Abstract
Regional real-world evidence on the safety and efficacy of tyrosine kinase inhibitors in patients with chronic myeloid leukemia (CML) is limited. This multicenter, observational, prospective study, ERASER, evaluated the safety and tolerability of nilotinib in routine clinical practice in Greece.
Adult patients with newly diagnosed BCR/ABL+ chronic phase (CP) CML and those with CP CML, resistant/intolerant to prior therapy were included in this study and followed up for 36 months. Nilotinib 300 mg/400 mg twice daily was prescribed, with appropriate dose adjustment by the investigator.
The analysis population (57 patients; median age, 55 years) remained in the study for a median of 34 months. Overall, 44 (77.2%) and 13 (22.8%) patients received nilotinib as first-line treatment and owing to resistance/intolerance to prior therapy, respectively. The most common adverse events (AEs) were thrombocytopenia in 8 (14%), neutropenia in 6 (10.5%), and blood bilirubin increased/hyperbilirubinemia in 10 (17.5%) patients. Permanent treatment discontinuation, including deaths and progression, occurred in 13 (22.8%) patients. Of 52 patients with available molecular response (MR), 30 achieved MR4.5 by end of the study.
The study affirms the long-term safety of nilotinib in real-world setting in Greece, in patients with newly diagnosed CML, and in those with resistance/intolerance to prior therapy.
Keywords
Ph+ CML, Nilotinib, Observational study, Real world evidence, Tyrosine kinase inhibitors
Introduction
Chronic myeloid leukemia (CML) is characterized by the presence of Philadelphia chromosome (Ph+) in the majority of cases, and definitely by the presence of the hybrid BCR/ABL1 proto-oncogene in all the patients [1,2]. The BCR::ABL1 fusion gene encodes for an oncoprotein that has an activated tyrosine kinase domain in the ABL region and affects crucial cellular activities, such as increased proliferation, loss of stromal adhesion, and resistance to apoptosis, through enhanced tyrosine phosphorylation of various cellular substrates [3]. Thus, molecular therapies targeting BCR::ABL1 tyrosine kinase oncoprotein have been proved a rational approach in the therapy of CML patients [3,4]. For almost two decades, tyrosine kinase inhibitors (TKIs) represent the cornerstone of treatment for patients with BCR::ABL1–positive CML [5,6]. For a minority of patients, refractory to many TKIs, or for those, whom accessibility to TKIs is not feasible, other types of treatment, including allogeneic stem cell transplantation [7], recombinant interferon alpha therapy [8], or different inhibitors such as omacetaxine [9], can be used.
The efficacy and safety of imatinib, the first TKI that specifically targeted the BCR::ABL1 oncogene, are well established as first-line therapy for patients with BCR::ABL1–positive CML. Imatinib has led to a marked improvement in the prognosis of these patients [10,11] and has prolonged overall survival, to almost that of the general population [12]. Recent evidence, however, indicates that not all patients achieve an optimum response to imatinib, and some patients may lose an initially obtained favorable response in later years, thereby being at risk of progressing to advanced phases of CML [11]. In the accelerated phase (AP), or blast crisis phase, responses are almost inevitably transient, and the prognosis remains poor, compared with chronic phase (CP) CML [13,14].
This possibility led to the evolution of TKIs with the emergence of second-generation molecules, such as dasatinib, nilotinib, and bosutinib, which have demonstrated effectiveness as second-line therapy and/or as an alternative first-line therapy [15]. Nilotinib is a rationally designed, second-generation TKI with improved target specificity over imatinib to the BCR::ABL1 kinase molecule, showing significant improvement in hematologic and cytogenetic responses [16-18]. Nilotinib has been approved for the treatment of adult patients with newly diagnosed BCR/ABL+ CML in CP (CML-CP), as well as for adult patients in CP and AP Ph+ CML with resistance or intolerance to prior therapy, including imatinib [16,19]. Despite ample clinical evidence, there are limited real?world data demonstrating the effectiveness and safety of nilotinib in these patients.
The ERASER study was designed to evaluate the safety and tolerability of nilotinib in adult patients with newly diagnosed CML-CP, as well as in CML-CP/AP patients, who would be resistant or intolerant to prior therapy, including imatinib. It is anticipated that the results of this study will improve the therapeutic use of nilotinib in patients with BCR/ABL+ CML in everyday clinical practice. The primary objective of the study was to evaluate the safety profile of nilotinib in routine clinical practice, when prescribed according to the summary of product characteristics (SmPC), in terms of the frequency (number) and type of adverse events (AEs) and serious AEs (SAEs) during treatment, irrespective of a causal relationship to the study medication.
The secondary objective of the study was to evaluate the tolerability profile of nilotinib, in terms of the number of patients discontinuing treatment due to hematologic and nonhematologic toxicity, number and proportion of patients receiving the recommended dose of nilotinib, dose delivered per patient cohort, number and proportion of patients with any dose adjustment/interruption, reasons for dose adjustments/interruptions, and type of concomitant medication. Although not designed for evaluating efficacy, parameters of treatment response were also explored.
Patients and Methods
Study design and patients
In this prospective, multicenter, non-interventional, observational study, patients with BCR/ABL+ CML were enrolled from 12 sites across Greece between July 2012 and December 2015. Approval was obtained from each site’s ethical and scientific committee, and informed consent was obtained from all patients before their enrollment in the study.
