Abstract
Introduction: Multidisciplinary treatment is widely applied for advanced hepatocellular carcinoma (HCC). Systemic therapy is recommended and can provide a modest prognosis for HCC in Barcelona Clinic Liver Cancer staging C. Lenvatinib is a newly developed multityrosine kinase inhibitor and has become a more preferred targeted drug than sorafenib to achieve conversion surgery due to its higher tumor necrosis effect. To our best knowledge, we have published 1st report of HCC patient undergoing conversion hepatectomy by multidisciplinary treatment including lenvatinib in October 2019.
Methods: Literature review was conducted of PubMed in recent 10 years. Keywords included lenvatinib, hepatocellular carcinoma, conversion therapy, and conversion surgery.
Results: Based on a randomized controlled phase-III REFLECT trial, the overall response rate was higher for lenvatinib than for sorafenib (41% vs. 12% assessed by modified Response Evaluation Criteria in Solid Tumors). Recently, a greater response rate of 45% was demonstrated in patients treated with lenvatinib in combination with pembrolizumab. Seven patients have been published treated with conversion surgery after multidisciplinary treatment including lenvatinib. All patients were judged as a partial response so that good tumor response was thought to be essential for conversion surgery. We have demonstrated the most impressive patient treated by ourselves. The patient had two huge HCCs, 24 cm in maximal diameter, with an excessive arterioportal shunt together with impaired liver function and extremely high level of the alpha-fetoprotein (1,008,021 ng/mL). She was treated with 4 times of transarterial chemoembolization and lenvatinib therapy followed by extended right hepatectomy. Her general condition is stable with no recurrences, 27 months after the initial therapy. The most important phenomena included the complete disappearance of remarkable arterioportal shunt followed by improvement of liver function.
Conclusions: Lenvatinib therapy is one of the quite beneficial options of conversion therapy for unresectable HCC.
Keywords
Arterioportal shunt, Conversion therapy, Conversion surgery, Hepatectomy, Hepatocellular carcinoma, Lenvatinib therapy
Abbreviations
HCC: Hepatocellular Carcinoma; TACE: Transarterial Chemoembolization; BCLC: Barcelona Clinic Liver Cancer; mRECIST: modified Response Evaluation Criteria in Solid Tumors; A-P shunt: Arterioportal shunt
Introduction
Multidisciplinary treatment is widely applied for hepatocellular carcinoma (HCC) using liver resection/ transplantation, local ablation therapy, transarterial chemoembolization (TACE), and systemic therapy [1-3]. Systemic therapy is recommended and can provide a modest prognosis for HCC in Barcelona Clinic Liver Cancer (BCLC) staging C [1-3].
Lenvatinib is a newly developed multityrosine kinase inhibitor recommended since 2018 firstly in Japan and later worldwide [3-8]. Several long-term survivors have been reported after conversion surgery using sorafenib in patients with advanced HCC [9-11]. Recently, lenvatinib has become a more preferred targeted therapy to achieve conversion surgery due to its higher tumor necrosis effect evaluated by the modified Response Evaluation Criteria in Solid Tumors (mRECIST) [12-16]. To our best knowledge, we have published 1st report of HCC patient undergoing conversion hepatectomy by multidisciplinary treatment, including lenvatinib, in October 2019 [12].
This paper summarized the current status of conversion therapy for advanced HCC using lenvatinib.
Impact of Lenvatinib in Conversion Therapy for Hepatocellular Carcinoma
Because advanced HCCs frequently show hypervascular nature, the therapeutic effect of systemic therapy is assessed not only by the tumor regression effect using the RECIST criteria but also tumor necrosis effect using the mRECIST criteria [17]. The higher response rate can provide a better liver resection rate in patients that underwent conversion surgery for initially unresectable colorectal liver metastases [18]. Consequently, similar tendency can be observed for HCC patients treated with liver resection following systemic therapy. Based on a randomized controlled phase-III REFLECT trial, the overall response rate was higher for lenvatinib than for sorafenib (41% vs. 12% and 19% vs. 7% for mRECIST and RECIST 1.1, respectively) [19].
