Background
Preventable medical errors and iatrogenic injuries remain significant contributors to mortality and morbidity, emphasizing the need for effective clinical training methodologies. Traditional teaching methods often inadequately prepare physicians for mastering procedural skills. Surgical procedures like nephrectomy for kidney tumor removal require intricate understanding of renal anatomy and meticulous technique. Close coordination among the specialists remains crucial to ensure optimal patient outcomes. Simulators offers an alternative solution to close coordination and a promising avenue for training in kidney tumor removal, with various existing products providing realistic models, interactive simulations, and performance feedback. A similar problem lies in kidney biopsy to a large extent. Simulation training has emerged as a promising approach to enhance procedural competencies. While historical medical models have evolved into sophisticated simulators, accurately replicating respiratory movements remains a challenge. Furthermore, current simulators lack the ability to replicate breathing movement, which impacts their fidelity in simulating real surgical environments. Integrating dynamic respiratory simulations into virtual reality platforms presents a potential solution to this limitation. By mimicking tumor movement during breathing, such simulations could offer a more comprehensive training experience closely resembling real-world surgical conditions.
Introduction
It is widely acknowledged that preventable medical errors constitute a significant contributor to mortality in the United States (US), leading to an estimated 400,000 deaths annually. Similarly, iatrogenic injuries leave approximately 3.5 million patients disabled each year in the US [1]. Conventional methods of teaching clinical procedures often fall short, leaving physicians inadequately prepared to master even fundamental procedural skills by the conclusion of their training. Simulation training, however, has demonstrated significant efficacy in enhancing procedural competencies [2,3].
Throughout history, ancient clay and stone models discovered worldwide served to illustrate clinical characteristics of different diseases (Figure 1) [4]. Over time, with technological progress, medical simulation has evolved into a more sophisticated practice. The inception of the first "modern" simulator dates back to around 1700, created by Gregoire and Gregoire, a father-son duo in Paris, France [5]. During the 1980s and 1990s, the swift development of computer hardware and software drove a parallel evolution in the complexity and capabilities of simulators. Simulators were being developed to extend training beyond simple interaction with a mannequin, focusing instead on real-life scenarios and providing live feedback, including haptic responses. Virtual reality stands out as a prime example of such capability in contemporary simulation training, offering a fully immersive experience that convincingly tricks the user's senses into perceiving themselves in a distinct environment detached from the physical world.
Figure 1. Ancient clay models of human anatomy.
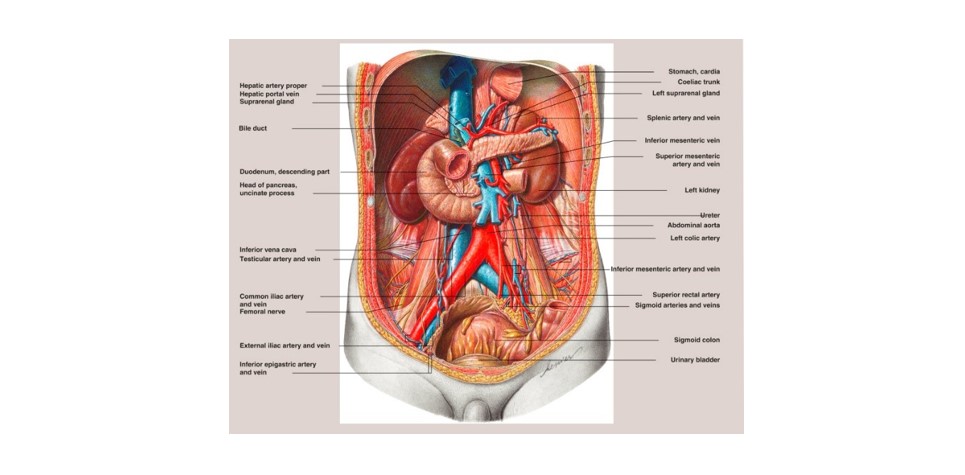
Removing a tumor from the kidney, typically through a surgical procedure known as nephrectomy, can be complex due to the intricate anatomy and function of the kidneys, as well as the surrounding structures. It requires a thorough understanding of renal anatomy [6] as shown in Figure 2, meticulous surgical technique, and careful consideration of potential risks and complications. Collaboration among urologists, radiologists, and other specialists is often necessary to ensure the best possible outcome for the patient.