Patients with newly diagnosed BCR/ABL+ CML-CP who had already started treatment with nilotinib for up to 3 months before enrollment, or those with CML?CP/AP who were resistant/intolerant to prior therapy, including imatinib, and had already received nilotinib up to 3 months prior to the start of the study, were eligible for participation. Patients with hypersensitivity to any nilotinib excipients as per contraindications mentioned in the SmPC, or those who were participating in any other clinical trial, were excluded.
Treatment and evaluations
Nilotinib was prescribed at the dose of 300 mg twice daily (bid) for newly diagnosed patients and of 400 mg bid for those, who were resistant/intolerant to prior therapy, with appropriate adjustment at the investigator’s discretion and the locally approved product’s SmPC. The planned duration for follow-up was 36 months.
The safety evaluation of nilotinib included frequency of AEs, according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 and of SAEs, which were defined according to the International Council for Harmonization (ICH) guidelines. All AEs were reported by the patients and/or investigators throughout the study. The investigators determined whether an AE was related to nilotinib treatment. If the CTCAE grading was not available (NA) for an AE, any of the following severity levels were used: mild, moderate, severe, and life-threatening, or grade 1 to 4. CTCAE grade 5 (death) was not used.
Cytogenetic evaluation was performed at baseline and at any subsequent visit at the investigator’s discretion, while molecular evaluation was done at baseline, 12 months, 24 months, and 36 months, and at any other visit at the discretion of the investigator.
Statistical analysis
Based on published literature from interventional studies on AE frequency, the proportion of patients experiencing any expected AE of any grade was assumed as 0.5 (50%, worst case scenario) with half-width 95% confidence interval (CI; maximum error) for a single proportion extending to 0.12 (12%) from the observed proportion of patients. The proposed sample size was 75 patients, including a 10% of dropout.
A descriptive statistical analysis was performed for all study data, including safety observations (blood parameters, biochemical profile, electrocardiogram) and other clinical assessments (physical examination, cytogenetic, and molecular evaluation) as recorded at baseline. Other characteristics, such as line of therapy, nilotinib dosage, CML history, prior medications, and current medical conditions, were also summarized. The safety population included all the study participants treated with at least one dose of nilotinib. All cases of treatment discontinuation for reasons of inefficiency, safety, or intolerance were recorded and analyzed. Efficacy analysis on molecular and cytogenetic responses, where available, was also described.
The significance of the prognostic factors was evaluated at first with univariate Cox regression analysis and all the variables that were found to be statistically significant (p<0.20) were included in a multivariate model. The association between categorical variables was assessed using the chi-square test or Fisher’s exact test, when appropriate. To examine the possible differences between continuous variables, the paired t-test or Wilcoxon signed rank test for related samples, and the t-test or Mann-Whitney U test for independent samples, were applied. Statistical analysis was performed using the IBM-SPSS v24.0 statistical software, and the Medical Dictionary for Regulatory Activities (MedDRA) v.21.1 was used for the medical coding of the recorded AEs.
Results
Patient characteristics
As on November 2018, 67 patients were enrolled, of which 10 patients were excluded as they did not meet the eligibility criteria (Table 1). The analysis population comprised 57 patients, who remained in the study for a median period of 34 months (interquartile range [IQR]: 27.0-36.0), with a median exposure to nilotinib of 35 months (IQR: 28.0-37.0). Most of the patients (n = 38, 66.7%) completed the study duration as per the protocol. Nineteen patients discontinued the treatment primarily due to AEs (8 of 19 patients [42.1%]) (Table 1).
|
Disposition |
n (%) of patients |
|---|---|
|
Enrolled patients |
67 |
|
Final analysis population |
57 |
|
Safety analysis population |
57 |
|
Patients excluded from analysis |
10 |
|
Reason for exclusion: inclusion criteria not met |
10a |
|
Study duration (months) median (min, max) |
34.0 (1.0, 41.0b) |
|
Exposure to nilotinib (months) median (min, max) |
35.0 (2.0, 42.0b) |
|
Patients who completed the 36-month observation period |
38 (66.7) |
|
Reason for discontinuation |
n = 19 |
|
Adverse event |
8 (42.1) |
|
Non-satisfactory therapeutic result |
3 (15.8) |
|
Lost to follow-up |
3 (15.8) |
|
Death |
2 (10.5) |
|
Otherc |
3 (15.8) |
|
aThree patients received nilotinib for more than 3 months prior to the enrollment, and seven patients did not receive nilotinib before signing consent form. bThere were 10 patients with a study duration of >36 months and two patients with nilotinib exposure for >36 + 3 months. cTwo patients completed follow-up visits before the study duration of 36 months, and one patient withdrew from the study after attaining complete molecular remission. Max: Maximum; min: Minimum. |
|
The median time from CML diagnosis to enrollment was 1 month (IQR: 0-3.5 months), and most of the patients (84.2%) were diagnosed less than 12 months prior to enrollment. Nilotinib was administered as first-line treatment in 44 (77.2%) patients, and 13 (22.8%) patients were administered nilotinib, owing to resistance or intolerance to prior anti-CML treatment (resistance, n = 8; intolerance, n = 5). At baseline, 10 (17.5%) patients had heart disease (coronary artery disease, n = 5 [8.8%]), seven (12.3%) had a history of other neoplasms, while depression and diabetes mellitus were recorded in three (5.3%) and two (3.5%) patients, respectively (Table 2). Prior to enrollment, 42 patients (73.7%) had received at least one previous treatment for CML. The most common prior medications other than hydroxyurea (81%) were imatinib (13 [31%] patients), dasatinib (two [5%] patients), and interferon-alpha (one [2.4%] patient) for a median duration of 18.7 months, 33.8 months, and 6 months, respectively. Toxicity to previous anti-CML treatment was reported by six of the 42 patients, of which four were treated with imatinib, and one each with hydroxyurea and dasatinib.