The efficacy and safety of lenvatinib were assessed for advanced HCC patients beyond the inclusion criteria of the REFLECT trial. The patients had a tumor with the main portal vein tumor thrombus (Vp4) or with >50% liver occupation. Response rates were 20.0% and 29.3% in the former and latter groups by mRECIST criteria, respectively [20]. Another study confirmed that the response rates according to Child–Pugh classification (A: 56.0% vs. B: 45.8%) or liver occupation of HCC (<50%: 56.3% vs. ≥50%: 33.3%) were not significantly different by mRECIST criteria [21]. Lenvatinib showed an excellent tumor necrosis effect inside and outside of the REFLECT criteria.
Recently, a greater response rate of 45% by mRECIST criteria was demonstrated in patients treated with lenvatinib in combination with pembrolizumab [22]. Their response rate is higher than those of patients undergoing hepatic arterial infusion chemotherapy (36%) or a novel standard systemic therapy with atezolizumab and bevacizumab (27%) [23,24]. The half-life of the lenvatinib plasma concentrations is short at approximately 28–35 h [25]. Therefore, 7–10 days is believed to be sufficient as a cessation time before conversion surgery [12]. Taken together, lenvatinib must be one of the recommendable options to be used for conversion therapy.
Conversion Surgery after Multidisciplinary Treatment Including Lenvatinib
The number of radical conversion surgery has increased after lenvatinib therapy in recent years [12-16]. Overall, five papers including seven patients have been published (Table 1). Conversion surgery included liver resection and thermal ablation with operative microwave and radiofrequency.
| Case No. / Ref. No. | 1.Our case Ref. [12] |
2. Our case Ref. [13] |
3. Ref. [14] | 4. Ref. [15] | 5. Ref. [16] | 6. Ref. [16] | 7. Ref. [16] |
| Age / Sex | 66 / F | 75 / M | 69 / M | 82 / F | 68 / F | 71 / F | 70 / M |
| Etiology | HCV | NonBNonC | HBV | NonBNonC | Alcohol | NASH | NASH |
| Tumor size (cm) | 24 | 17 | 15 | 10 | 7.3 | 5.8 | 5.2 |
| Tumor number | 2 | 3 | 1 | 1 | many | many | many |
| Distant metastasis | none | none | none | lung | none | none | none |
| BCLC stage | C (A-Pshunt) |
B | B / C | C | B | B | B |
| Child-Pugh grade | 8B→ 6A | 5A | 8 B | 5 A | 5 A | 5 A | 5 A |
| ALBI grade | 3→2a | 1 | NE | NE | 2a | 2a | 1 |
| Previous Tx and number | TACE4 | TACE 5 RFA, LDEV | TACE 1 Sorafenib |
none | TACE 1 | TACE 1 | TACE 1 |
| Duration of LEN Tx (month) | 4.5 | 1.2 | 5 | 13 | 6 | 6 | 6 |
| Assessment of LEN therapy (mRECIST / RECIST) | PR/PR | CR/PR | NE/SD | PR/SD | PR/PR | PR/PR | PR/PR |
| Combination drug with LEN | none | none | Nivolumab | none | none | none | none |
| Conversion surgery | Hepatectomy | MWA | Hepatectomy | Hepatectomy | Hepatectomy | Hepatectomy | MWA |
| Postoperative Tx | No | TACE | LEN | No | No | No | LEN |
| Survival months after initial Tx | 27Alive | 18 Dead | 11 Alive | 5 Alive | 6 Alive | 3 Alive | 6 Alive |
| Recurrence | none | none | none | none | none | none | none |
| Ref.: Reference; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NASH: Nonalcoholic Steatohepatitis; Tx.: Treatment; TACE: Transarterial Chemoembolization; RFA: Radiofrequency Ablation; LDEV: Laparoscopic Devascularization; MWA: Microwave Ablation; BCLC; Barcelona Clinic Liver Cancer; A-P shunt: Arterioportal shunt; ALBI: Albumin-bilirubin; NE: Not Evaluated; LEN: Lenvatinib; mRECIST: modified Response Evaluation Criteria in Solid Tumour; PR: Partial Response; SD: Stable Disease | |||||||
The median age of the patients was 70 years (range, 66–82 years) and three patients (43%) were male. Median tumor size and number was 10 cm (range: 5.2–24) and 3 (range: 1 to many), respectively. Five patients (72%) had non-hepatitis B and C, one (14%) patient had distant metastasis, three (43%) patients were in BCLC stage C, and all patients except one had previous treatments for HCC and Child–Pugh A immediately before conversion surgery. All patients and five of seven patients were judged as a partial response by mRECIST and RECIST criteria, respectively. Good tumor response was thought to be essential for conversion surgery. The median duration of lenvatinib therapy was 10 months (range, 1.2–13). In addition, conversion surgery included five hepatectomies and two microwave ablations. Postoperative therapies included two lenvatinib therapies and one TACE. One patient expired of postoperative complications for primary lung cancer without HCC recurrence [13]. The remaining patients were alive without recurrence for 3–21 months. However, the observational period is not long enough to assess the true survival benefit of conversion surgery. Considering strict definition of conversion therapy that make unresectable HCC to be resectable, case 1, 4, 5, and 6 are true conversion cases.
