Abstract
The major issue in kidney transplantation remains the suppression of allograft rejection. Immunosuppressant decrease both donor-specific responsiveness and the risk of rejection in the months after transplantation and are maintained. The current immunosuppressant medications, although potent against short-time graft complications including acute renal rejection but are still less effective to ensure long-term graft survival. Also, immunosuppressive medications are associated with cumulative side effects, including increased risks of heart disease, infection, cancer, and diabetes. The important element is that the response of the host T cells becomes less to donor antigens when antigen persists, and immunosuppression is maintained. The antigen persistence with inadequate co-stimulation triggers adaptations that limit T-cell responsiveness. Therefore, the key to successful allograft function is the development of an immunosuppressive medication that suppresses the target cells, which in turn suppresses the cumulative side effects associated with currently available immunosuppressants.
Regulatory T cells may also be able to control allo immune responses, by analogy with their ability to suppress autoimmunity. The main goal of immunosuppression is to induce tolerance by preventing allograft loss without the consequences of infection or toxicity and, most importantly, to achieve a long-term graft result. There are several studies explaining the role of regulatory cells in the prognosis, diagnosis, and treatment of kidney transplant recipients. While several types of regulatory lymphocyte populations have been described, CD4 T cells expressing the Foxp3 transcription factor (Foxp3+ Tregs) are the best understood. Foxp3+ Tregs constitute 5 to 10% of peripheral CD4+ T cells in both mice and humans and are critical for maintaining immune homeostasis. Evidence exists that Tregs expand after peri transplantation lymphopenia, inhibit graft rejection, and induce and maintain tolerance. Reducing the dosage of current immunosuppressants or combination therapy with the Tregs may eliminate the long term side effects of immunosupressants.
Keywords
Kidney transplantation, Allograft function, Allograft rejection, Immunosuppression, Regulatory T cells
Abbreviations
Tregs: Regulatory T cells; CTLA-4: Cytotoxic T Lymphocyte Associated protein 4; LAG 3: Lymphocyte Activation Gene 3; AMP: Adenosine Monophosphate; TGF-β: Transforming Growth Factor beta; IL-10: Interleukin 10; Bcl-2: B cell lymphoma 2; GZB: Granzyme; PRF: Perforin; ROC: Receiver Operating Curve; AUC: Area Under the Curve
Introduction
The central issue in Kidney transplantation is the suppression of alloimmune response. Thus, development of immune suppression drug is the key to successful allograft function [1]. Although short-term graft and patient survival rates post-transplantation have improved dramatically over recent times, this has not been seen by similarly improved 10-year and long-term outcomes [2]. These drugs are nonspecific, require lifelong use, favor the development of opportunistic infections and tumors, directly damage transplanted organs, and significantly increase cardiovascular morbidity and mortality [3,4]. All these complications may necessitate reduction or even withdrawal of immune suppression that leads to graft rejection and graft loss. Therefore, the key to improving immunosuppression after transplantation is to selectively block the immune responses against the graft without impeding other protective immune functions or causing non-specific toxicities [5]. The CD4+ CD25high FoxP3+ T regulatory cells (Tregs) are immunodominant suppressors in the immune system. To control immune responses, Tregs act by various mechanisms. Preclinical data from animal models have confirmed the huge therapeutic potential of Tregs in many immune-mediated diseases [6].
Biology and Mechanism of Action of Tregs
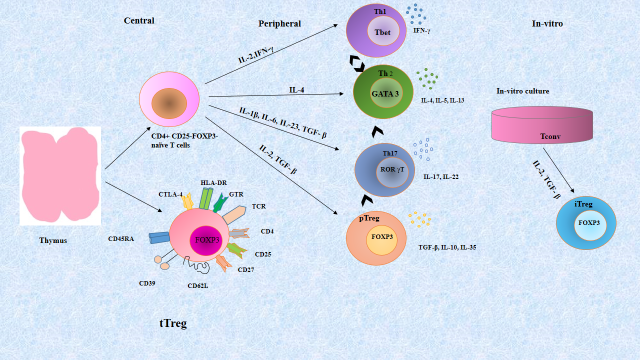
Regulatory T cells [CD4 (+) CD25 (+) Foxp3 (+)] are the subset of T cells, are essential to the balance between pro and anti-inflammatory responses at mucosal surfaces. There are two subsets of Treg cells, “natural” Treg (nTreg) cells and “induced” Treg (iTreg) cells. A distinct lineage of nTreg cells develops in the thymus, whereas iTreg cells are derived from peripheral naive conventional T (Tconv) cells (Figure 1). Developing Tregs are subject to both positive and negative selection processes during thymic ontogeny, which together creates a repertoire that is self–major histocompatibility complex (MHC) restricted (positive selection) but eliminates many auto-reactive T cells (negative selection) [7]. According to current data, developing thymocyte with modest affinity for self-peptide plus MHC are induced to express Foxp3 and differentiate into Tregs [8]. It is believed that these thymically derived Tregs, called tTregs (formerly nTregs), and are important for suppressing the response of auto reactive T cells that escape negative selection. They may suppress antigen-independently once activated. Peripheral T cells (nave non-Tregs) can be induced to become pTregs, another population of Tregs. The inducible Tregs population is created by stimulating T cells with high levels of TGF- β in vitro [9]. There are other factors that support Tregs induction, such as vitamin D, retinoic acid, vitamin C, and inhibition of Phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI-3 kinase) [10,11].