|
Baseline characteristics |
n (%) of patients |
|---|---|
|
Age (years), median, range (min-max) |
55 (18-87) |
|
<50 years |
23 (40.4) |
|
50-65 years |
15 (26.3) |
|
≥65 years |
19 (33.3) |
|
Sex, n (%) |
n = 57 |
|
Male |
32 (56.1) |
|
Female |
25 (43.9) |
|
Race, n (%) |
n = 57 |
|
Caucasian |
56 (98.2) |
|
Asian |
1 (1.8) |
|
Time from CML diagnosis (months), median (min, max) |
1.0 (0.0, 151.0) |
|
Diagnosis in <12 months since enrollment |
48 (84.2) |
|
Setting of nilotinib treatment |
n = 57 |
|
Newly diagnosed in chronic phase |
44 (77.2) |
|
Resistant/intolerant to previous therapy |
13 (22.8) |
|
Resistant, chronic phase |
8 (61.5) |
|
Intolerant, chronic phase |
5 (38.5) |
|
ECOG performance status, quantitative |
n = 54 |
|
0 |
42 (73.7) |
|
1 |
11 (19.2) |
|
2 |
1 (1.8) |
|
Cytogenetic status at baseline (% of Ph+) |
n = 57 |
|
0 |
4 (7) |
|
66%-95% |
36 (63.2) |
|
96%-100% |
5 (8.8) |
|
Not availablea |
12 (21) |
|
Heart disease |
10 (17.5) |
|
aNot available includes not available, not done, and unknown; one patient had an unsuccessful karyotype. CML: Chronic Myeloid Leukemia; ECOG: Eastern Cooperative Oncology Group; max: Maximum; min: Minimum; Ph: Philadelphia chromosome |
|
Safety and tolerability
Of the 38 patients, who successfully completed the study, 33 remained on the initial recommended nilotinib dose. Twenty-two (38.6%) patients underwent 59 dose modifications/interruptions of nilotinib, primarily due to hematologic toxicities (38 dose interruptions/modifications in 11 patients) (Table 3). Of these 11 patients, five (45.5%) were re?escalated to the recommended dose. The serum glucose levels were increased in 2 patients (3.5%; treatment-related in 1 patient [1.8%]). No incidence of high-grade glucose elevation was documented in the present study.
|
Patients with dose interruptions/modifications, n (%) (n = 57) |
22 (38.6) |
|
|
Total number of interruptions/modifications |
59 |
|
|
Reason for interruption/modification |
No. of interruptions/ |
n (%) of patients, (n = 57) |
|
Toxicity (hematologic) |
38 |
11 (19.3) |
|
Toxicity (nonhematologic) |
8 |
5 (8.8) |
|
Toxicity (hematologic and nonhematologic) |
4 |
3 (5.3) |
|
Poor compliance |
3 |
2 (3.5) |
|
Others |
6 |
6 (10.5) |
|
Complete molecular remission |
2 |
2 (3.5) |
|
Non-satisfactory therapeutic result |
1 |
1 (1.8) |
|
Transition to accelerated phase (from 600 to 800 mg/day) |
1 |
1 (1.8) |
|
Disease progression (loss of molecular remission) |
1 |
1 (1.8) |
|
Disease progression: blast crisis, acute lymphoblastic leukemia |
1 |
1 (1.8) |
Over the 36-month observation period, 45 of the 57 evaluable patients (78.9%) reported at least one AE, with 22.8% being grade 3 or 4 AEs. In total, 23 of the 57 patients (40.4%) reported SAEs. The median number of AEs per patient was 4.0 (range, 1.0-18.0), with a median time to first AE of 2.7 months (95% CI: 1.0?5.8). The percentage of patients with their first AE occurring from month 32 onward was stable at 20.9% (Figure 1; Table 4). Most study patients (35 [61.4%]) experienced a mild AE, and 29 (50.9%) patients had a recovered AE. Fifteen (26.3%) patients experienced at least one hematologic AE, with 7% being grade ≥3. Most of the hematologic AEs occurred during the first 3 months of treatment. Common hematologic AEs occurring during the first 3 months of treatment were thrombocytopenia (8 [14.0%] patients) and neutropenia (5 [10.5%] patients). Permanent discontinuation due to an AE was recorded for 13 (22.8%) patients: eight (14%) due to toxicities, three (5.3%) due to disease progression, and two (3.5%) due to patient death. Of these 13 patients that discontinued treatment due to AEs, nine were receiving nilotinib as first-line and four as second-line treatment. Thirty-three (57.9%) patients reported an event that was suspected to be related to nilotinib, at the investigator’s judgment.