Figure 2. The posterior abdominal wall and topographical relationships of the kidneys, ureters, and suprarenal.
Potential Challenges to Build an Efficient Simulator
Here are several anatomical complexities involved in removing a kidney tumor [7-9]:
- Location of the Kidney: The kidneys are located deep within the abdomen, retroperitoneally, which means they are situated behind the peritoneum, the membrane that lines the abdominal cavity. Accessing the kidney for surgery requires careful navigation through layers of tissue and organs without causing damage to surrounding structures.
- Vascular Anatomy: The kidneys have a rich blood supply through the renal arteries and veins, which branch extensively within the kidney to supply oxygenated blood and remove waste products. The intricate vascular anatomy presents challenges during surgery, as preserving blood flow to the kidney while removing the tumor is crucial to maintain kidney function.
- Ureter and Urinary Tract: The ureter, the tube that carries urine from the kidney to the bladder, is intimately associated with the kidney. Surgeons must carefully identify and preserve the ureter to prevent injury and maintain urinary function during tumor removal. Additionally, ensuring a secure closure of the ureter after tumor removal is essential to prevent urine leakage and complications.
- Adjacent Structures: The kidneys are surrounded by other vital structures such as the adrenal glands, major blood vessels (e.g., aorta and inferior vena cava), and the intestines. Surgeons must navigate around these structures to access the kidney and remove the tumor while minimizing damage to nearby organs and tissues.
- Renal Parenchyma and Capsule: The kidney's outer layer, known as the renal capsule, encloses the renal parenchyma, which contains the functional units of the kidney, including the nephrons and collecting ducts. Preserving as much healthy renal tissue as possible while removing the tumor is essential to maintain kidney function post-surgery.
- Potential Complications: Surgery to remove a kidney tumor carries inherent risks, including bleeding, infection, damage to surrounding structures, and postoperative complications such as decreased kidney function. Surgeons must carefully weigh the risks and benefits of the procedure and take precautions to minimize complications.
- Impact of Breathing: The diaphragm's continuous movement during breathing can make it difficult to maintain a stable target for needle insertion, increasing the risk of inadvertent puncture of surrounding organs. Breathing, especially deep breaths, can shift the position of the kidneys and surrounding organs, further complicating needle targeting and raising the risk of unintended punctures.
Reducing morbidity linked to nephrectomy involves meticulous surgical planning and adherence to fundamental anatomical and surgical principles [10]. This underscores the significance of surgical training simulators to be used in complex heart, liver, brain, and other key organ surgeries.
Methodology
Although the field of surgical training simulators continues to expand, there are some popular interventional radiology virtual simulators (as shown in Table 1) available for kidney biopsy, each possessing unique strengths and limitations. These solutions offer a range of features, including realistic anatomical models, interactive simulations, haptic feedback, and performance analytics, to facilitate effective training in kidney tumor removal and other urological procedures.