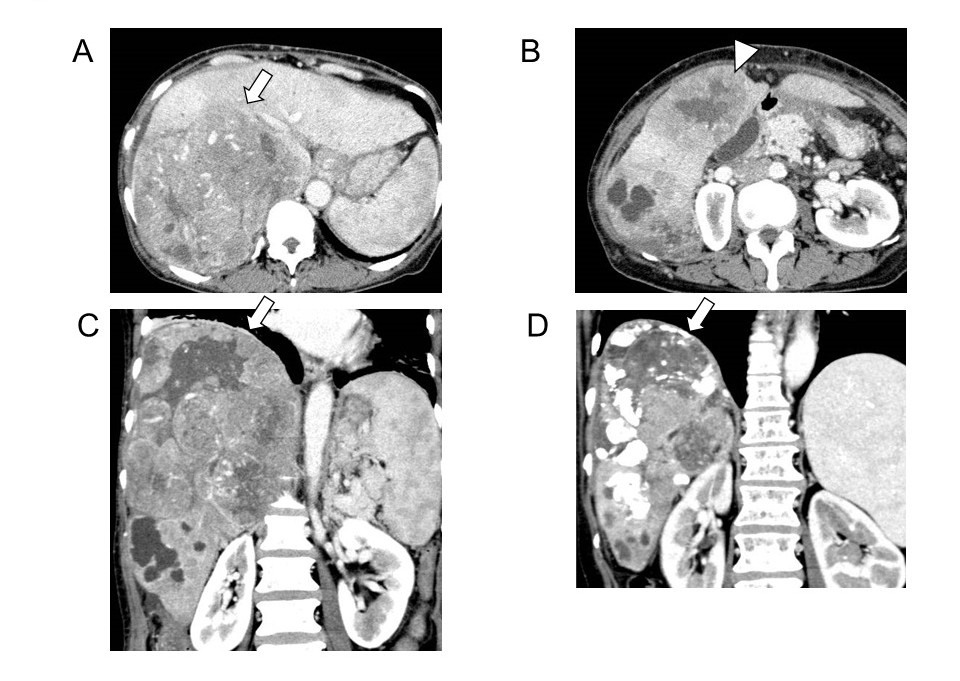
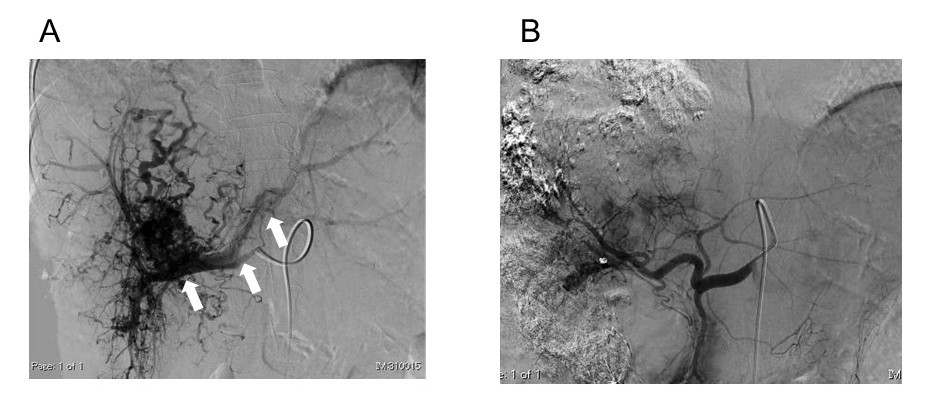
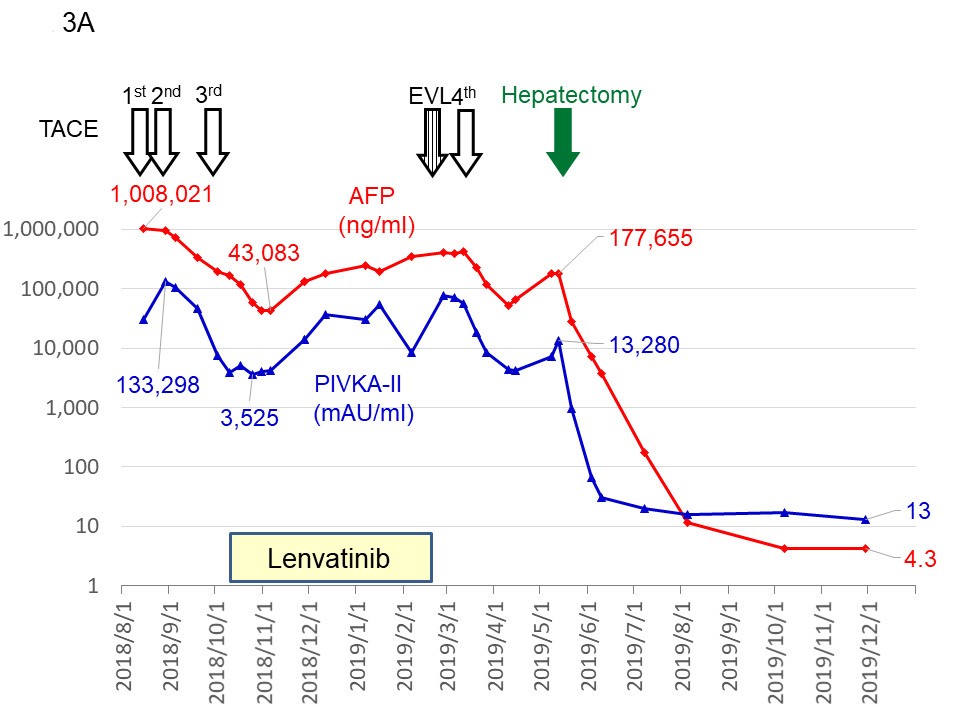
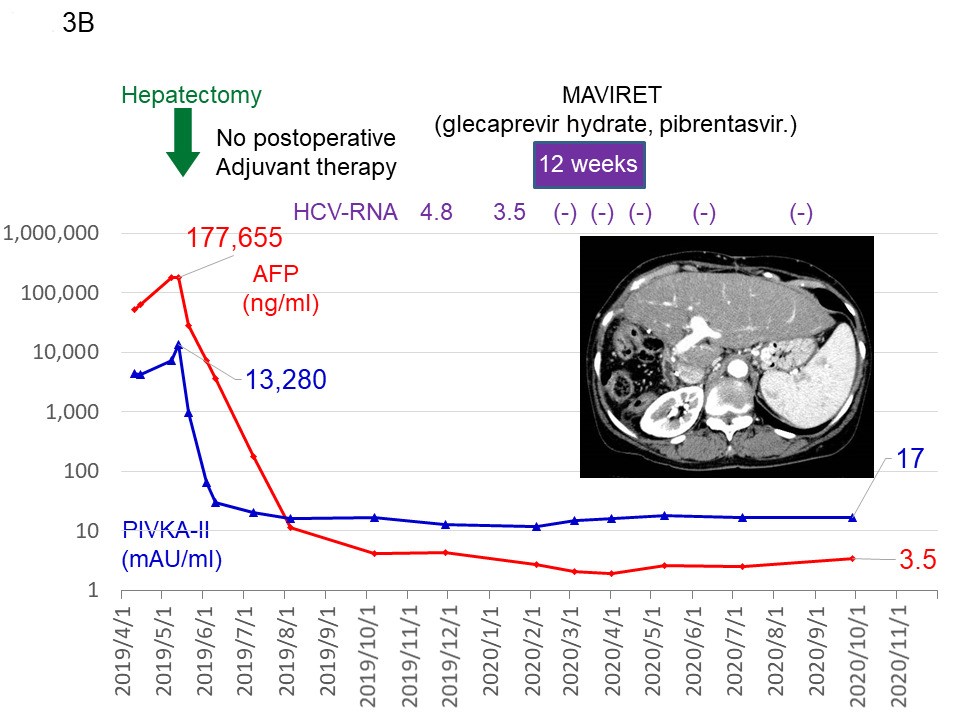
Two patients were treated in our institution [12,13] and the most impressive patient is introduced [12]. The patient had two huge HCCs, 24 cm in maximal diameter, with an excessive arterioportal shunt (A-P shunt) (Figure 1A–1C and Figure 2A). Moreover, the patient had impaired liver function consisting of 2.8 g/dL of serum albumin level (normal range, 4.1–5.1), 2.6 mg of total bilirubin (normal range, 0.4–1.5), and 80.7% of prothrombin activity (normal range, 70%–140%). Indocyanine green retention rate at 15 min (ICG R15) was 24.0% (normal range, ≤10%), and Child–Pugh score was classified as B (7 points). The levels of tumor markers on admission were extremely high; the alpha-fetoprotein level was 1,008,021 (≤ 10 ng/mL) and protein induced by the absence of vitamin K or antagonist- II was 133,298 (≤ 40 mAU/mL). The patient was judged to be completely unresectable. Targeted therapy and TACE with drug-eluting beads was out of indications due to liver dysfunction and A-P shunt, respectively. Therefore, mild