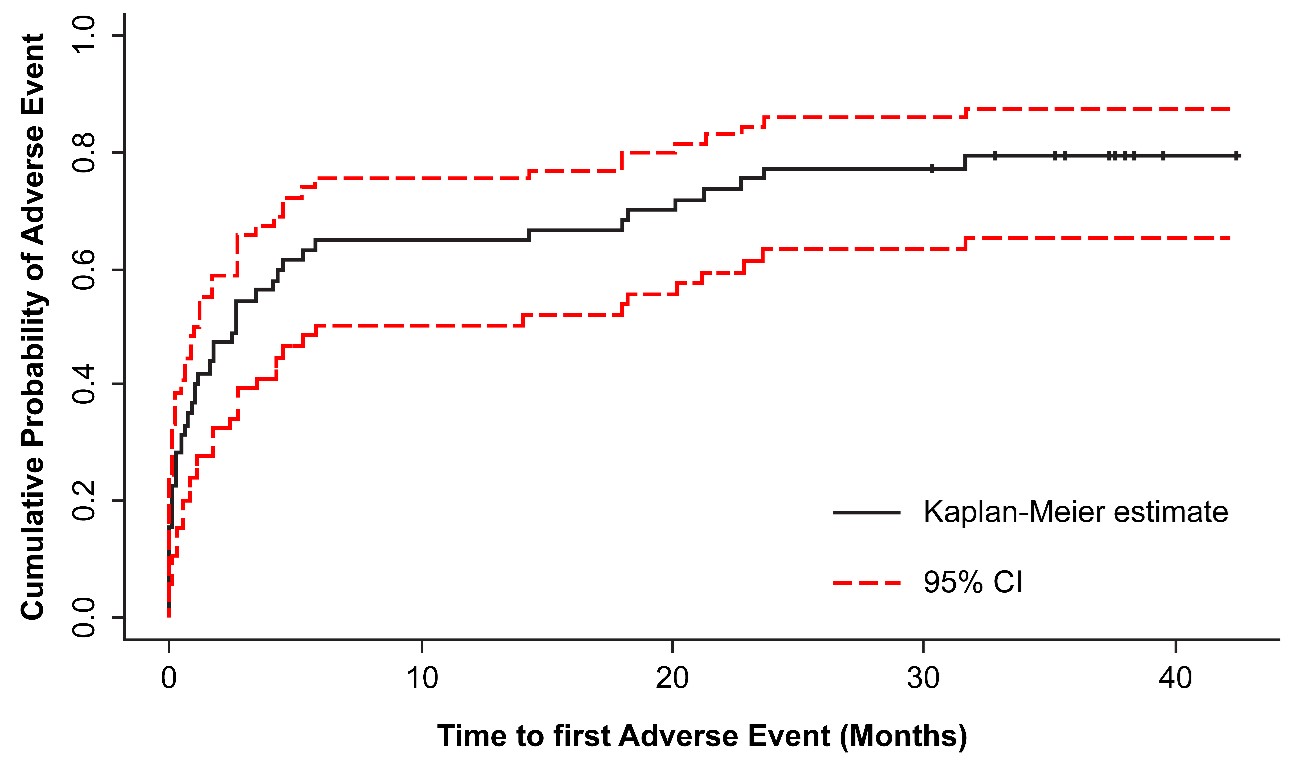
Figure 1. Cumulative survival probability estimates and 95% CI of patients’ first adverse event during the study (censored observations marked with +). CI: Confidence Interval.
|
Preferred term |
All AEs N = 57 (%)a |
Related to nilotinib |
|||
|
Anemia |
5 (8.8) |
2 (3.5) |
|||
|
Leukopenia |
4 (7) |
3 (5.3) |
|||
|
Neutropenia |
6 (10.5) |
4 (7) |
|||
|
Thrombocytopenia |
8 (14) |
8 (14) |
|||
|
Abdominal pain upper |
3 (5.3) |
2 (3.5) |
|||
|
Nausea |
3 (5.3) |
2 (3.5) |
|||
|
Fatigue (one unknown) |
3 (5.3) |
0 |
|||
|
Edema peripheral |
3 (5.3) |
1 (1.8) |
|||
|
Pyrexia |
5 (8.8) |
1 (1.8) |
|||
|
Hyperbilirubinemia/blood bilirubin increased |
10 (17.5) |
7 (12.3) |
|||
|
Gastroenteritis |
3 (5.3) |
0 |
|||
|
Respiratory tract infections |
4 (7) |
1 (1.8) |
|||
|
Alanine aminotransferase increased |
5 (8.8) |
5 (8.8) |
|||
|
Aspartate aminotransferase increased |
4 (7) |
4 (7) |
|||
|
Gamma-glutamyl transferase increased |
3 (5.3) |
3 (5.3) |
|||
|
Hypercholesterolemia |
4 (7) |
3 (5.3) |
|||
|
Arthralgia |
5 (8.8) |
1 (1.8) |
|||
|
Alopecia |
3 (5.3) |
3 (5.3) |
|||
|
Rash |
5 (8.8) |
5 (8.8) |
|||
|
Rash pruritic |
3 (5.3) |
2 (3.5) |
|||
|
aNine patients received a different dosage scheme than that recommended by the product’s SmPC. AE: Adverse Event; SmPC: Summary of Product Characteristics |
|||||
The most frequently reported AEs, including patients receiving treatment regimen outside the SmPC (off-label use) in nine (15.8%), were thrombocytopenia (n = 8 [14%]), neutropenia (n = 6 [10.5%]), and blood bilirubin increased/hyperbilirubinemia (n = 10 [17.5%]) (Table 4). Thirteen patients reported abnormal biochemical laboratory values during the study, the most common being increased serum bilirubin in seven (12.3%) patients. Only one laboratory abnormality was of grade ≥3 severity (increase in the hepatic enzymes, alanine aminotransferase, and aspartate aminotransferase of >3 of the upper limit of normal), which led to permanent discontinuation of nilotinib. Cardiac AEs were identified in seven (12.3%) patients, of which the most common were atrial fibrillation and palpitations, two each (3.5%), leading to permanent discontinuation of nilotinib in one patient each. One patient died due to coronary artery disease of grade ≥ 3. When a logistic regression analysis was performed, to assess the correlation between clinical and baseline factors and the presence of AEs, the previous CML treatment was found to be significantly associated with the presence of AEs (p = 0.028).