|
Existing Products |
Respective Features |
|
KBVTrainer (Kidney Biopsy Virtual Trainer) [11] |
Open-source, affordable, realistic kidney and vasculature models, haptic feedback for needle interaction, customizable scenarios, performance feedback. |
|
Simbionix Angioscopy Simulator [12] |
High-fidelity haptic feedback with force and tissue resistance, realistic needle interaction, diverse case scenarios with bleeding and complications. |
|
EchoPixel Virtual Ultrasound Simulator [13] |
Real-time ultrasound simulation with probe movement and tissue deformation, customizable kidney models with pathology, breathing simulation. |
|
CAE Healthcare Sim&Cath 3D Interventional Radiology Simulator [14] |
Advanced haptic feedback with various tissue textures, realistic needle interaction and vascular pulsation, diverse interventional procedures including kidney biopsy. |
|
3D Systems Touch Haptic Device with iMSTK Software [15] |
Affordable haptic device, open-source software integration with various virtual environments, customizable for different interventional procedures like kidney biopsy. |
While these solutions provide a variety of features like live anatomical models, they may not entirely mirror the intricacies and variations experienced in real surgical environments; useful simulators need to be interactive, should have haptic feedback, and performance analytics enhancing the training for kidney tumor removal. Discrepancies in tissue texture, haptic feedback, and instrument manipulation between simulated and actual surgeries can impact the applicability of skills acquired in simulation to the operating theater. These simulators are yet to be proven replicating the movement due to patient breathing. In surgical simulations, the respiratory movements significantly impact the organ positioning (involving the abdomen or thoracic cavity) and visibility. Replicating the intricate movements of the lungs and diaphragm during breathing poses technical challenges for simulator developers [16].
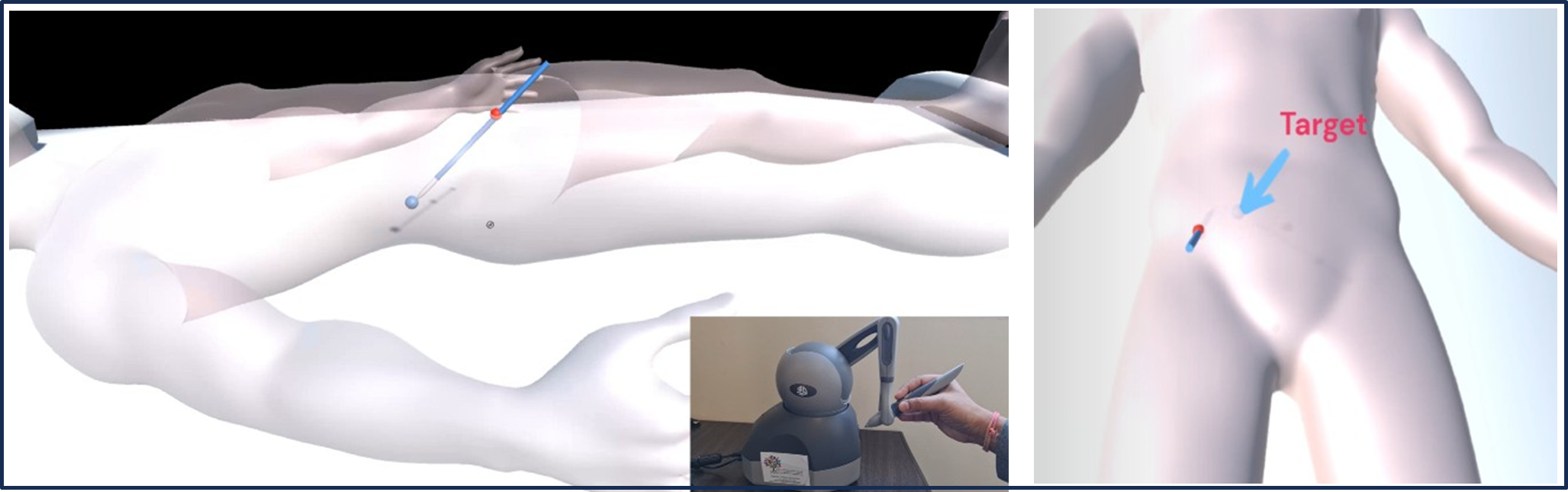
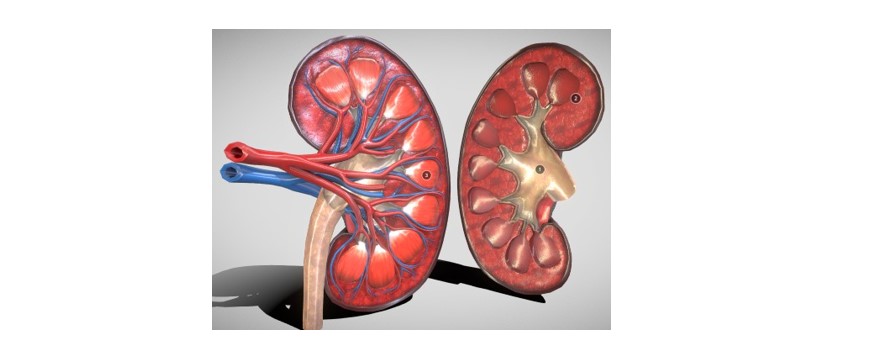
We have developed a simulator for biopsy training mimicking tumor movement caused by breathing patterns (Figure 3). The trajectory of lesion movement is determined through a derived mathematical equation. Virtual reality (VR) platforms offer immersive environments where users can engage with simulated anatomical structures and perform surgical procedures in real-time. Integrating dynamic respiratory simulations into VR environments allows simulators to offer a more comprehensive training experience, closely resembling real-world surgical conditions. Achieving haptic feedback involves assigning various materials to the 3D kidney model structures (Figures 4 and 5), enabling the virtual tools to provide diverse haptic sensations for a realistic and interactive simulation. Thus, when the trainee touches any unnecessary tissue or vessel, the tissue gets highlighted, and a warning noise is heard alarming the trainee to change the trajectory. These unnecessary tissues [17] or vessels inside the kidney are individually segmented by various conventional [18-21] and artificial intelligence- based methods [22-25].
