Abstract
MicroRNAs (miRNAs) play important roles in gene regulation and have been implicated in various human diseases, including cancer. MiRNAs can be packaged in exosomes and transferred between cells. These exosomal miRNAs regulate intercellular communication and influence almost all aspects of cancer biology, including proliferation, apoptosis, invasion, metastasis, and angiogenesis. Over the last two decades, the association between exosomal miRNAs and paclitaxel resistance has been widely studied. However, the mechanisms underlying the effect of exosomal miRNAs on paclitaxel sensitivity require further research. In this review, we summarize the paclitaxel sensitivity-modulating mechanisms of exosomal miRNAs and discuss exosomal miRNAs as a novel therapeutic tool for paclitaxel resistance.
Keywords
MicroRNA, Paclitaxel, Exosome, Cancer, Drug-resistance
Abbreviations
miRNA: MicroRNA; CAA: Cancer-Associated Adipocyte; CAF: Cancer-Associated Fibroblast; BC: Breast Cancer; OC: Ovarian Cancer; GI: Gastrointestinal; G3BP2: GTPase-activated SH3 domain-Binding Protein 2; EV: Extracellular Vehicle; HCC: Hepatocellular Carcinoma; EMT: Epithelial-Mesenchymal Transition; NPC: Nasopharyngeal Carcinoma
Introduction
MicroRNAs (miRNAs) are a class of single-stranded RNAs that participate in the post-transcriptional regulation of gene expression. They are powerful regulators of various cellular activities, including cell growth, differentiation, development, and apoptosis [1], thereby influencing the occurrence, development, invasion, and migration of different types of cancer [2,3]. Exosomes are extracellular vesicles that contain not only proteins but also nucleic acids such as DNA, mRNA, miRNA, and non-coding RNA, which play roles in angiogenesis, exocytosis, and tumorigenesis by modulating gene expression and protein translation [4-8]. Exosomes perform their roles in cellular communication by releasing bioactive molecules, fusing with recipient cell membranes, or interacting with cell surface receptors. As integral components of the tumor microenvironment, exosomes participate in cell proliferation, angiogenesis, metastasis, immune regulation, drug resistance, and the formation of a pre-metastatic environment [9-12]. Previous studies have shown that exosomes produced in the tumor microenvironment in response to chemotherapy promote a chemotherapy-resistant phenotype in tumors [13]. Evidence suggests that miRNAs can be encapsulated in exosomes and transferred between cells via a microencapsulation-dependent mechanism [14,15], indicating their potential to influence chemoresistance by regulating intercellular communication. Paclitaxel, a compound derived from the bark of the western yew (Taxus brevifolia), is commonly used to treat various cancers. However, frequent cancer cell resistance significantly decreases its anti-cancer efficacy [16]. A recent study suggests that miRNAs transferred by paclitaxel-sensitive cell-derived exosomes may affect paclitaxel resistance [17]. Nevertheless, studies on exosomal miRNAs in the context of paclitaxel treatment are limited. Moreover, the mechanisms underlying the effects of exosome-derived miRNAs on paclitaxel sensitivity require further investigation. Therefore, it is of considerable scientific significance to analyze and summarize research on exosomal miRNAs to provide a preliminary basis for subsequent research and to create a new mechanistic therapeutic approach to overcome chemoresistance in paclitaxel treatment. In this paper, we discuss the paclitaxel sensitivity-modulating mechanisms of exosome-derived miRNAs, summarize their regulatory roles in various cancer species, and evaluate the application of exosome-derived miRNAs as a novel therapeutic method for inhibiting paclitaxel resistance.
Mechanisms
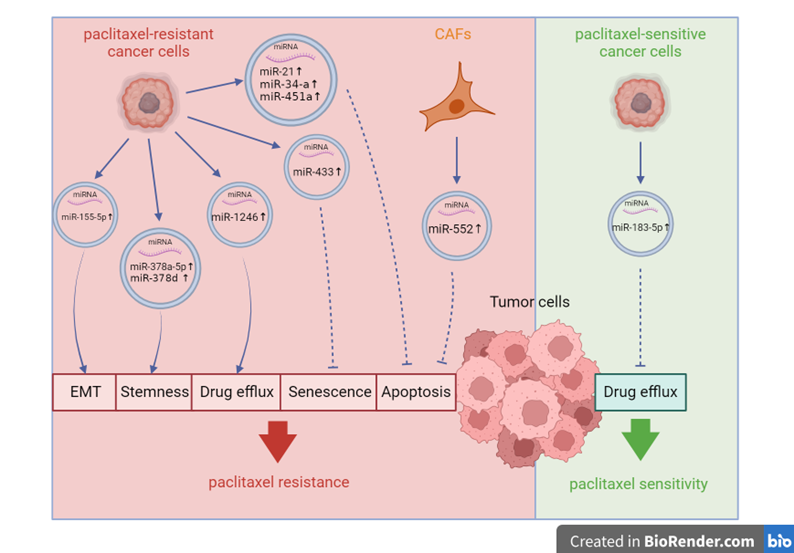
In vitro and in vivo studies have indicated that alterations in microtubule-protein interactions [18], the expression and activity of multidrug efflux transporters of the ABC superfamily, including P-glycoprotein (P-gp/ABCB1) [19], and changes in the expression of PKC, bcl-2, p53, and ErbB2 in the apoptotic pathway [20,21], are the primary mechanisms contributing to paclitaxel resistance. Researchers have found that exosome-derived miRNAs can bidirectionally regulate paclitaxel sensitivity by intervening in the aforementioned pathways, contingent upon whether exosome-secreting cells are sensitive or resistant to paclitaxel [17,22]. Exosomes containing miRNAs can be derived from paclitaxel-resistant cancer cells. After internalization by paclitaxel-sensitive cancer