Figure 1. Regulatory T cell populations. In the thymus, CD4+ T cells and natural Tregs are selected. In the periphery, naive CD4+ T cells can differentiate into several distinct T cell subsets, Th1, Th2, Th17, and induced Tregs. These differentiation programs are controlled by different cytokines and each separate CD4+ T cell subset can be identified from their lineage-specific transcription factors responsible for the regulation and maintenance of their individual functions. Each subset has its own immunological role in vivo. Even though all of these cells appear to have terminal differentiation, they cannot be considered committed to one cell fate. Lineage plasticity following differentiation is depicted by the arrows between the cells. In vitro generation of Tregs in the presence of IL-2 and TGF-β polarizing conditions leads to the development of iTregs.
Abbreviations: APC: Antigen Presenting Cells; CD: Cluster of Differentiation; CTLA-4: Cytotoxic T-Lymphocyte-Associated protein 4; FOXP3: Forkhead Box P3; IFN: Interferon; IL: Interleukin; IRF: Interferon Regulatory Factor; iTreg: induced Treg; nTreg: natural Treg; pTreg: peripheral Treg; RORγt: Retinoid related Orphan Receptor γ; T-bet: T box transcription factor; TCR: T cell Receptor; TGF-β: Transforming Growth Factor-β; Th: T helper cell; Treg: regulatory T cell: Tconv: naïve conventional T cells
Tregs mainly act through four major mechanisms to induce immune suppression. Inhibition of Tregs activation requires cytokine production, including TGF-β, Interleukin-35 and interleukin-10. Another mechanism is found in lineages Type 1 Tregs that secrete Granzyme B (GZB) and perforin (PRF) which act specifically in myeloid precursors. As a result of GZB's cytolytic activity, target cells undergo apoptosis through the caspase pathway [12]. Through gap junctions, adenosine monophosphate (AMP) inhibits the proliferation and differentiation of target cells. The Bcl-2 pathway induces apoptosis in target cells by competing for growth factors, in particular IL-2 and CD25 expressed by Tregs. Another mechanism is the contact dependent, mediated by the surface markers cytotoxic T lymphocyte associated protein 4 (CTLA 4), and lymphocyte activation gene 3 (LAG 3) which interact with molecules CD80/86 and MHC II on the target cells respectively to regulate the cell function (Figure 2).
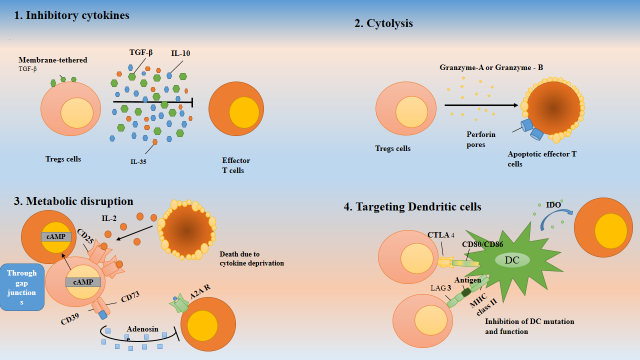
Figure 2. Mechanism of action of regulatory T cells: Tregs cells can utilize four major mechanisms to induce immunosuppression. 1. The secretion of inhibitory cytokines, 2. Cytolysis by secretig Granzyme A or Granzyme B, 3. The metabolic disruption of effector T cells, 4. By targeting antigen presenting cells (Dendritic cells).