Efficacy
Cytogenetic evaluation was performed in 37 (64.9%) patients throughout the study at the discretion of the physician. Among the patients with cytogenetic evaluations recorded, the most common change was from “none” at first response to “complete” at last recorded response in 10 of 37 patients; nine of whom serum were newly diagnosed. Of 25 patients with more than one cytogenetic evaluation recorded, 10 (40%) achieved an early cytogenetic response (patient achieves partial cytogenetic response [PCyR] at 3 months), and seven (28%) had a complete response that persisted throughout the study; one patient (4%) who had a history of resistance to imatinib before his enrollment in the study, was also resistant. Newly diagnosed patients achieved a complete cytogenetic response (CCyR) significantly earlier than those, who were resistant/intolerant to previous therapy (5 months [95% CI: 4-6] vs 12 months [95% CI: 3 months to ΝΑ]; p = 0.022), while there was no significant difference between those being resistant and those being intolerant to prior therapy (p = 0.356). Overall, 24 of 28 newly diagnosed patients with available cytogenetic data (85.7%) and six of nine patients who were resistant/intolerant to previous therapy (66.7%) achieved a major cytogenetic response.
Among the prognostic factors analyzed in univariate model (age, time from CML diagnosis, history of heart disease, and diastolic blood pressure), duration of CML diagnosis was found to have the highest prognostic value in the multivariate analysis for attaining CCyR (p = 0.008). Newly diagnosed patients had approximately 70% higher probability of achieving CCyR than those who were resistant/intolerant to previous therapies.
Most of the patients with available data on molecular response (MR; 37 of 52 patients, 71.2%) had an improvement at the end of the study. Among patients who were resistant/intolerant to prior therapy, nilotinib exhibited an improved response in 8 of 12 patients with change in BCR::ABL1 >10% to ≤1% (n = 1), major molecular response (MMR) (n = 2), undetectable BCR::ABL1 (n = 2); and from MMR to MR4 (n = 1) and undetectable in 2 patients.
In newly diagnosed patients, the median time to MMR was 6 months (95% CI: 4-10), the median time to MR 4 (BCR::ABL1 ≤ 0.01% on the International Scale [MR4]) was 20 months (95% CI: 18-28), and the median time to MR 4.5 (BCR::ABL1 ≤ 0.0032% on the International Scale [MR4.5]); or undetectable BCR::ABL1 was 18 months (95% CI: 13 months-NA, p: non-significant). In patients who were resistant/intolerant to previous therapy, the median time to MMR, MR4, and MR4.5 or undetectable BCR::ABL1 achievement was 13 months (95% CI: 5 months-NA), 30 months (95% CI: 14 months-NA), and 25 months (95% CI: 13 months to NA), respectively (Table 5, Figure 2). The attainment of the best MR was associated with patient’s age of <65 years (p = 0.055) (Table 6).
|
Patients by response |
Newly diagnosed patients |
Patients resistant/intolerant to prior therapy |
Log-rank test (p-value) |
|
Patients with molecular response data, n |
39 |
13 |
|
|
Patients with improvement in molecular response at end of the study, n (%) |
29 (74.4) |
8 (61.5) |
|
|
Patients who achieved MMR by the end of the studya, n (%) |
31 (79.5) |
8 (61.5) |
|
|