cells, these miRNAs activate paclitaxel resistance-related pathways, including stem cell pathways (WNT and NOTCH) [23] and inhibit paclitaxel sensitivity-related pathways, including cellular apoptosis (targeting BCL-2) [24] and cellular senescence (targeting CDK6) [25]. A recent study revealed that paclitaxel-sensitive cancer cells can transfer exosomal miRNAs to paclitaxel-resistant cancer cells, mediating the reversal of paclitaxel resistance by negatively regulating the drug transporter P-gp [17]. This may provide new targets for reversing the resistance to paclitaxel therapy. However, studies on exosomal miRNAs that reverse paclitaxel resistance in paclitaxel-sensitive cancer cells are limited. In addition to cancer cells, the tumor microenvironment comprises other cells, such as cancer-associated adipocytes (CAAs) and fibroblasts (CAFs); however, whether exosomal miRNAs derived from these cells play a role in paclitaxel resistance requires further exploration. Given that CAFs are the most abundant and critically involved cell type in cancer progression, their role in paclitaxel resistance requires further discussion [26]. Growing evidence suggests that CAFs support paclitaxel resistance by secreting various bioactive substances, including exosomes. Exosomal miRNAs derived from CAFs suppress cancer apoptosis [27] and ferroptosis [28]. The recent discovery of CAFs that promote paclitaxel resistance via exosomal miRNAs not only enhances our understanding of the tumor microenvironment’s modulatory role in paclitaxel sensitivity but also underscores the potential of CAF-targeting therapies for addressing paclitaxel resistance. Figure 1 summarizes the mechanisms through which exosomal miRNAs influence paclitaxel sensitivity in tumor cells.
Figure 1. Mechanisms by which exosomal microRNAs influence paclitaxel sensitivity of tumor cells.
Exosome-derived MicroRNAs and Response to Paclitaxel in Various Cancer Types
Paclitaxel is one of the most effective chemotherapeutic drugs, demonstrating significant efficacy across a broad range of cancers, including breast, ovarian, gastrointestinal, and prostate cancers [29-32]. In this section, we summarize the regulatory functions of exosomal miRNAs in paclitaxel sensitivity, classified by cancer type. Table 1 provides a summary of the results of studies that have assessed the role of exosome-derived miRNAs in modulating the response of various cancer types to paclitaxel.
|
Cancer |
miRNA & Expression Pattern |
Assessed cell line |
Animal |
Human |
Derived cells |
Signaling pathways |
Outcome |
Reference |
|
gastrointestinal cancers (GC) |
miR-522(Up) |
SGC7901, MGC803, MKN45 |
6–8 weeks male BALB/c-nu nude mice |
45 pairs tumor tissue samples and plasma samples of patients with GC |
cancer-associated fibroblasts (CAFs) |
USP7/hnRNPA1 axis |
Activating of USP7/hnRNPA1 pathway could lead to ALOX15 suppression and decreased lipid-ROS accumulation in tumor cells, eventually inhibiting ferroptosis, thus inducing resistance to paclitaxel in GC cells. |
[28] |
|
GC |
miR?155?5p(Up) |
MGC-803 |
—— |
—— |
paclitaxel-resistant gastric cancer cells |
miR-155-5p/GATA3 and miR-155-5p/TP53INP1 axis |
Suppressing of GATA3 and TP53INP1 could lead to the active of epithelial-mesenchymal transition (EMT) in tumor cells, thus inducing resistance to paclitaxel in GC cells. |
[51] |
|
breast cancer (BC) |
miR-378a-3p;miR-378d(Up) |
CAL51, MDA-MB-231, MCF-7 |
NSG mice |
45 pairs tumor tissue samples from patients who had received 4–8 neoadjuvant chemotherapy cycles |
breast cancer cells |
WNT/β-catenin and Notch pathways |
The activation of the WNT and NOTCH stem cell pathways via the targeting of DKK3 and NUMB could promote the stemness effects of cancer cells, eventually inducing paclitaxel resistance of BC cells. |
[23] |
|
ovarian |
miR-1246(Up) |
HeyA8, SKOV3-ip1, A2780, HeyA8-MDR, SKOV3-TR, A2780-CP20,THP-1,HIO180 |
6 weeks female athymic nude mice |
OC tissue samples (n?=?15) and normal ovarian surface epithelium specimens (n?=?7) |
ovarian cancer cells |
Cav1/p-gp/M2-type macrophage axis |
Overexpression of Cav1 and p-gp, significantly increased P-gp-mediated drug efflux in cancer cells, eventually inducing paclitaxel resistance of OC cells. |
[40] |
|
OC |
miR-21(Up) |
A2780, ALST, OVCA432, OVCA433, OVCAR5, HeyA8, HeyA8-MDR, SKOV3ip, SKOV3-TR, SKOV3 |
6 weeks female BALB/c athymic nude mice |
4 samples of ovarian cancer tissue |
cancer-associated adipocytes (CAAs) and fibroblasts (CAFs) |
miR21/APAF1 axis |
Inhibition of APAF1 mRNA expression could lead to apoptosis suppression of cancer cells, directly inducing paclitaxel-resistance of OC cells. |
