity, females and younger individuals develop tumors with poor expression of driver mutations, with younger females showing compounding effects of MHC-based selection [85]. Interestingly, females had less TMB which could explain worse responses to ICI compared to males in some studies [84].
Sex steroid hormones impact the behavior of immune cells, influencing how they respond to ICI. For example, estrogen has been shown to modulate immune cell activity in ways that may enhance anticancer responses, while testosterone can suppress immune responses, potentially affecting treatment outcomes. Hormonal influences also shift with age, especially in postmenopausal women and older men, further complicating ICI treatment responses. The role of sex hormones in ICI response suggests that targeting estrogen or androgen pathways, could improve immunotherapy outcomes by reprogramming immune responses based on sex? [86-88]. The androgen receptor (AR) plays a role in suppressing the CD8+ T cell activity in the TME. AR regulates key immune pathways, including the Tcf7/TCF1 transcription factor, which is critical for the development and function of progenitor exhausted CD8+ T cells in tumors. This contributes to sex differences in cancer immunity, with male tumors showing higher AR activity and reduced immune responses compared to females. When AR was deleted from CD8+ T cells, male mice experienced the same level of tumor protection as female mice, indicating AR suppresses effective CD8+ T cell responses in males, suggesting that targeting AR could improve immunotherapy outcomes, particularly in male patients [87,88]. Estrogen enhances the activity of these CD8+ T cells, promoting more effective tumor cell killing in some cases. Estrogen signaling can also shape the TME by influencing immune cell infiltration and function [73].
Recently, Anobile et al. reported the effect of a cancer cell autocrine 17-β-estradiol (E2)/estrogen receptor alpha (ERα) signaling loop on NSCLC progression and response to ICI [89]. Here, aromatase generates endogenous E2, which in conjunction with EGFR-downstream effectors AKT and ERK1/2, activates ERα upregulating CD274/PD-L1 transcript and protein expression [89]. High ERa expression was a stronger predictor of response to ICI (pembrolizumab) than either gender or PD-L1 expression, with authors suggesting a four-biomarker signature (EGFR activity, E2, ERα, and PD-L1) to identify NSCLC patients most likely to benefit from ICI or ICI/E2-inhibition. NSCLC patient-derived xenografts possessing high E2/ERα/PD-L1 expression were more responsive to ICI (pembrolizumab), an effect that was further increased in female-derived xenografts. Finally, co-administration of the aromatase inhibitor letrozole augmented ICI efficacy, a finding that again was stronger in females. Collectively, the E2 pathway likely promotes both tumor intrinsic as well as extrinsic (immune-mediated) mechanisms of immunosuppression within the TME leading to increased tumor growth and decreased response to immunotherapy [90]. Notably, fulvestrant, an ER antagonist was found to reduce mesenchymal features of NSCLC and enhance immune cytotoxicity [91]. Thus, the combination of E2 inhibition with ICI may provide additional benefit for the treatment of a subset of refractory NCSLS patients.
The inconsistency of sex-differences in ICI efficacy among published studies highlights the need for further research to understand the mechanism by which sex influences ICI outcomes. Additionally, addressing the historical underrepresentation of women in immunotherapy clinical trials will be crucial to adequately perform rigorous sex-specific analyses that could inform sex-specific treatment strategies [77]. Incorporating sex-based factors into cancer cachexia basic mechanistic studies will also be important and could ultimately lead to a sex-tailored approach, such as hormone manipulation or metabolic reprogramming, to optimize immunotherapeutic approaches separately for both men and women, ultimately improving patient outcomes.