Reduced function of pTregs correlated with decreased expression of CTLA-4, interleukin-10, and transforming growth factor-β, and mediates the loss of immune tolerance and promotes allograft rejection [32]. The expressions of co-inhibitory receptors (CTLA-4, PD-1, Tim-3, LAG-3 and TIGIT) on Tregs and the levels of the relative cytokines (TGF-β and IL-10) were significantly higher in tacrolimus+MMF therapy patients than that in tacrolimus monotherapy and control group [33].
Prognostic and Diagnostic Role of Tregs in Renal Transplantation
Expressed transcription factor by regulatory T (Treg) cells FoxP3, also known as forkhead box protein P3, is crucial for regulating host-commensal flora interaction, promoting tissue healing, and establishing immune tolerance to self-tissues. Several studies have shown the reduction in the frequency of Tregs in peripheral blood samples in patients during the early post-transplantation period [13-15]. The use of immunosuppressive drugs could be the possible cause of depletion of Tregs in the early period of post-transplantation [16]. According to Vondran et al., following Basiliximab administration, a distinct loss of Tregs cells was observed, lasting for at least six weeks [17]. Other studies have shown that the decrease of Tregs during chronic graft failure as compared to those with stable graft function when monitored up to 12 months post-transplantation [18,19]. Inomata et al. reported that renal transplant recipients with low Tregs have a higher risk of acute graft rejection [20]. However few studies have shown either no change or increase in Tregs in patients with graft failure [21,22]. In addition, some studies have observed that the measurement of Tregs can predict the clinical outcome of the patients, whereas the other studies showed contrary results as measured by multivariate analysis [23]. The size of the Treg population and regulatory FOXP3 gene expression in the early period after transplantation are associated with kidney transplant outcome [31]. A recent study by Katyayani et al. showed that peripheral Tregs% was decreased in early post-transplant period and during active graft rejection. Tregs% was stabilizing at 6 months of post-transplant with normal graft function. The ROC analysis of Tregs% with the risk of graft rejection showed that AUC was 0.80 [24].
Cell Based Therapies Involving Tregs in Renal Transplantation
Natural immune regulatory mechanisms using cell based therapies is the goal in solid organ transplantation. Various types of T cells have been shown to contribute to transplant tolerance (Tregs, IL-10 producing Tr1, CD8+ 28- T cells, and anergic T cells) [5]. With the rapidly growing knowledge on regulatory T cells as mediators of immune homeostasis, there is now increasing confidence that Tregs can serve as a rational target for a new generation of immune – modulatory therapy. The generation of autologous, highly pure nTregs products is feasible and qualifies patients awaiting or having received allogenic kidney transplantation for nTregs therapy [25].
Ongoing Clinical Trials Involving Tregs Adoptive Immunotherapy in Kidney Transplantation
Several clinical trials were published utilizing Tregs immunoadoptive therapy in solid organ transplantation. Out of them, few were involved with liver transplantation, and others involved differing approaches to Tregs therapy in renal transplantation [26]. Chandran et al. tested the safety and feasibility of autologous polyclonal Tregs therapy in patients with sub-clinical inflammation on 6-month surveillance biopsies. Three renal transplant recipients with 6-month biopsies demonstrating 5–25% inflammation and no evidence of rejection received 320 × 106 autologous CD4+CD127loCD25+ polyclonal Tregs isolated and expanded. Infusion was well-tolerated and infused Tregs were detectable in the peripheral blood for 3 months post-infusion [27]. Mathew et al. reported a phase I trial in which nine alemtuzumab-induced living-donor renal transplant recipients received 0.5, 1, or 5 × 109 autologous polyclonal Tregs at day 60 post-transplant and expanded [28]. Tregs infusion was safe. Sirolimus and mycophenolate were maintained as conventional immunosuppressants. In 2 years of follow-up, one patient developed sub-clinical rejection, and two patients developed de novo donor specific antibodies (DSA), which describe the presence of antibodies specific to donor’s HLA-Molecules. The ONE study demonstrated that regulatory cell therapy is achievable and safe in living-donor kidney transplant recipients, and is associated with fewer infectious complications, but similar rejection rates in the first year. Therefore, immune cell therapy is a potentially useful therapeutic approach in recipients of kidney transplant to minimize the burden of general immunosuppression [29]. Tang et al. reported that the primary efficacy endpoint was calcineurin inhibitor dose reduction by 75% with stable liver function tests for at least 12 weeks. Among 10 individuals who initiated immunosuppression withdrawal, 1 experienced rejection before planned donor alloantigen-reactive Treg (darTreg) infusion, 5 received darTregs, and 4 were not infused because of failure to manufacture the minimal infusible dose of 100 × 106 cells. darTreg infusion was not associated with adverse events. Two darTreg-infused participants reached the primary endpoint, but an insufficient number of recipients were treated for assessing the efficacy of darTregs [30].