[27] |
|
OC |
miR-433(Up) |
A2780, PEO1, PEO4 |
—— |
—— |
miR-433 overexpressing ovarian cancer cell line |
miR-433/CDK6 axis |
The loss of CDK6 inhibits cellular senescence of cancer cells, subsequently inducing paclitaxel resistance of OC cells. |
[25] |
|
prostate cancer (PC) |
miR-34a(Up) |
22Rv1RD, DU145RD, PC3RD |
—— |
21 pairs of prostate cancer tissues from publicly available datasets |
docetaxel resistant prostate cancer cells |
miR-34a/BCL-2 |
Upregulation of BCL-2 gene induces suppressed apoptosis of cancer cells, leading to paclitaxel resistance of PC cells. |
[24] |
|
hepatocellular carcinoma (HCC) |
miR-451a(Up) |
Hep3B, MHCC-97 H, HB611, HepG2, SMMC-7721 |
—— |
92 samples of HCC cancer tissues |
human umbilical cord mesenchymal stem cells (hucMSCs) |
miR-451a/ADAM10 axis |
The downregulation of ADAM10 promotes apoptosis of HCC cells, thus suppressing paclitaxel resistance. |
[55] |
|
nasopharyngeal carcinoma (NPC) |
miR-183-5p(Up) |
HNE3, C666-1 |
3 weeks BALB/c nude mice (38 males and 38 |
—— |
62 blood samples and |
miR-183-5p/P-gp axis |
Enhancing the anti-proliferative effects of paclitaxel by inhibiting P-gp-mediated drug efflux, eventually suppressing paclitaxel resistance of NPC cells. |
[17] |
Breast cancer
Paclitaxel resistance in breast cancer (BC) primarily manifests in its effects on BC cell metastasis and invasiveness. Notably, paclitaxel-treated BC-derived exosomes have been identified as potential drivers of resistance. Xia et al. revealed that paclitaxel-treated BC-derived exosomes promotes BC cell resistance by activating the circBACH1/miR-217/G3BP2 signaling pathway. MiR-217 interacts with circBACH1 and targets GTPase-activated SH3 domain-binding protein 2 (G3BP2) in BC cells [33]. The overexpression of G3BP2 promotes metastasis in BC [34,35], thereby inducing paclitaxel resistance. MiR-378 is another upregulated miRNA in the exosomes derived from paclitaxel-resistant BC cells. Paclitaxel activates the EZH2/STAT3 axis in BC cells. Activated BC cells secrete chemotherapy-elicited exosomes enriched in miR-378a-3p and miR-378d. These exosomes are absorbed by chemotherapy-surviving BC cells, leading to activation of the WNT and NOTCH stem cell pathways by targeting DKK3 and NUMB [23]. Wnt/β-catenin and Notch signaling can act in a synergistically to regulate cell development and differentiation [36]. Activation of Wnt/β-catenin and Notch signaling is in part responsible for the stemness effects of cancer cells [37], thus resulting in drug resistance.
Ovarian cancer
The mechanisms underlying paclitaxel resistance in ovarian cancer (OC) include P-gp-mediated drug efflux, apoptosis evasion, and cell cycle dysregulation [38,39]. Several exosomal miRNAs participate in these mechanisms and influence paclitaxel resistance in ovarian cancer. For instance, miR-1246 expression is significantly higher in paclitaxel-resistant OC exosomes than in their sensitive counterparts. Exosomal miR-1246 confers chemoresistance by targeting the Cav1/p-gp/M2-type macrophage axis. Overexpression of Cav1, a direct miR-1246 target, and anti-miR-1246 treatment significantly sensitize OC cells to paclitaxel [40]. Additionally, MiR21 expression is increased in exosomes from CAAs and CAFs. Transferred from CAAs or CAFs to cancer cells, miR21 confers paclitaxel resistance by binding to its target, apoptotic peptidase activating factor 1 (APAF1). APAF1 sensitizes OC cells to paclitaxel treatment, and miR21 inhibits APAF1 mRNA expression by binding to the APAF1 coding sequence, thereby inducing paclitaxel resistance [27]. Weiner-Gorzel et al. showed that cells expressing high levels of miR-433 release miR-433 into growth media via exosomes and induce a senescence bystander effect that modulates the tumor microenvironment, ultimately promoting resistance to paclitaxel through the induction of cellular senescence in OC cells. MiR-433-induced inhibition of cellular senescence may be attributed to the loss of CDK6, a critical mediator of cellular transition into the S phase [41]. The induction of cellular senescence and subsequent alterations in cell signaling have been shown to correlate with changes in the epigenome of cells, promoting cancer progression [42], and mediate paclitaxel resistance in OC cells [25]. Although miRNAs can participate in the mechanisms involved in paclitaxel resistance in OC, including intracellular drug inactivation, DNA damage repair, activation of cancer stem cells, and epithelial-mesenchymal transition (EMT) [43,44], the role of exosomal miRNAs in these processes remains unclear.