Conclusions
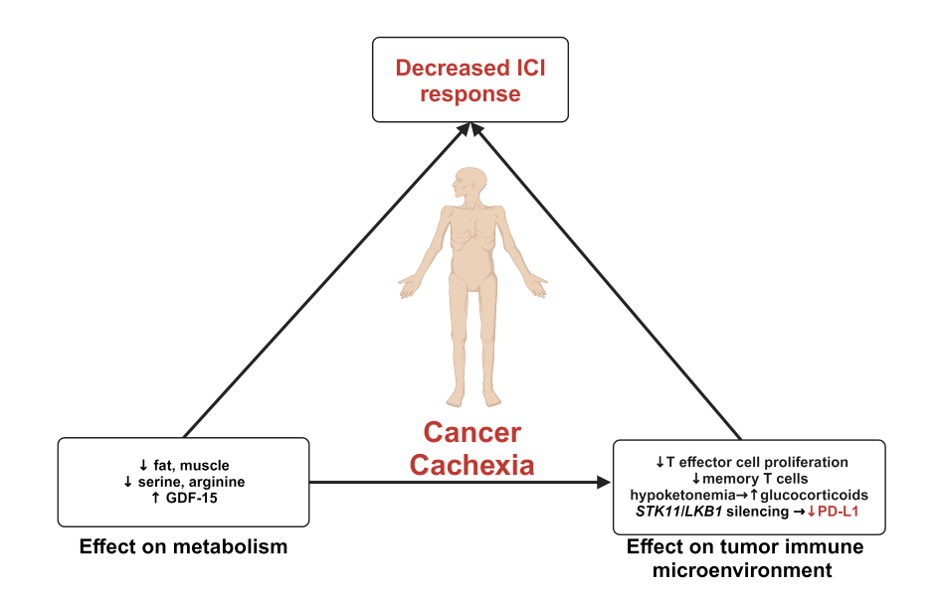
In summary, these findings highlight the inherent complex interplay between body weight, sex, and ICI efficacy in NSCLC patients as summarized in Figure 1. Body weight, whether obesity or cachexia, alters the metabolic and immune landscape to, in turn, affect response to ICI. While the impact of patient sex on ICI efficacy is still not fully understood, emerging evidence suggests that an estradiol/ER/PD-L1 signaling loop may play a critical role. Additionally, cancer cachexia may manifest differently in females, with hormonal factors and estrogen signaling influencing disease severity and response to therapy. As such, considering sex-specific factors when treating and managing cancer-associated cachexia may improve patient outcomes and response to ICIs.
Figure 1. In the cancer cachexia state, enhanced breakdown of adipocytes and muscle cells, along with reductions in key amino acids such as serine and arginine, limits effector and memory T cell proliferation. Additionally, stress-induced growth factor differentiation factor 15 (GDF-15) levels are elevated in cachexic patients, further influencing immune response. This metabolic imbalance predisposes to hypoketonemia and elevated glucocorticoid levels, fostering an immunosuppressive tumor immune microenvironment. Silencing of STK11/LKB1 reduces PD-L1 expression, further compromising immune function. Together, these alterations contribute to reduced efficacy of immune checkpoint inhibitors (ICI) in cachectic patients which may be further influenced by sex hormone signaling pathways.
Conflicts of Interest
The authors have no conflicts of interest.
Funding Statement
The authors have no relevant financial disclosures or financial/material support.
Acknowledgements
The authors acknowledge the original authors of the publication “Male sex and pretreatment weight loss are associated with poor outcome in patients with advanced non-small cell lung cancer treated with immunotherapy: a retrospective study”. This commentary would not be possible without their input.
Author Contributions
All authors contributed equally to writing, editing, and reviewing the manuscript.
References
2. Lee JJK, Jung YL, Cheong TC, Espejo Valle-Inclan J, Chu C, Gulhan DC, et al. ERα-associated translocations underlie oncogene amplifications in breast cancer. Nature. 2023 Jun;618(7967):1024-32.
3. Dou DR, Zhao Y, Belk JA, Zhao Y, Casey KM, Chen DC, et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. 2024 Feb 1;187(3):733-49.e16.
4. Smith R, Gee KN, Kalvapudi S, Pachimatla A, Swamidoss R, Vedire Y, et al. Sex-based Differences in the Lung Immune Microenvironment Are Associated with an Increased Risk of Lung Cancer in Women. J Thorac Cardiovasc Surg. 2024 Jul 15:S0022-5223(24)00617-2.
5. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016 Oct;16(10):626-38.
6. Manuel RSJ, Liang Y. Sexual dimorphism in immunometabolism and autoimmunity: Impact on personalized medicine. Autoimmun Rev. 2021 Apr;20(4):102775.
7. DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021 Dec;21(12):785-97.
8. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N Engl J Med. 2003 Apr 24;348(17):1625-38.
9. Sanikini H, Yuan JM, Butler LM, Koh WP, Gao YT, Steffen A, et al. Body mass index and lung cancer risk: a pooled analysis based on nested case-control studies from four cohort studies. BMC Cancer. 2018 Feb 23;18(1):220.