conventional TACE using cisplatin lipiodol and gelatin sponge was started [26]. Four times of TACE in combination with lenvatinib therapy was quite effective and provided markedly tumor regression and complete disappearance of major A-P shunt (Figure 1D and Figure 2B). Thus, the liver function had dramatically improved. Serum albumin, total bilirubin, and prothrombin activity was 3.52 g/dL, 0.4 mg/dL, and 111.4%, respectively. The Child–Pugh classification improved to A (6 points). ICG R15 and liver resection rate was estimated as 12.8% and 21.4%, respectively. Therefore, extended right hepatectomy was successfully completed without major postoperative complications. No postoperative adjuvant therapy was performed. All tumor markers normalized within 5 months. MAVIRET (glecaprevir hydrate and pibrentasvir), an antiviral therapy for hepatitis C, was administered for 12 weeks, after which hepatitis C virus- RNA immediately turned negative. Changes in the levels of tumor markers and treatments are summarized in Figure 3A and 3B. Recent enhanced CT showed good liver regeneration and no tumor recurrence (Figure 3B). The condition of the patient was stable with normal levels of tumor markers 27 and 20 months after the initial TACE and hepatectomy, respectively.
Figure 1. Contrast-enhanced computed tomography findings. Axial images of the portal phase on admission (A, B). Coronal images of the portal phase on admission (C) and before conversion surgery (D). Main (arrow) and satellite (arrow head) hepatocellular carcinomas were located in the right liver and in segment 5, respectively. The main tumor size decreased from 24 cm to 15 cm (37.5% reduction in maximal diameter). Reproduced from Beppu, et al. [12].
Figure 2. Digital subtraction angiography. Digital subtraction angiography on the first (A) and the 4th (B) transarterial chemoembolization. A-P shunt (arrows) almost disappeared after lenvatinib administration. Reproduced from Beppu, et al. [12].
Figure 3. Changes in the levels of tumor markers and treatments. The beginnings to the end of 2019 (A) and thereafter until now (B). AFP: Alpha-fetoprotein; PIVKA-II: Protein Induced by the Absence of Vitamin K or antagonist-II; TACE: Transarterial Chemoembolization; EVL: Endoscopic Variceal Ligation. Reproduced from Beppu, et al. [12].