Gastrointestinal cancers
The complex mechanisms underlying paclitaxel resistance in gastrointestinal (GI) cancers involve the inactivation of apoptotic signaling pathways, loss of cell cycle checkpoint control, accelerated cell proliferation and autophagy flux, enhanced DNA damage repair capacity, diminished uptake and/or increased efflux of drugs, activation of cancer stem cells, and EMT [45-47]. The regulatory effects of exosomal miRNAs on apoptosis and EMT signaling pathways have been confirmed in GI cancer cell lines. The effects of CAF-derived exosomes on the chemoresistance of GI cancer cells are significant [48]. Zhang et al. analyzed CAF-secreted exo-miRNAs and found that paclitaxel can promote miR-522 secretion by activating the USP7/hnRNPA1 axis. This leads to arachidonate lipoxygenase 15 (ALOX15) suppression and decreased lipid peroxide (lipid-ROS) accumulation in cancer cells. The decrease in iron-dependent lipid ROS leads to the inhibition of ferroptosis, a novel form of regulated cell death, ultimately resulting in decreased paclitaxel sensitivity [28]. Another study in GI cancer generated a paclitaxel-resistant GI cell line (MGC-803R) and found that MGC-803R-exosomes deliver miR-155-5p into chemosensitive cancer cells, inducing paclitaxel-resistant phenotypes. Exosomal delivery of miR-155-5p may induce chemoresistance by suppressing the expression of GATA-binding protein 3 (GATA3) and tumor protein p53-inducible nuclear protein 1 (TP53INP1). Both of these proteins are associated with the regulation of EMT [49,50], a classical paclitaxel resistance mechanism in GI cancer. Moreover, knockdown of miR?155?5p reversed the chemoresistant phenotypes of MGC?803R cells, potentially via the upregulation of GATA3 and TP53INP1. Targeting miR?155?5p may be a promising strategy to overcome paclitaxel resistance in GI cancer [51]. Future translational studies or clinical trials are warranted to develop exosomal miRNA-based therapeutic interventions along with traditional chemotherapy, which may improve the prognosis of patients with GI cancer by overcoming drug resistance.
Prostate cancer
Given that paclitaxel is commonly used in prostate cancer treatment, extensive research has been conducted on the mechanisms of resistance. Activation of oncogenic pathways such as STAT3, NF-κB, β-catenin, and EHZ2 contributes to the development of chemotherapy resistance in prostate cancer, while tumor-suppressor factors like PTEN are downregulated [52]. There is limited research on the role of exosomal miRNAs in prostate cancer, and follow-up studies can be initiated from these pathways. Corcoran et al. investigated intracellular and extracellular (exosomal) miRNAs related to docetaxel resistance and found that downregulation of the tumor suppressor gene miR-34a can cause chemoresistance. Exosomal miR-34a has been suggested to be a predictive biomarker for the response to docetaxel because it regulates the anti-apoptotic gene BCL-2 [24]. This is the first time that exosomal miRNAs have been suggested as possible adjuvants to paclitaxel for the treatment of prostate cancer. Recent studies have reported that treatment of prostate cancer with paclitaxel often fails owing to the development of chemoresistance; however, there is limited research on the regulation of paclitaxel resistance in prostate cancer by exosomal miRNAs. Several miRNAs, such as miR-223-3p, miR-323, miR-375, and miR-148a, have been linked to taxane resistance by interfering with apoptosis. Moreover, miR-21 is often overexpressed in cancer and renders prostate cancer resistant to docetaxel by interfering with pro-apoptotic proteins and downstream pathways [53]. Given the significant roles of miRNAs in altering apoptosis-related pathways and influencing the response of prostate cancer cells to paclitaxel, further studies are warranted to investigate the detection of these miRNAs in exosomes and their impact on the sensitivity of target cancer cells to paclitaxel. Moreover, it is crucial to determine whether this effect is caused by interventions in apoptosis-related pathways.
Other cancers
Furthermore, exosomal miRNAs modulate the response of other cancer types to paclitaxel, including hepatocellular carcinoma (HCC) and nasopharyngeal carcinoma (NPC). Xu et al. investigated the molecular mechanism of human umbilical cord mesenchymal stem cell (hucMSC)-derived exosomal miR-451a in HCC and found that hucMSC-derived exosomal-induced upregulation of miR-451a can downregulate metalloprotease 10 (ADAM10). Inhibition of ADAM10 promotes apoptosis [54] and suppresses paclitaxel resistance of HCC cells [55]. A recent study on NPC suggested that paclitaxel-sensitive cancer cells can transfer exosomal miRNAs to paclitaxel-resistant cancer cells, mediating the reversal of paclitaxel resistance. In this previous study, we elucidated the role of miR-183-5p delivered by extracellular vehicles (EVs). EVs transferred miR-183-5p from paclitaxel-sensitive NPC cells to paclitaxel-resistant NPC cells and enhanced the anti-proliferative effects of paclitaxel by targeting P-glycoprotein (P-gp). P-gp expression was lower in the miR-183-high group, indicating an increased sensitivity to paclitaxel treatment. These findings indicate that miR-183-5p-based therapeutics may constitute a key component of personalized medicine to enhance therapeutic outcomes [17]. In addition to the aforementioned cancer types, exosomes have also been reported to play synergistic roles in miRNA?mediated drug resistance in oral squamous cell carcinoma [56]. This suggests that exosomal miRNAs play a therapeutic role in paclitaxel resistance in various cancer types. Although paclitaxel has been used in non-small cell lung cancer, pancreatic cancer, esophageal cancer, soft tissue sarcoma, and other malignant tumors, the influence of exosomal miRNAs on paclitaxel sensitivity in these cancer types requires further exploration.