10. Hidayat K, Du X, Chen G, Shi M, Shi B. Abdominal Obesity and Lung Cancer Risk: Systematic Review and Meta-Analysis of Prospective Studies. Nutrients. 2016 Dec 15;8(12):810.
11. Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018 Jan;68(1):31-54.
12. Islami F, Goding Sauer A, Gapstur SM, Jemal A. Proportion of Cancer Cases Attributable to Excess Body Weight by US State, 2011-2015. JAMA Oncol. 2019 Mar 1;5(3):384-92.
13. Rogado J, Romero-Laorden N, Sanchez-Torres JM, Ramos-Levi AM, Pacheco-Barcia V, Ballesteros AI, et al. Effect of excess weight and immune-related adverse events on the efficacy of cancer immunotherapy with anti-PD-1 antibodies. Oncoimmunology. 2020 Apr 16;9(1):1751548.
14. Cortellini A, Bersanelli M, Buti S, Cannita K, Santini D, Perrone F, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J Immunother Cancer. 2019 Feb 27;7(1):57.
15. Antoun S, Lanoy E, Ammari S, Farhane S, Martin L, Robert C, et al. Protective effect of obesity on survival in cancers treated with immunotherapy vanishes when controlling for type of cancer, weight loss and reduced skeletal muscle. Eur J Cancer. 2023 Jan;178:49-59.
16. Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2020 Apr 1;6(4):512-18.
17. McQuade JL, Daniel CR, Hess KR, Mak C, Wang DY, Rai RR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018 Mar;19(3):310-22.
18. Kajimura S. Advances in the understanding of adipose tissue biology. Nat Rev Endocrinol. 2017 Feb;13(2):69-70.
19. Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022 Jan;12(1):31-46.
20. Moore LL, Chadid S, Singer MR, Kreger BE, Denis G V. Metabolic health reduces risk of obesity-related cancer in framingham study adults. Cancer Epidemiol Biomarkers Prev. 2014 Oct;23(10):2057-65.
21. Hahn AW, Venkatesh N, Msaouel P, McQuade JL. The Influence of Obesity on Outcomes with Immune Checkpoint Blockade: Clinical Evidence and Potential Biological Mechanisms. Cells. 2023 Oct 31;12(21):2551.
22. Ichihara E, Harada D, Inoue K, Sato K, Hosokawa S, Kishino D, et al. The impact of body mass index on the efficacy of anti-PD-1/PD-L1 antibodies in patients with non-small cell lung cancer. Lung Cancer. 2020 Jan 1;139:140-5.
23. Spyrou N, Vallianou N, Kadillari J, Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin Cancer Biol. 2021 Aug 1;73:356-76.
24. Ihara Y, Sawa K, Imai T, Bito T, Shimomura Y, Kawai R, et al. Immunotherapy and Overall Survival Among Patients With Advanced Non–Small Cell Lung Cancer and Obesity. JAMA Netw Open. 2024 Aug 1;7(8):e2425363.
25. Vick L V., Rosario S, Riess JW, Canter RJ, Mukherjee S, Monjazeb AM, et al. Potential roles of sex-linked differences in obesity and cancer immunotherapy: revisiting the obesity paradox. NPJ Metab Health Dis. 2024;2(1):5.
26. Wang Z, Aguilar EG, Luna JI, Dunai C, Khuat LT, Le CT, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019 Jan;25(1):141-51.
27. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016 May 23;11:421-49.
28. Amjadi F, Javanmard SH, Zarkesh-Esfahani H, Khazaei M, Narimani M. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. J Exp Clin Cancer Res. 2011 Feb 21;30(1):21.
29. Albiges L, Ari Hakimi A, Xie W, McKay RR, Simantov R, Lin X, et al. Body mass index and metastatic renal cell carcinoma: Clinical and biological correlations. J Clin Oncol. 2016 Oct 20;34(30):3655-63.
30. Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025.
31. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, et al. Pro-Inflammatory CD11c+CD206+ Adipose Tissue Macrophages Are Associated With Insulin Resistance in Human Obesity. Diabetes. 2010 Jul;59(7):1648-56.
32. Iyengar NM, Brown KA, Zhou XK, Gucalp A, Subbaramaiah K, Giri DD, et al. Metabolic Obesity, Adipose Inflammation and Elevated Breast Aromatase in Women with Normal Body Mass Index. Cancer Prev Res (Phila). 2017 Apr;10(4):235-43.