Future Perspectives
Despite the better understanding and exponential growth in the number of studies on Tregs, the biology of these cells is still complex. The characterization of these cells provide a good benefit for future studies such as phenotypic modifications from CD4+CD25+ T cells to the CD4+ T cells with the highest expression of CD25+ receptors, the discovery of the plasticity of T cells with possible links between Tregs and Th17 cells, and the findings around the expression of foxp3 gene and its epigenetic changes in Tregs must be taken into account when manufacturing medicinal Tregs. The transfer of Tregs therapy from a scientific model to the clinic requires cooperation between scientists, clinicians, and regulatory authorities for efficacious and safe therapy. Funding support to the scientific studies including the registration process must be increased. Academia should also change the attitude towards more flexible thinking around the studies with cellular therapies [6].
Conclusion
The focus of this commentary are recent studies that advance our understanding of the role of Tregs in kidney transplantation. Cells characterized by the CD4+ CD25high FoxP3+ T regulatory cells (Tregs) phenotype are responsible for maintaining immune tolerance and suppression of immune responses. And, during graft rejection in kidney transplantation due to imbalanced immune homeostasis, Tregs% decreases. Tregs can selectively block the immune responses against graft without impeding other protective immune functions or causing non-specific toxicities. Hence, Tregs can be used to predict the graft outcome and to diagnose graft rejection. As a part of cell based therapy, Tregs can be used as adjuvant therapy with the standard immune suppressants to reduce the toxic effects of long term use of immune suppressants.
References
2. Meier‐Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. American Journal of Transplantation. 2004 Mar;4(3):378-83.
3. Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Critical Reviews in Oncology/Hematology. 2005 Oct 1;56(1):23-46.
4. Marcén R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009 Nov;69:2227-43.
5. Tang Q, Bluestone JA. Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harbor Perspectives in Medicine. 2013 Nov;3(11):a015552.
6. Gliwiński M, Iwaszkiewicz-Grześ D, Trzonkowski P. Cell-based therapies with T regulatory cells. BioDrugs. 2017 Aug;31:335-47.
7. Alessandrini A, Turka LA. FOXP3-positive regulatory T cells and kidney allograft tolerance. American Journal of Kidney Diseases. 2017 May 1;69(5):667-74.
8. Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005 Jun 2;435(7042):598-604.
9. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. The Journal of Experimental Medicine. 2003 Dec 15;198(12):1875-86.
10. Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, et al. Control of PI (3) kinase in Treg cells maintains homeostasis and lineage stability. Nature Immunology. 2015 Feb;16(2):188-96.
11. Yue X, Trifari S, Äijö T, Tsagaratou A, Pastor WA, Zepeda-Martínez JA, et al. Control of Foxp3 stability through modulation of TET activity. Journal of Experimental Medicine. 2016 Mar 7;213(3):377-97.
12. Pereira LM, Gomes ST, Ishak R, Vallinoto AC. Regulatory T cell and forkhead box protein 3 as modulators of immune homeostasis. Frontiers in Immunology. 2017 May 26;8:605.
13. Alwahaibi NY, Alissaei HK, Alshihi SA, Alabri N, Albalushi SS, Albalooshi M. Serum levels of TNF-α, IL-6 and IL-10 in haemodialysis and renal transplant patients and in healthy subjects. Port J Nephrol Hypert. 2016 Sep;30(3):194-8.
14. Hoerning A, Köhler S, Jun C, Tebbe B, Fu J, Menke J, et al. Peripherally circulating CD4+ FOXP3+ CXCR3+ T regulatory cells correlate with renal allograft function. Scandinavian Journal of Immunology. 2012 Sep;76(3):320-8.
15. Hu M, Rogers NM, Li J, Zhang GY, Wang YM, Shaw K, et al. Antigen specific regulatory T cells in kidney transplantation and other tolerance settings. Frontiers in Immunology. 2021 Aug 26;12:717594.
16. Presser D, Sester U, Mohrbach J, Janssen M, Köhler H, Sester M. Differential kinetics of effector and regulatory T cells in patients on calcineurin inhibitor–based drug regimens. Kidney International. 2009 Sep 1;76(5):557-66.