Clinical Treatment
Primary or secondary resistance to paclitaxel presents a challenge in clinical treatment, leading to aggressive cancer behavior and poor outcomes [57,58]. Researchers are constantly attempting to overcome paclitaxel resistance in clinical treatment. Mosca et al. have delineated possible modalities to overcome chemoresistance to taxanes, such as increasing drug solubility, delivery, and pharmacokinetics; overcoming microtubule alterations or mitotic slippage; inhibiting drug efflux pumps or drug metabolism; and targeting redox metabolism, immune response, and other cellular functions [59]. Exosomal miRNAs inhibit drug efflux pumps and target cellular functions (cellular apoptosis, senescence, and ferroptosis). Significant progress has been made in the use of exosomal miRNAs as therapies for paclitaxel resistance and in potential regenerative medicine in preclinical trials. Based on preclinical research, several clinical trials have been planned and conducted. These clinical trials involve various cancer types, including GC (NCT01779583, NCT02662621 and NCT02565264), BC (NCT03262311 and NCT01299038), HCC (NCT05375604), pancreatic cancer (NCT03608631), and lung cancer (NCT01159288 and NCT03108677) [60]. The findings from these clinical trials suggest that EVs can overcome chemoresistance, including paclitaxel vincristine (VCR) and cisplatin [33,61,62]. Xia et al. suggests that exosomal miRNAs derived from paclitaxel-treated BC cells could induce paclitaxel resistance, indicating the possibility of using exosomes to treat paclitaxel resistance [33].
Compared with traditional gene carriers, exosomes offer the following advantages: (1) as endogenous vesicles, exosomes have low immunogenicity; (2) their biocompatible structure facilitates efficient cellular entry, enabling more effective gene delivery; (3) their robust membrane can protect therapeutic miRNAs from degradation; and (4) their ability to avoid phagocytosis and bypass engulfment by lysosomes makes them well tolerated in body fluids, enhancing their therapeutic effects, especially in GI cancers [63-66].
However, several challenges remain associated with the translation of exosomal miRNA-based therapies from preclinical studies to clinical applications, including issues related to their scalability, delivery, and safety. Regarding the scalable manufacturing of therapeutic exosomal miRNAs, the selection of proper exosome-producing cells, development of efficient cargo-loading technology, and establishment of large-scale production and purification systems should be considered for successful clinical translation. The choice of exosome-producing cells should be carefully considered to minimize the incorporation of unwanted cellular bioactive cargoes and maximize the loading of desired therapeutic miRNAs on a large scale [67]. The delivery of miRNAs with the ability to regulate paclitaxel resistance in patients presents another challenge. Although exosomes confer stability to miRNAs, controlling their destinations remains to be investigated [68]. Anti-cancer therapeutic exosomes could be targeted to cancer cells or tissues passively or actively. Passive targeting occurs when exosomes naturally target tumors based in their cellular origin [69]. However, Smyth et al. suggested that tumor-derived exosomes exhibit tumor targetability only when injected locally into the tumor parenchyma [70]. Jung et al. also showed that hypoxic cancer cell-derived exosomes exhibited increased delivery to hypoxic cancer cells in vitro but not in vivo [71]. Further studies are required to elucidate the mechanisms underlying the targetability of tumor cell-derived exosomes to their parental cells.
Active targeting of anti-cancer therapeutic exosomes to cancer cells or tissues can be achieved through two main strategies: a non-genetic approach that directly engineers the surface of exosomes via diverse exogenous methods and a genetic approach that non-directly engineers exosomes by genetically modifying exosome-producing cells. Indirect engineering of exosomes expresses specific cancer-targeting moieties on the surface of exosomes by conjugation with exosomal membrane-associated domains, such as the GPI, C1C2 domain, Lamp2b, and tetraspanins, which could serve as a promising strategy for the active targeting of anti-cancer therapeutic exosomes to cancer cells and tissues [72]. This indicates that the delivery problem may be resolved through active targeting, achieved by targeted exosome engineering. Additionally, tumor-derived exosomes may raise safety concerns in terms of delivering tumorigenic factors to non-tumorigenic cells, which could promote tumor metastasis by inducing the formation of a pre-metastatic niche at potential metastatic sites [73-75]. Therefore, careful consideration is required when using tumor-derived exosomes as delivery vehicles for anti-cancer agents. The use of toxic chemicals for exosomal surface engineering requires caution when applied in clinical settings [67].
Discussion
Paclitaxel is a chemotherapeutic agent extensively used for treating human cancers. Resistance to this drug is regarded as a major problem in clinical settings. The dysregulation of exosome-derived miRNA shuttling from paclitaxel-resistant cancer cells to paclitaxel-sensitive cells has been implicated in this problem. Moreover, further studies are needed to determine whether exosomal miRNAs derived from paclitaxel-sensitive cells can fulfil their role after being transported to paclitaxel-resistant cells. Therefore, the insights gained from these studies offer novel avenues for promoting the efficacy of paclitaxel treatment. Considering that ovarian, breast, and gastrointestinal cancers are commonly treated with paclitaxel, these cancer types have been extensively studied regarding the role of exosomal miRNAs in conferring resistance to this drug. Stem cell pathways (Wnt/β-catenin and NOTCH), P-gp axis, and pathways related to cellular apoptosis, senescence, and ferroptosis are among the pathways involved in the modulation of paclitaxel sensitivity by exosomal miRNAs. Other classical apoptotic pathways such as PKC, p53, and ErbB2 also play roles in exosomal miRNA-mediated resistance to paclitaxel. Considering the significant role of exosomal miRNAs in altering the response of cancer cells to paclitaxel, targeted therapies against these transcripts represent putative treatment modalities for combating paclitaxel resistance and enhancing patient survival. Exosome-mediated delivery of miRNAs for gene therapy presents a novel clinical approach to reverse paclitaxel resistance. Moreover, certain exosomal miRNAs provide insights into the cell type from which they are derived, the target, and the cellular state, including therapy resistance. This enables the monitoring and regulation of tumor resistance for personalized therapy. However, this review has several limitations. Current research has yet to elucidate the role of exosomal miRNAs derived from other cells in the tumor microenvironment. Moreover, while in vivo studies support the feasibility of exosomal miRNA therapy in animal models, these modalities have not undergone clinical evaluation. In terms of clinical use, paclitaxel is a first-line drug for late-stage non-small cell lung cancer; however, the role of exosomal miRNAs in modulating the response of lung cells to paclitaxel treatment is still unclear, which could provide valuable insights for future clinical trials benefiting patients with lung cancer.