Median time to achieve MMR, months, (95% CI) |
6 (4-10) |
13 (5-NA) |
p = 0.095 |
|
Patients who achieved MR4 by the end of the study, n (%)a |
24 (61.5) |
5 (38.5) |
|
|
Median time to achieve MR4, months, (95% CI) |
20 (18-28) |
30 (14-NA) |
p = 0.121 |
|
Patients who achieved MR4.5 by the end of the study, n (%)a |
23 (59) |
7 (53.8) |
|
|
Median time to achieve MR4.5, months (95% CI) |
18 (13-NA) |
25 (13-NA) |
p = 0.814 |
|
aThe number of patients who achieved MMR, MR4, and MR4.5 by the end of the study represents the number of patients who achieved MMR, MR4, and MR4.5 at any timepoint during the study, including the patients whose first recorded molecular response was one of the aforementioned responses (not necessarily at baseline). CI: Confidence interval; MMR: Major molecular response; MR4: Molecular response 4 (BCR::ABL1 ≤ 0.01% on the International Scale); MR4.5: Molecular response 4.5 (BCR::ABL1 ≤ 0.0032% on the International Scale); NA: Not available |
|||
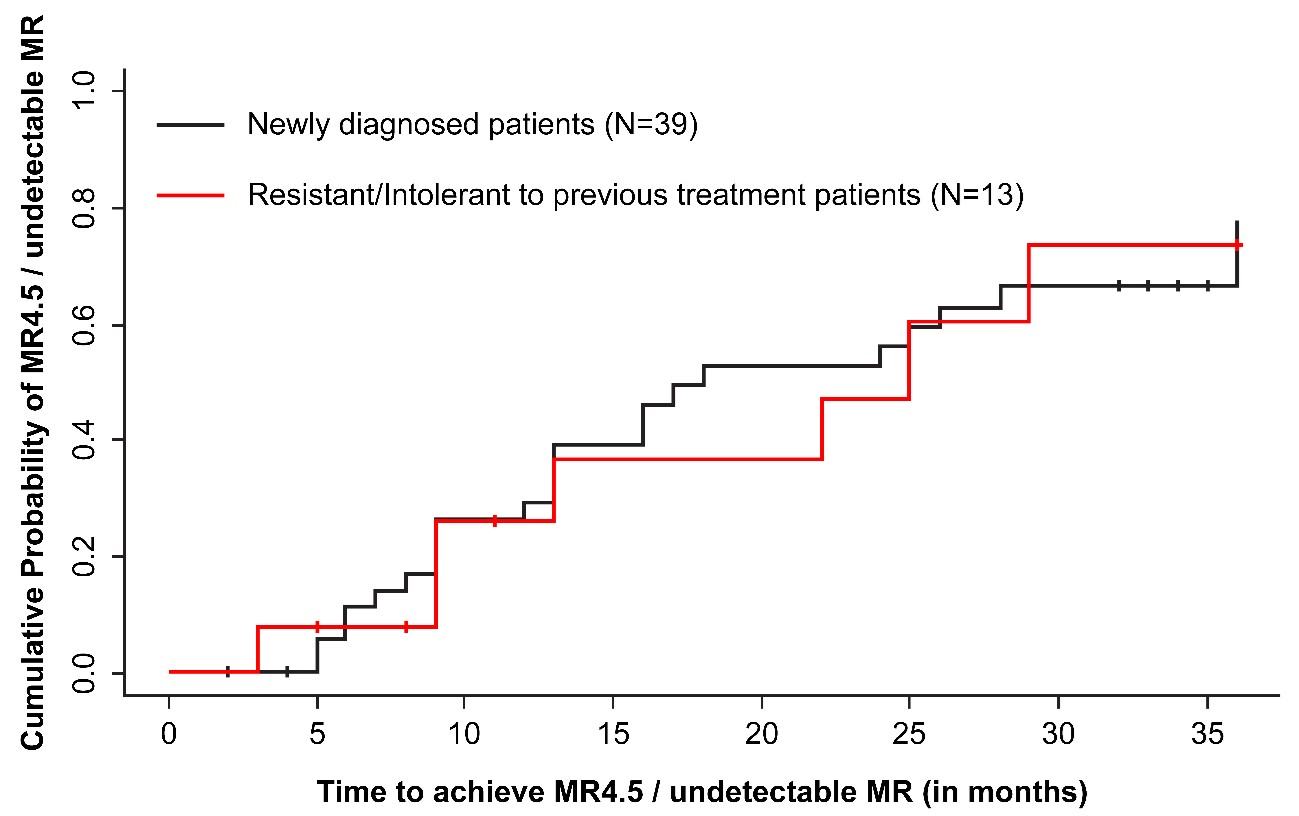
Figure 2. Cumulative survival probability estimates of patients who achieved MR4.5. MR: Molecular Response; MR4.5: Molecular Response 4.5 (BCR::ABL1 ≤ 0.0032% on the International Scale).
|
Molecular Response |
Patients by age group, n (%) |
||
|
<50 years (n = 20) |
50-65 years (n = 14) |
≥ 65 years (n = 18) |
|
|
BCR::ABL1>10%/ |
2 (3.5) |
1 (1.8) |
7 (12.3) |
|
MMR |
6 (10.5) |
0 |
2 (3.5) |
|
MR4 |
1 (1.8) |
2 (3.5) |
1 (1.8) |
|
MR4.5 |
1 (1.8) |
0 |
0 |
|
Undetectable BCR::ABL1 |
10 (17.5) |
11 (19.3) |
8 (14) |
|
Chi-square X2 (p-value) |
0.055 |
||
|
MMR: Major Molecular Response; MR4: Molecular Response 4 (BCR::ABL1 ≤ 0.01% on the International Scale); MR4.5: Molecular Response 4.5 (BCR::ABL1 ≤ 0.0032% on the International Scale). |
|||
Discussion
CML is a chronic myeloproliferative neoplasm, requiring long-term treatment with TKIs, and real-world data, along with data from controlled clinical trials, could further enhance the understanding of disease management and response to treatment. The ERASER study demonstrated that nilotinib was well tolerated in adult patients with BCR/ABL+ CML-CP from a wide geographical area of Greece. High rates of response were achieved within the first 12 months of treatment, which translated into optimal outcomes for most of the patients. This study also confirmed the good tolerability and long-term durability of nilotinib responses at 3 years in newly diagnosed as well as in those, who were resistant/intolerant to previous therapies.
For newly diagnosed patients, the median time to achieve CCyR and MMR was 5 months and 6 months, respectively. This observation is in line with the results of the ENESTnd study, in which the median time to MMR was 8.3 months in the 2-year follow-up study [20,21]. Discontinuation due to an AE occurred in 10% of patients in the ENESTnd 3-year analysis, primarily due to disease progression, treatment failure, or suboptimal response [22]. In our study, 13 (22.8%) patients discontinued treatment due to AEs, of which eight (14%) were due to toxicities and three (5.3%) due to disease progression. The percentage of deaths at 36 months (3.5%, n = 2) is comparable with the 3.7% of overall deaths reported in the 3?year follow?up of the ENESTnd study [22].