33. Wang L, Zhao R peng, Song X yu, Wu W fu. Targeting ERβ in Macrophage Reduces Crown-like Structures in Adipose Tissue by Inhibiting Osteopontin and HIF-1α. Sci Rep. 2019 Oct 31;9(1):15762.
34. Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Györi G, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond). 2007 Sep;31(9):1420-8.
35. Haase J, Weyer U, Immig K, Klöting N, Blüher M, Eilers J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014 Mar;57(3):562-71.
36. Mayi TH, Daoudi M, Derudas B, Gross B, Bories G, Wouters K, et al. Human Adipose Tissue Macrophages Display Activation of Cancer-related Pathways. Journal of Biological Chemistry. 2012 Jun 22;287(26):21904-13.
37. Springer NL, Iyengar NM, Bareja R, Verma A, Jochelson MS, Giri DD, et al. Obesity-Associated Extracellular Matrix Remodeling Promotes a Macrophage Phenotype Similar to Tumor-Associated Macrophages. Am J Pathol. 2019 Oct 1;189(10):2019–35.
38. Dudzinski SO, Bader JE, Beckermann KE, Young KL, Hongo R, Madden MZ, et al. Leptin Augments Antitumor Immunity in Obesity by Repolarizing Tumor-Associated Macrophages. J Immunol. 2021 Dec 15;207(12):3122-30.
39. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017 May 25;545(7655):495-9.
40. Baazim H, Antonio-Herrera L, Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat Rev Immunol. 2022 May;22(5):309-21.
41. Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, et al. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016 Nov 8;24(5):672-84.
42. Vissers YL, Dejong CH, Luiking YC, Fearon KC, von Meyenfeldt MF, Deutz NE. Plasma arginine concentrations are reduced in cancer patients: evidence for arginine deficiency? Am J Clin Nutr. 2005 May;81(5):1142-6.
43. Ragni M, Fornelli C, Nisoli E, Penna F. Amino Acids in Cancer and Cachexia: An Integrated View. Cancers (Basel). 2022 Nov 19;14(22):5691.
44. Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016 Oct 20;167(3):829-42.e13.
45. Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, Samborska B, et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017 Feb 7;25(2):345-57.
46. Lu SW, Pan HC, Hsu YH, Chang KC, Wu LW, Chen WY, et al. IL-20 antagonist suppresses PD-L1 expression and prolongs survival in pancreatic cancer models. Nat Commun. 2020 Sep 14;11(1):4611.
47. Advani SM, Advani PG, Vonville HM, Jafri SH. Pharmacological management of cachexia in adult cancer patients: A systematic review of clinical trials. BMC Cancer. 2018 Nov 27;18(1):1174.
48. Cuenca AG, Cuenca AL, Winfield RD, Joiner DN, Gentile L, Delano MJ, et al. Novel Role for Tumor-Induced Expansion of Myeloid-Derived Cells in Cancer Cachexia. The Journal of Immunology. 2014 Jun 15;192(12):6111-9.
49. Narsale A, Moya R, Ma J, Anderson LJ, Wu D, Garcia JM, et al. Cancer-driven changes link T cell frequency to muscle strength in people with cancer: a pilot study. J Cachexia Sarcopenia Muscle. 2019 Aug 1;10(4):827-43.
50. Costamagna D, Duelen R, Penna F, Neumann D, Costelli P, Sampaolesi M. Interleukin-4 administration improves muscle function, adult myogenesis, and lifespan of colon carcinoma-bearing mice. J Cachexia Sarcopenia Muscle. 2020 Jun 1;11(3):783-801.
51. Burfeind KG, Zhu X, Norgard MA, Levasseur PR, Huisman C, Michaelis KA, et al. Microglia in the hypothalamus respond to tumor-derived factors and are protective against cachexia during pancreatic cancer. Glia. 2020 Jul 1;68(7):1479-94.
52. Erdem M, Möckel D, Jumpertz S, John C, Fragoulis A, Rudolph I, et al. Macrophages protect against loss of adipose tissue during cancer cachexia. J Cachexia Sarcopenia Muscle. 2019 Oct 1;10(5):1128–42.