17. Vondran FW, Timrott K, Tross J, Kollrich S, Schwarz A, Lehner F, et al. Impact of Basiliximab on regulatory T-cells early after kidney transplantation: down-regulation of CD25 by receptor modulation. Transplant International. 2010 May;23(5):514-23.
18. Louis S, Braudeau C, Giral M, Dupont A, Moizant F, Robillard N, et al. Contrasting CD25hiCD4+ T cells/FOXP3 patterns in chronic rejection and operational drug-free tolerance. Transplantation. 2006 Feb 15;81(3):398-407.
19. Segundo DS, Fernández‐Fresnedo G, Ruiz JC, Rodrigo E, Benito MJ, Arias M, et al. Two‐year follow‐up of a prospective study of circulating regulatory T cells in renal transplant patients. Clinical Transplantation. 2010 May;24(3):386-93.
20. Inomata T, Hua J, Di Zazzo A, Dana R. Impaired function of peripherally induced regulatory T cells in hosts at high risk of graft rejection. Scientific Reports. 2016 Dec 23;6(1):39924.
21. Game DS, Hernandez-Fuentes MP, Chaudhry AN, Lechler RI. CD4+ CD25+ regulatory T cells do not significantly contribute to direct pathway hyporesponsiveness in stable renal transplant patients. Journal of the American Society of Nephrology. 2003 Jun 1;14(6):1652-61.
22. Braudeau C, Racape M, Giral M, Louis S, Moreau A, Berthelot L, et al. Variation in numbers of CD4+CD25highFOXP3+ T cells with normal immuno-regulatory properties in long-term graft outcome. Transpl Int. 2007 Oct;20(10):845-55.
23. Chung BH, Oh HJ, Piao SG, Hwang HS, Sun IO, Choi SR, et al. Clinical significance of the ratio between FOXP3 positive regulatory T cell and interleukin‐17 secreting cell in renal allograft biopsies with acute T‐cell‐mediated rejection. Immunology. 2012 Jul;136(3):344-51.
24. Bejugama K, Taduri G, Guditi S. Effect of regulatory T cells on short-term graft outcome in kidney transplant recipients, a prospective observational, single-center study. Transplant Immunology. 2022 Aug 1;73:101630.
25. Landwehr-Kenzel S, Zobel A, Hoffmann H, Landwehr N, Schmueck-Henneresse M, Schachtner T, et al. Ex vivo expanded natural regulatory T cells from patients with end-stage renal disease or kidney transplantation are useful for autologous cell therapy. Kidney International. 2018 Jun 1;93(6):1452-64.
26. El-Ayachi I, Washburn WK, Schenk AD. Recent progress in T reg biology and transplant therapeutics. Current Transplantation Reports. 2020 Jun;7:131-9.
27. Chandran S, Tang Q, Sarwal M, Laszik ZG, Putnam AL, Lee K, et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. American Journal of Transplantation. 2017 Nov 1;17(11):2945-54.
28. Mathew JM, H.-Voss J, LeFever A, Konieczna I, Stratton C, He J, et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Scientific Reports. 2018 May 9;8(1):7428.
29. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. The Lancet. 2020 May 23;395(10237):1627-39.
30. Tang Q, Leung J, Peng Y, Sanchez-Fueyo A, Lozano JJ, Lam A, et al. Selective decrease of donor-reactive Tregs after liver transplantation limits Treg therapy for promoting allograft tolerance in humans. Science Translational Medicine. 2022 Nov 2;14(669):eabo2628.
31. Krajewska M, Kościelska-Kasprzak K, Kamińska D, Żabińska M, Myszka-Kozłowska M, Gomułkiewicz A, et al. Kidney transplant outcome is associated with regulatory T cell population and gene expression early after transplantation. Journal of immunology research. 2019 Jan 8;2019:7452019.
32. Inomata T, Hua J, Di Zazzo A, Dana R. Impaired function of peripherally induced regulatory T cells in hosts at high risk of graft rejection. Scientific Reports. 2016 Dec 23;6(1):39924.
33. Zeng Q, Yuan XY, Li W, Liu BW, Zhao X, Ren GJ, et al. Effects of tacrolimus (FK506) and mycophenolate mofetil (MMF) on regulatory T cells and co-inhibitory receptors in the peripheral blood of human liver allograft patients. Immunopharmacology and Immunotoxicology. 2019 May 4;41(3):380-5.