No incidence of high-grade glucose elevation was documented in the present study, while other published data report incidences ranging from 1% [23] to 6% in the ENESTnd 12?month and 2-year follow-up [20,21], 5.4% to 6.1% in the 3-year ENESTnd study [22], and 6.9% to 7.2% in the 5-year ENESTnd study [24]. In an observational, multicenter, prospective study conducted in France, 183 patients with newly diagnosed CML-CP were evaluated for outcomes after 24 months of treatment with nilotinib. The study reported an MMR of 95.5% with nilotinib as a first-line treatment option for patients with CML-CP [25]. Another European real-world study from Italy affirmed the safety and efficacy of second-generation TKIs, including nilotinib, for a treatment duration of up to 50 months [26]. In a real-world study from Taiwan, the median time to achieve CCyR and MMR was 5 months and 9 months, respectively, which is comparable with our findings [27]. The study also demonstrated that nilotinib was well tolerated and effective in patients resistant or intolerant to imatinib in CP as well as AP of CML, with a reported MMR of 56.8%, similar to the 61.5% observed in our study on this subset [28].
There are some limitations in our study. AEs that might have occurred before the study enrollment when patients were on nilotinib treatment were not retrospectively captured at baseline. As the follow-up duration was restricted to only 3 years, potential long?term AEs may not have been recorded. Further, quality of life and patient satisfaction information were not recorded. Since this was a purely non-interventional, observational study, there was no control group, and data were collected retrospectively from the patient medical records, and prospectively during the study visits, and thus, patient selection bias and missing data might have occurred. Nevertheless, every effort was made to minimize the inherent bias in patient selection. In addition, the investigators’ decision to administer nilotinib to a patient was based on the current medical practice and preceded the consideration of the patient’s eligibility for enrollment into the study. Furthermore, the potential influence of confounding factors on the outcomes of this study was accounted for in the statistical analyses, using robust multivariable analyses. The study took place in normal clinical settings under real-life conditions and thus is more representative of the study population of interest and the clinical outcomes under observation.
This study contributes important real-world evidence on therapeutic patterns, effectiveness, and safety of nilotinib. With higher patient-years of treatment?free remission reported with nilotinib, it may be the treatment of choice in CML for limiting the toxicity associated with long-term use of TKIs and improving cost?effectiveness [29].
Conclusion
This study from Greece provides a reference for the use of nilotinib in the long-term management of patients with BCR/ABL+ CML, either newly diagnosed or resistant/intolerant to previous therapies. The safety profile of nilotinib was comparable with that reported in controlled clinical trials and that of other TKIs in use. In the present study, high rates of responses were achieved from the first 12 months of treatment, and long-term durability of responses was observed at 3 years in BCR/ABL+ CML patients treated with nilotinib. This real-world evidence supports the effectiveness and safety of nilotinib and provides a therapeutic pattern of nilotinib use in Greece.
Conflicts of Interest
AS reports receiving grants through institution from AbbVie, Amgen, Astellas, BMS, Demo, Enorasis, Genesis, Gilead, Glaxo, Integris, Janssen, MSD, Novartis, Pfizer, Rafarm, Roche, Sanofi, Takeda, Vianex, Win Medica; honoraria from AbbVie, Amgen, BMS, Genesis, Gilead, Glaxo, Janssen, Novartis, Pfizer, Roche, Sanofi, Win Medica; Support for attending meetings and/or travel grants from AbbVie, BMS, Genesis, Gilead, Janssen, Roche, Sanofi; Participation on a Data Safety Monitoring Board or Advisory Board from AbbVie, Amgen, BMS, Genesis, Gilead, Janssen, Novartis, Pfizer, Roche, Sanofi, Takeda.
AA reports participation in clinical trials sponsored by AbbVie, Amgen, Astellas, Celgene, GSK, Incyte, Janssen, Novartis, Oncopeptides, Roche, Sanofi, Takeda; support for attending meetings and/or travel from Novartis.
GK reports honoraria, travel support for attending meetings, and participation on a Data Safety Monitoring Board or Advisory Board from Novartis.
NAV reports grants/contracts from AbbVie, BMS, Takeda and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from Astellas, BMS, Janssen, Novartis, Sandoz, Takeda.
TM was in advisory board for Novartis, AbbVie and received speaker honorarium from Sanofi.
VP reports grants from AbbVie, Celgene, Janssen; honoraria from AbbVie, Amgen, Genesis Pharma, Gilead, Janssen, Novartis; travel support from AbbVie, Amgen, Genesis, Gilead, Janssen, Novartis, Roche; and is a member of the Administrative Board of the Hellenic Society of Hematology.
MT is a full-time employee of Novartis.
DK, DM, EK, GV, MD, MX have no conflicts of interest to declare outside the published work.
Authors' Contributions
All authors apart from MT have worked as the investigators in this study; they have treated their patients and provided patients’ information. All authors have read, commented, and approved the final manuscript for submission. All authors have participated in reviewing and revising the manuscript.
Acknowledgments
This research was sponsored by Novartis (Hellas) S.A.C.I. The authors thank all the patients and physicians who participated in the study. The authors acknowledge Kavita Garg, PhD CMPP™, of Novartis Healthcare Pvt Ltd for providing medical writing assistance with this manuscript.