53. Wang Z, Zhao C, Moya R, Davies JD. A novel role for CD4+ T cells in the control of cachexia. J Immunol. 2008 Oct 1;181(7):4676-84.
54. Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nature Reviews Disease Primers. Nat Rev Dis Primers. 2018;4:17105.
55. Shukuya T, Takahashi K, Shintani Y, Miura K, Sekine I, Takayama K, et al. Epidemiology, risk factors and impact of cachexia on patient outcome: Results from the Japanese Lung Cancer Registry Study. J Cachexia Sarcopenia Muscle. 2023 Jun 10;14(3):1274-85.
56. Shiroyama T, Nagatomo I, Koyama S, Hirata H, Nishida S, Miyake K, et al. Impact of sarcopenia in patients with advanced non–small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci Rep. 2019 Feb 21;9(1):2447.
57. Rounis K, Makrakis D, Tsigkas AP, Georgiou A, Galanakis N, Papadaki C, et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl Lung Cancer Res. 2021 Aug 1;10(8):3538-49.
58. Iyengar P, Gandhi AY, Granados J, Guo T, Gupta A, Yu J, et al. Tumor loss-of-function mutations in STK11/LKB1 induce cachexia. JCI Insight. 2023 Apr 24;8(8):e165419.
59. Pons-Tostivint E, Lugat A, Fontenau JF, Denis MG, Bennouna J. STK11/LKB1 Modulation of the Immune Response in Lung Cancer: From Biology to Therapeutic Impact. Cells. 2021 Nov 11;10(11):3129.
60. Al-Sawaf O, Weiss J, Skrzypski M, Lam JM, Karasaki T, Zambrana F, et al. Body composition and lung cancer-associated cachexia in TRACERx. Nat Med. 2023 Apr 12;29(4):846-58.
61. Crawford J, Calle RA, Collins SM, Weng Y, Lubaczewski SL, Buckeridge C, et al. A Phase Ib First-In-Patient Study Assessing the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Ponsegromabin Participants with Cancer and Cachexia. Clinical Cancer Research. 2024 Feb 1;30(3):489-97.
62. Groarke JD, Crawford J, Collins SM, Lubaczewski S, Roeland EJ, Naito T, et al. Ponsegromab for the Treatment of Cancer Cachexia. N Engl J Med. 2024 Sep 14.
63. Groarke JD, Crawford J, Collins SM, Lubaczewski SL, Breen DM, Harrington MA, et al. Phase 2 study of the efficacy and safety of ponsegromab in patients with cancer cachexia: PROACC-1 study design. J Cachexia Sarcopenia Muscle. 2024 Jun 1;15(3):1054-61.
64. Zhong X, Zimmers TA. Sex Differences in Cancer Cachexia. Curr Osteoporos Rep. 2020 Dec;18(6):646-54.
65. Queiroz AL, Dantas E, Ramsamooj S, Murthy A, Ahmed M, Zunica ERM, et al. Blocking ActRIIB and restoring appetite reverses cachexia and improves survival in mice with lung cancer. Nat Commun. 2022 Aug 8;13(1):4633.
66. Wang R, Bhat-Nakshatri P, Zhong X, Zimmers T, Nakshatri H. Hormonally regulated myogenic miR-486 influences sex-specific differences in cancer-induced skeletal muscle defects. Endocrinology. 2021 Oct 1;162(10):bqab142.
67. Caliman E, Petrella MC, Rossi V, Mazzoni F, Grosso AM, Fancelli S, et al. Gender Matters. Sex-related Differences in Immunotherapy Outcome in Patients with Non-small Cell Lung Cancer. Curr Cancer Drug Targets. 2022 Aug 31.
68. Brennan M, DeBruin D, Nwokolo C, Hunt KS, Piening A, Donlin MJ, et al. T-Cell Expression of CXCL13 is Associated with Immunotherapy Response in a Sex-Dependent Manner in Patients with Lung Cancer. Cancer Immunol Res. 2024 Aug 1;12(8):956-63.
69. Somasundaram A, Rothenberger NJ, Stabile LP. The Impact of Estrogen in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1277:33-52.
70. Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never-smokers. J Clin Oncol. 2007 Feb 10;25(5):472-8.