Conclusion
Exosomal miRNAs play a pivotal role in modulating cancer cell response to paclitaxel. Several classic miRNAs have been identified as functional miRNAs in this regard. For instance, miR-433 and miR-21 in OC [25,27] and miR-34a in prostate cancer [24] are functional miRNAs that exert regulatory effects on cancer cell responses to paclitaxel through exosomes. Given the significant role of exosomal miRNAs in altering cancer cell response to paclitaxel, targeted therapies against these transcripts represent potential treatment modalities to combat paclitaxel resistance. Although in vivo studies support the feasibility of exosomal miRNA-based therapies in animal models, these modalities have not been examined in clinical settings.
Cancer treatment remains a significant challenge. Paclitaxel has been widely used to treat various types of cancers and has shown good therapeutic effects. Nevertheless, most patients eventually acquire drug resistance, leading to a poor prognosis. Therefore, understanding the mechanism underlying paclitaxel resistance and identifying strategies to reverse it are urgently required. Exosomal miRNAs offer promising targets for therapies against paclitaxel resistance, which may benefit patients with cancer.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by grants from the Science and Technology Major Project of Liaoning Province (No. 2020JH1/10300002) and Xingliao Yingcai Program of Liaoning Province (No. YXMJ(XY)-JC-001), Liaoning Natural Science Foundation Joint Fund (No.1700306705485). The graphic abstract was created using BioRender.
References
2. Hill M, Tran N. miRNA interplay: Mechanisms and consequences in cancer. Disease Models & Mechanisms. 2021 Apr 1;14(4):dmm047662.
3. Ping WA, Liu XM, Lei DI, Zhang XJ. mTOR signaling-related MicroRNAs and Cancer involvement. Journal of Cancer. 2018;9(4):667-73.
4. Bryant R, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, et al. Changes in circulating microRNA levels associated with prostate cancer. British Journal of Cancer. 2012 Feb;106(4):768-74.
5. Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non‐coding RNAs: regulators of disease. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2010 Jan;220(2):126-39.
6. Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. International Journal of Molecular Sciences. 2013 Jul 9;14(7):14240-69.
7. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007 Jun;9(6):654-9.
8. Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Research. 2014 Jun;24(6):766-9.
9. Ge R, Tan E, Sharghi-Namini S, Asada HH. Exosomes in cancer microenvironment and beyond: have we overlooked these extracellular messengers?. Cancer Microenvironment. 2012 Dec;5:323-32.
10. Beach A, Zhang HG, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. Journal of Ovarian Research. 2014 Dec;7:14.
11. Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Research. 2007 Apr 1;67(7):2912-5.
12. Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature Reviews Cancer. 2009 Apr;9(4):285-93.
13. Sharma A. Chemoresistance in cancer cells: exosomes as potential regulators of therapeutic tumor heterogeneity. Nanomedicine. 2017 Sep;12(17):2137-48.
14. Mihelich BL, Dambal S, Lin S, Nonn L. miR-182, of the miR-183 cluster family, is packaged in exosomes and is detected in human exosomes from serum, breast cells and prostate cells. Oncology letters. 2016 Aug 1;12(2):1197-203.
15. Guo Z, Wang X, Yang Y, Chen W, Zhang K, Teng B, et al. Hypoxic tumor-derived exosomal long noncoding RNA UCA1 promotes angiogenesis via miR-96-5p/AMOTL2 in pancreatic cancer. Molecular Therapy-Nucleic Acids. 2020 Dec 4;22:179-95.
16. Smith ER, Wang JQ, Yang DH, Xu XX. Paclitaxel resistance related to nuclear envelope structural sturdiness. Drug Resistance Updates. 2022 Dec 1;65:100881.
17. Cui X, Chen Y, Zhao L, Ding X. Extracellular vesicles derived from paclitaxel-sensitive nasopharyngeal carcinoma cells deliver miR-183-5p and impart paclitaxel sensitivity through a mechanism involving P-gp. Cell Biology and Toxicology. 2023 Jun;39(6):2953-70.
18. Wang Y, Zhou Y, Zheng Z, Li J, Yan Y, Wu W. Sulforaphane metabolites reduce resistance to paclitaxel via microtubule disruption. Cell Death & Disease. 2018 Nov 14;9(11):1134.
19. Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature Reviews Cancer. 2002 Jan 1;2(1):48-58.