Figure 3. Dynamic respiratory simulation integrated with VR and Haptic.
Figure 4. Basic blood vessel model.
Figure 5. Kidney 3D model.
The advancement of simulation technology holds immense promise for revolutionizing medical training, particularly in complex procedures such as kidney tumor removal. A simulation training should ideally offer a dynamic and interactive platform for physicians to hone their skills in a risk-free environment. We have presented proof of concept by integrating dynamic respiratory simulations into virtual reality environments that can replicate the complexities of real-world surgical scenarios, including the movement of tumors during breathing. Additionally, the incorporation of haptic feedback further enhances the realism and interactivity of the simulation, providing trainees with valuable tactile sensations akin to those experienced in actual surgeries. As the field of surgical simulation continues to evolve, ongoing research and innovation will be essential in refining simulation technologies and maximizing their potential to improve patient outcomes through better-trained healthcare professionals.
Acknowledgement
This work was supported by the Medical Research Center, Hamad Medical Corporation Doha, Qatar, under Grant MRC-01-23-367.
References
2. Mrug M, Bissler JJ. Simulation of real-time ultrasound-guided renal biopsy. Kidney International. 2010 Oct 1;78(7):705-7.
3. Aebersold M. The history of simulation and its impact on the future. AACN Advanced Critical Care. 2016 Feb 1;27(1):56-61.
4. Jones F, Passos-Neto CE, Braghiroli OF. Simulation in medical education: brief history and methodology. Principles and Practice of Clinical Research. 2015 Sep 16;1(2).
5. Buck GH. Development of simulators in medical education. Gesnerus. 1991 Nov 25;48(1):7-28.
6. Mahadevan V. Anatomy of the kidney and ureter. Surgery (Oxford). 2019 Jul 1;37(7):359-64.
7. Klatte T, Ficarra V, Gratzke C, Kaouk J, Kutikov A, Macchi V, et al. A literature review of renal surgical anatomy and surgical strategies for partial nephrectomy. European Urology. 2015 Dec 1;68(6):980-92.
8. Sattar S, Shaik SZ, Khan ZA. Impact of respiration on kidney movement during percutaneous ultrasound-guided renal biopsy. Journal of Medical Ultrasonics (India). 2014;32(3):189-92.
9. Al-Ani N, Shah R, Singh S, Sharma T. Colonic perforation after percutaneous renal biopsy. Nephron Clinical Practice. 2006;103(4):199-202.