71. Wang F, Tan F, Wu Z, Cao W, Yang Z, Yu Y, et al. Lung cancer risk in non-smoking females with a familial history of cancer: a multi-center prospective cohort study in China. J Natl Cancer Cent. 2021 Jul 25;1(3):108-14.
72. Guo L, Meng Q, Zheng L, Chen Q, Liu Y, Xu H, et al. Lung Cancer Risk Prediction Nomogram in Nonsmoking Chinese Women: Retrospective Cross-sectional Cohort Study. JMIR Public Health Surveill. 2023 Jan 6;9:e41640.
73. Smida T, Bruno TC, Stabile LP. Influence of Estrogen on the NSCLC Microenvironment: A Comprehensive Picture and Clinical Implications. Front Oncol. 2020 Feb 18;10:137.
74. Forsyth KS, Jiwrajka N, Lovell CD, Toothacre NE, Anguera MC. The conneXion between sex and immune responses. Nat Rev Immunol. 2024 Jul;24(7):487-502.
75. Ramos-Casals M, Brito-Zerón P, Kostov B, Sisó-Almirall A, Bosch X, Buss D, et al. Google-driven search for big data in autoimmune geoepidemiology: analysis of 394,827 patients with systemic autoimmune diseases. Autoimmun Rev. 2015 Aug;14(8):670-9.
76. Fish EN. Human illnesses affect men and women differently. Nat Rev Immunol. 2008;8(SEPTEmBER):737-44.
77. Wang S, Cowley LA, Liu XS. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules. 2019 Sep 4;24(18):3214.
78. Conforti F, Pala L, Bagnardi V, De Pas T, Martinetti M, Viale G, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018 Jun;19(6):737-46.
79. Conforti F, Pala L, Bagnardi V, Viale G, De Pas T, Pagan E, et al. Sex-Based Heterogeneity in Response to Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2019 Aug 1;111(8):772-81.
80. Hu X, Liu Y, He Y, Wang Z, Zhang H, Yang W, et al. Relationship between Patients’ Baseline Characteristics and Survival Benefits in Immunotherapy-Treated Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. J Oncol. 2022 May 19;2022:3601942.
81. Lang D, Brauner A, Huemer F, Rinnerthaler G, Horner A, Wass R, et al. Sex-Based Clinical Outcome in Advanced NSCLC Patients Undergoing PD-1/PD-L1 Inhibitor Therapy-A Retrospective Bi-Centric Cohort Study. Cancers (Basel). 2021 Dec 24;14(1):93.
82. Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, Lambe M. Sex and survival in non-small cell lung cancer: A nationwide cohort study. PLoS One. 2019 Jun 27;14(6):e0219206.
83. Irelli A, Sirufo MM, D’Ugo C, Ginaldi L, De Martinis M. Sex and Gender Influences on Cancer Immunotherapy Response. Biomedicines. 2020 Jul 21;8(7):232.
84. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther. 2017 Nov;16(11):2598-608.
85. Castro A, Pyke RM, Zhang X, Thompson WK, Day CP, Alexandrov LB, et al. Strength of immune selection in tumors varies with sex and age. Nat Commun. 2020 Aug 17;11(1):4128.
86. Zhao J, Wang Q, Tan AF, Loh CJL, Toh HC. Sex differences in cancer and immunotherapy outcomes: the role of androgen receptor. Front Immunol. 2024 May 28;15:1416941.
87. Li H, Jiang W, Liu S, Yang M, Chen S, Pan Y, et al. Connecting the mechanisms of tumor sex differences with cancer therapy. Mol Cell Biochem. 2024 Feb;479(2):213-31.
88. Özdemir BC, Dotto GP. Sex hormones and anticancer immunity. Clin Cancer Res. 2019 Aug 1;25(15):4603-10.
89. Anobile DP, Salaroglio IC, Tabbò F, Vecchia S La, Akman M, Napoli F, et al. Autocrine 17-β-Estradiol/Estrogen Receptor-α Loop Determines the Response to Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer. Clin Cancer Res. 2023 Oct 2;29(19):3958-73.
90. Rothenberger NJ, Somasundaram A, Stabile LP. The role of the estrogen pathway in the tumor microenvironment. Int J Mol Sci. 2018 Feb 19;19(2):611.
91. Hamilton DH, Griner LM, Keller JM, Hu X, Southall N, Marugan J, et al. Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clinical Cancer Research. 2016 Dec 15;22(24):6204-16.