20. Inoue S, Salah-Eldin AE, Omoteyama K. Apoptosis and anticancer drug resistance. Human Cell. 2001 Sep 1;14(3):211-21.
21. Yu D. Mechanisms of ErbB2-mediated paclitaxel resistance and trastuzumab-mediated paclitaxel sensitization in ErbB2-overexpressing breast cancers. Seminars in Oncology. 2001 Oct 1;28(5 Suppl 16):12-7.
22. Ghafouri-Fard S, Shoorei H, Abak A, Raza SH, Pichler M, Taheri M. Role of non-coding RNAs in modulating the response of cancer cells to paclitaxel treatment. Biomedicine & Pharmacotherapy. 2021 Feb 1;134:111172.
23. Yang Q, Zhao S, Shi Z, Cao L, Liu J, Pan T, et al. Chemotherapy-elicited exosomal miR-378a-3p and miR-378d promote breast cancer stemness and chemoresistance via the activation of EZH2/STAT3 signaling. Journal of Experimental & Clinical Cancer Research. 2021 Dec;40(1):120.
24. Corcoran C, Rani S, O'Driscoll L. miR‐34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. The Prostate. 2014 Sep;74(13):1320-34.
25. Weiner‐Gorzel K, Dempsey E, Milewska M, McGoldrick A, Toh V, Walsh A, et al. Overexpression of the microRNA miR‐433 promotes resistance to paclitaxel through the induction of cellular senescence in ovarian cancer cells. Cancer Medicine. 2015 May;4(5):745-58.
26. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nature Reviews Drug Discovery. 2019 Feb;18(2):99-115.
27. Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS, et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nature Communications. 2016 Mar 29;7(1):11150.
28. Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Molecular Cancer. 2020 Dec;19(1):43.
29. Seidman AD. Single-agent use of Taxol (paclitaxel) in breast cancer. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 1994 Jan 1;5:S17-22.
30. Einzig AI. Review of phase II trials of Taxol (paclitaxel) in patients with advanced ovarian cancer. Annals of Oncology: Official Journal of the European Society for Medical Oncology. 1994 Jan 1;5:S29-32.
31. Sakamoto J, Matsui T, Kodera Y. Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer. 2009 Jun;12:69-78.
32. Smith DC, Pienta KJ. Paclitaxel in the treatment of hormone-refractory prostate cancer. Seminars in Oncology. 1999 Feb 1;26(1 Suppl 2):109-11.
33. Xia W, Chen W, Ni C, Meng X, Wu J, Yang Q, et al. Chemotherapy-induced exosomal circBACH1 promotes breast cancer resistance and stemness via miR-217/G3BP2 signaling pathway. Breast Cancer Research. 2023 Jul 17;25(1):85.
34. Gupta N, Badeaux M, Liu Y, Naxerova K, Sgroi D, Munn LL, et al. Stress granule-associated protein G3BP2 regulates breast tumor initiation. Proceedings of the National Academy of Sciences. 2017 Jan 31;114(5):1033-8.
35. Alam U, Kennedy D. G3BP1 and G3BP2 regulate translation of interferon-stimulated genes: IFITM1, IFITM2 and IFITM3 in the cancer cell line MCF7. Molecular and Cellular Biochemistry. 2019 Sep 15;459(1-2):189-204.
36. Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, The Wnt/β-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Developmental cell. 2010 Jun 15;18(6):938-49.
37. Jiang N, Zou C, Zhu Y, Luo Y, Chen L, Lei Y, et al. HIF-1ɑ-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/β-catenin and Notch signaling. Theranostics. 2020;10(6):2553-70.
38. Christie EL, Pattnaik S, Beach J, Copeland A, Rashoo N, Fereday S, et al. Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nature Communications. 2019 Mar 20;10(1):1295.
39. Liang F, Ren C, Wang J, Wang S, Yang L, Han X, et al. The crosstalk between STAT3 and p53/RAS signaling controls cancer cell metastasis and cisplatin resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT and autophagy. Oncogenesis. 2019 Oct 9;8(10):59.
40. Kanlikilicer P, Bayraktar R, Denizli M, Rashed MH, Ivan C, Aslan B,. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine. 2018 Dec 1;38:100-12.
41. Goel S, Bergholz JS, Zhao JJ. Targeting CDK4 and CDK6 in cancer. Nature Reviews Cancer. 2022 Jun;22(6):356-72.
42. Cruickshanks HA, McBryan T, Nelson DM, VanderKraats ND, Shah PP, Van Tuyn J, et al. Senescent cells harbour features of the cancer epigenome. Nature cell biology. 2013 Dec;15(12):1495-506.
43. Belur Nagaraj A, Knarr M, Sekhar S, Connor RS, Joseph P, Kovalenko O, et al. The miR–181a–SFRP4 Axis Regulates Wnt Activation to Drive Stemness and Platinum Resistance in Ovarian Cancer. Cancer research. 2021 Apr 15;81(8):2044-55.
44. Chiappa M, Guffanti F, Bertoni F, Colombo I, Damia G. Overcoming PARPi resistance: Preclinical and clinical evidence in ovarian cancer. Drug Resistance Updates. 2021 Mar 1;55:100744.
45. Nies AT, Magdy T, Schwab M, Zanger UM. Role of ABC transporters in fluoropyrimidine-based chemotherapy response. Advances in Cancer Research. 2015 Jan 1;125:217-43.
46. Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Therapeutic Advances in Medical Oncology. 2016 Jan;8(1):57-84.
47. Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nature reviews Molecular Cell Biology. 2014 Jan;15(1):49-63.