10. Pahernik S, Bergsträßer C, Teber D, Hohenfellner M. Nephrectomy: complication management. Der Urologe. 2014 May;53:706-9.
11. Park H, Kim HK, Jeong MH, Cho JY, Lee KH, Sim DS, et al. Clinical impacts of inhibition of renin-angiotensin system in patients with acute ST-segment elevation myocardial infarction who underwent successful late percutaneous coronary intervention. Journal of Cardiology. 2017 Jan 1;69(1):216-21.
12. Villard PF, Vidal FP, Ap Cenydd L, Holbrey R, Pisharody S, Johnson S, et al. Interventional radiology virtual simulator for liver biopsy. International Journal of Computer Assisted Radiology and Surgery. 2014 Mar;9:255-67.
13. Villanueva AG, Hernandez-Prera JA, Fernandez-Fernandez MJ, Hernandez-Gea R, Lopez-Canovas AE. Virtual reality simulation for training in ultrasound-guided liver biopsy: Preliminary experience. World Journal of Gastroenterology. 2013;19(37):6052-8.
14. Kim HJ, Ha SW, Kim TW, Kwon HY, Park JH. Development and validation of a virtual reality simulator for ultrasound-guided needle biopsy. Simulation in Healthcare. 2015;10(4):189-98.
15. Enquobahrie A, Ko JH. Advanced virtual simulator for real-time ultrasound-guided renal biopsy training. SBIR.gov. 2020.
16. Akhtar KS, Chen A, Standfield NJ, Gupte CM. The role of simulation in developing surgical skills. Current Reviews in Musculoskeletal Medicine. 2014 Jun;7:155-60.
17. Zhai X, Eslami M, Hussein ES, Filali MS, Shalaby ST, Amira A, et al. Real-time Automated Image Segmentation Technique for Cerebral Aneurysm on Reconfigurable System-On-Chip. Journal of Computational Science. 2018 Jul;27:35-45, 2018.
18. Al-Kababji A, Bensaali F, Dakua SP. Scheduling techniques for liver segmentation: Reducelronplateau vs onecyclelr. International Conference on Intelligent Systems and Pattern Recognition. 2022 Mar 24; pp. 204-12.
19. Dakua SP, Sahambi JS. LV contour extraction from cardiac MR images using random walks approach. 2009 IEEE International Advance Computing Conference. 2009 Mar 6; pp. 228-33.
20. Akhtar Y, Dakua SP, Abdalla A, Aboumarzouk OM, Ansari MY, Abinahed J, et al. Risk assessment of computer-aided diagnostic software for hepatic resection. IEEE Transactions on Radiation and Plasma Medical Sciences. 2021 Apr 5;6(6):667-77.
21. Mohanty S, Dakua SP. Toward computing cross-modality symmetric non-rigid medical image registration. IEEE Access. 2022 Feb 25;10:24528-39.
22. Ansari MY, Yang Y, Balakrishnan S, Abinahed J, Al-Ansari A, Warfa M, et al. A lightweight neural network with multiscale feature enhancement for liver CT segmentation. Scientific Reports. 2022 Aug 19;12(1):14153.
23. Ansari MY, Yang Y, Meher PK, Dakua SP. Dense-PSP-UNet: A neural network for fast inference liver ultrasound segmentation. Computers in Biology and Medicine. 2023 Feb 1;153:106478.
24. Ansari MY, Chandrasekar V, Singh AV, Dakua SP. Re-routing drugs to blood brain barrier: A comprehensive analysis of Machine Learning approaches with fingerprint amalgamation and data balancing. IEEE Access. 2022 Dec 29;11:9890-906.
25. Chandrasekar V, Ansari MY, Singh AV, Uddin S, Prabhu KS, Dash S, et al. Investigating the Use of Machine Learning Models to Understand the Drugs Permeability Across Placenta. IEEE Access. 2023 May 4;11: 52726-39.