References
2. Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005 Mar;5(3):172-83.
3. Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001 Apr 5;344(14):1084-6.
4. Druker BJ. Inhibition of the Bcr-Abl tyrosine kinase as a therapeutic strategy for CML. Oncogene. 2002 Dec 9;21(56):8541-6.
5. García-Gutiérrez V, Hernández-Boluda JC. Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol. 2019;9:603.
6. Massimino M, Stella S, Tirrò E, Pennisi MS, Vitale SR, Puma A, et al. ABL1-Directed inhibitors for CML: Efficacy, resistance and future perspectives. Anticancer Res. 2020 May;40(5):2457-65.
7. Innes AJ, Milojkovic D, Apperley JF. Allogeneic transplantation for CML in the TKI era: striking the right balance. Nat Rev Clin Oncol. 2016 Feb;13(2):79-91.
8. Kujawski LA, Talpaz M. The role of interferon-alpha in the treatment of chronic myeloid leukemia. Cytokine Growth Factor Rev. 2007 Oct-Dec;18(5-6):459-71.
9. Gandhi V, Plunkett W, Cortes JE. Omacetaxine: a protein translation inhibitor for treatment of chronic myelogenous leukemia. Clin Cancer Res. 2014 Apr 1;20(7):1735-40.
10. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017 Mar 9;376(10):917-27.
11. Hochhaus A, O'Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009 Jun;23(6):1054-61.
12. Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016 Aug 20;34(24):2851-7.
13. Silver RT, Talpaz M, Sawyers CL, Druker BJ, Hochhaus A, Schiffer CA, et al. Four years of follow-up of 1027 patients with late chronic phase (L-CP), accelerated phase (AP), or blast crisis (BC) chronic myeloid leukemia (CML) treated with imatinib in three large Phase II trials. Blood. 2004;104(11):23-.
14. le Coutre PD, Giles FJ, Hochhaus A, Apperley JF, Ossenkoppele GJ, Blakesley R, et al. Nilotinib in patients with Ph+ chronic myeloid leukemia in accelerated phase following imatinib resistance or intolerance: 24-month follow-up results. Leukemia. 2012 Jun;26(6):1189-94.
15. Hochhaus A, Ernst T, Eigendorff E, La Rosée P. Causes of resistance and treatment choices of second- and third-line treatment in chronic myelogenous leukemia patients. Ann Hematol. 2015 Apr;94 Suppl 2:S133-40.
16. Giles FJ, Kantarjian HM, le Coutre PD, Baccarani M, Mahon FX, Blakesley RE, et al. Nilotinib is effective in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blastic phase. Leukemia. 2012 May;26(5):959-62.
17. Giles FJ, le Coutre PD, Pinilla-Ibarz J, Larson RA, Gattermann N, Ottmann OG, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013 Jan;27(1):107-12.
18. Hughes TP, Lipton JH, Spector N, Cervantes F, Pasquini R, Clementino NC, et al. Deep molecular responses achieved in patients with CML-CP who are switched to nilotinib after long-term imatinib. Blood. 2014 Jul 31;124(5):729-36.
19. Tokuhira M, Kimura Y, Sugimoto K, Nakazato T, Ishikawa M, Fujioka I, et al. Efficacy and safety of nilotinib therapy in patients with newly diagnosed chronic myeloid leukemia in the chronic phase. Med Oncol. 2018 Feb 13;35(3):38.
20. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010 Jun 17;362(24):2251-9.
21. Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011 Sep;12(9):841-51.
22. Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012 Oct;26(10):2197-203.
23. Suh KJ, Lee JY, Shin DY, Koh Y, Bang SM, Yoon SS, et al. Analysis of adverse events associated with dasatinib and nilotinib treatments in chronic-phase chronic myeloid leukemia patients outside clinical trials. Int J Hematol. 2017 Aug;106(2):229-39.
24. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016 May;30(5):1044-54.
25. Huguet F, Cayuela JM, Cambier N, Carpentier N, Tindel M, Violet I, et al. Nilotinib efficacy, safety, adherence and impact on quality of life in newly diagnosed patients with chronic myeloid leukaemia in chronic phase: a prospective observational study in daily clinical practice. Br J Haematol. 2019 Dec;187(5):615-26.
26. Fava C, Rege-Cambrin G, Dogliotti I, Cerrano M, Berchialla P, Dragani M, et al. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019 Aug;104(8):1589-96.
27. Wen-Li H, Han S-M, Lin S-Y, Chang M-C, Bai L-Y, Hsiao P-C, et al. NOVEL-1st: Interim analysis of a non-interventional, multi-center observational study to assess the safety and efficacy of nilotinib in newly diagnosed patients with Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia in chronic phase (CML-CP) in Taiwan. Blood. 2018;132(Supplement 1):3019-.
28. Kuo CY, Wang PN, Hwang WL, Tzeng CH, Bai LY, Tang JL, et al. Safety and efficacy of nilotinib in routine clinical practice in patients with chronic myeloid leukemia in chronic or accelerated phase with resistance or intolerance to imatinib: results from the NOVEL study. Ther Adv Hematol. 2018 Mar;9(3):65-78.
29. Hochhaus A, Masszi T, Giles FJ, Radich JP, Ross DM, Gómez Casares MT, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017 Jul;31(7):1525-31.