48. Bhome R, Goh RW, Bullock MD, Pillar N, Thirdborough SM, Mellone M, et al. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: role in driving cancer progression. Aging (Albany NY). 2017 Dec;9(12):2666-94.
49. Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K, et al. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nature Communications. 2013 Jan 22;4(1):1393.
50. Yu SJ, Yang L, Hong Q, Kuang XY, Di GH, Shao ZM. MicroRNA-200a confers chemoresistance by antagonizing TP53INP1 and YAP1 in human breast cancer. BMc cancer. 2018 Dec;18(1):74.
51. Wang M, Qiu R, Yu S, Xu X, Li G, Gu R, et al. Paclitaxel resistant gastric cancer MGC 803 cells promote epithelial to mesenchymal transition and chemoresistance in paclitaxel sensitive cells via exosomal delivery of miR 155 5p. International Journal of Oncology. 2019 Jan 1;54(1):326-38.
52. Hashemi M, Zandieh MA, Talebi Y, Rahmanian P, Shafiee SS, Nejad MM, et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies. Biomedicine & Pharmacotherapy. 2023 Apr 1;160:114392.
53. Hussein MA, Munirathinam G. MicroRNAs in Prostate Cancer: Implications for Treatment Response and Therapeutic Targets. Cancers. 2023 Oct 17;15(20):5023.
54. Zhang W, Liu S, Liu K, Ji B, Wang Y, Liu Y. Knockout of ADAM10 enhances sorafenib antitumor activity of hepatocellular carcinoma in vitro and in vivo. Oncology Reports. 2014 Nov 1;32(5):1913-22.
55. Xu Y, Lai Y, Cao L, Li Y, Chen G, Chen L, et al. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-451a represses epithelial–mesenchymal transition of hepatocellular carcinoma cells by inhibiting ADAM10. RNA Biology. 2021 Oct 3;18(10):1408-23.
56. Liu T, Chen G, Sun D, Lei M, Li Y, Zhou C, et al. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta biochimica et biophysica Sinica. 2017 Sep 1;49(9):808-16.
57. Kim JJ, Yin B, Christudass CS, Terada N, Rajagopalan K, Fabry B, et al. Acquisition of paclitaxel resistance is associated with a more aggressive and invasive phenotype in prostate cancer. Journal of Cellular Biochemistry. 2013 Jun;114(6):1286-93.
58. Kim HS, Kim TJ, Chung HH, Kim JW, Kim BG, Park NH, et al. In vitro extreme drug resistance assay to taxanes or platinum compounds for the prediction of clinical outcomes in epithelial ovarian cancer: a prospective cohort study. Journal of Cancer Research and Clinical Oncology. 2009 Nov;135:1513-20.
59. Mosca L, Ilari A, Fazi F, Assaraf YG, Colotti G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resistance Updates. 2021 Jan 1;54:100742.
60. Kumar MA, Baba SK, Sadida HQ, Marzooqi SA, Jerobin J, Altemani FH, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduction and Targeted Therapy. 2024 Feb 5;9(1):27.
61. Wang X, Qiao D, Chen L, Xu M, Chen S, Huang L, et al. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Molecular Cancer. 2019 Dec;18(1):182.
62. Zhou G, Gu Y, Zhu Z, Zhang H, Liu W, Xu B, et al. Exosome mediated cytosolic cisplatin delivery through clathrin-independent endocytosis and enhanced anti-cancer effect via avoiding endosome trapping in cisplatin-resistant ovarian cancer. Frontiers in Medicine. 2022 May 3;9:810761.
63. Pirisinu M, Pham TC, Zhang DX, Hong TN, Nguyen LT, Le MT. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: Recent advances, current obstacles, and challenges for clinical translation. Seminars in Cancer Biology. 2022 May 1;80:340-55.
64. Herrmann IK, Wood MJ, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nature Nanotechnology. 2021 Jul;16(7):748-59.
65. Ullah M, Kodam SP, Mu Q, Akbar A. Microbubbles versus extracellular vesicles as therapeutic cargo for targeting drug delivery. ACS nano. 2021 Mar 5;15(3):3612-20.
66. Yao X, Lyu P, Yoo K, Yadav MK, Singh R, Atala A, et al. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. Journal of Extracellular Vesicles. 2021 Mar;10(5):e12076.
67. Choi H, Yim H, Park C, Ahn SH, Ahn Y, Lee A, et al. Targeted delivery of exosomes armed with anti-cancer therapeutics. Membranes. 2022 Jan 13;12(1):85.
68. Zhou L, Lv T, Zhang Q, Zhu Q, Zhan P, Zhu S, et al. The biology, function and clinical implications of exosomes in lung cancer. Cancer letters. 2017 Oct 28;407:84-92.
69. Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue engineering and regenerative medicine. 2021 Aug;18(4):499-511.
70. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. Journal of Controlled Release. 2015 Feb 10;199:145-55.
71. Jung KO, Jo H, Yu JH, Gambhir SS, Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018 Sep 1;177:139-48.
72. Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotechnology. 2011 Apr;29(4):341-5.
73. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology. 2015 Jun;17(6):816-26.
74. Guo Y, Ji X, Liu J, Fan D, Zhou Q, Chen C, Wang W, Wang G, Wang H, Yuan W, Ji Z. Effects of exosomes on pre-metastatic niche formation in tumors. Molecular Cancer. 2019 Dec;18(1):39.
75. Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Advanced Drug Delivery Reviews. 2013 Mar 1;65(3):383-90.