The most impressive phenomena in this conversion surgery after lenvatinib therapy included the complete disappearance of remarkable A-P shunt followed by improvement of liver function. A-P shunt is well known to decrease efficient portal flow. Therefore, the disappearance of the A-P shunt could provide improved liver functional reserve. In addition, lenvatinib is a multiple oral tyrosine kinase inhibitor that targets vascular endothelial growth factor receptors 1–3, fibroblast growth factor receptors 1–4, and platelet-derived growth factor receptor β [4-6]. Therefore, the A-P shunt could be diminished. Consequently, more patients maintained or improved their liver functional reserves when treated with lenvatinib than those treated with sorafenib (79% vs. 61%) [27]. Interestingly, advanced portal hypertension was the only independent predictor of good progression-free survival after lenvatinib therapy limited for patients with albumin bilirubin of grades 2 or 3 [28]. Another prospective cohort study showed that the congestion index (portal venous area/portal venous flow velocity) significantly worsened after lenvatinib therapy, which reflects the pathophysiological hemodynamics of the portal venous system [29]. The direct effect of lenvatinib therapy on A-P shunt is still unclear. Further studies will be required focusing on the hemodynamics of liver blood flow.
In conclusion, lenvatinib therapy is one of the quite beneficial options of conversion therapy for advanced HCC. In addition, novel combination therapies can provide more opportunities for conversion surgery.
Conflict of Interests
Authors declare no Conflict of Interests for this article.
References
2. Naugler WE, Alsina AE, Frenette CT, Rossaro L, Sellers MT. Building the multidisciplinary team for management of patients with hepatocellular carcinoma. Clinical Gastroenterology and Hepatology. 2015 May 1;13(5):827- 35.
3. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. The Lancet. 2018 Mar 31;391(10127):1301-14.
4. Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Medicine. 2018 Jun;7(6):2641-53.
5. Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. Journal of Gastroenterology. 2017 Apr 1;52(4):512-9.
6. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. 2018 Mar 24;391(10126):1163-73.
7. Kudo M. Systemic therapy for hepatocellular carcinoma: Latest Advances. Cancers. 2018 Nov;10(11):412.
8. Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology. 2019;97(5):277-85.
9. Tanaka K, Shimada M, Kudo M. Characteristics of longterm survivors following sorafenib treatment for advanced hepatocellular carcinoma: report of a workshop at the 50th Annual Meeting of the Liver Cancer Study Group of Japan. Oncology. 2014;87(Suppl. 1):104-9.
10. Takeyama H, Beppu T, Higashi T, Kaida T, Arima K, Taki K, et al. Impact of surgical treatment after sorafenib therapy for advanced hepatocellular carcinoma. Surgery Today. 2018 Apr 1;48(4):431-8.
11. Yoshimoto T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Iwahashi S, et al. The outcome of Sorafenib therapy on Unresectable hepatocellular carcinoma: experience of conversion and salvage hepatectomy. Anticancer Research. 2018 Jan 1;38(1):501-7.
12. Sato N, Beppu T, Kinoshita K, Yuki H, Suyama K, Chiyonaga S, et al. Conversion hepatectomy for huge hepatocellular carcinoma with arterioportal shunt after chemoembolization and lenvatinib therapy. Anticancer Research. 2019 Oct 1;39(10):5695-701.
13. Yamamura K, Beppu T, Sato N, Oda E, Kinoshita K, Yuki H, et al. Huge hepatocellular carcinoma with extrahepatic collateral arteries successfully treated by multidisciplinary treatment including laparoscopic devascularization: a case report. Clinical Journal of Gastroenterology. 2020 Nov 12:1-7.
14. Chen X, Zhang Y, Zhang N, Ge Y, Jia W. Lenvatinib combined nivolumab injection followed by extended right hepatectomy is a feasible treatment for patients with massive hepatocellular carcinoma: a case report. OncoTargets and therapy. 2019;12: 7355-9.
15. Matsuki R, Kawai K, Suzuki Y, Kogure M, Nakazato T, Naruge D, et al. Pathological Complete Response in Conversion Hepatectomy Induced by Lenvatinib for Advanced Hepatocellular Carcinoma. Liver Cancer. 2020;9(3):358-60.
16. Tomonari T, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Sogabe M, et al. Conversion therapy for unresectable hepatocellular carcinoma after lenvatinib: three case reports. Medicine. 2020 Oct 16;99(42):e22782.
17. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. InSeminars in Liver Disease 2010 Feb (Vol. 30, No. 01, pp. 052-060).
18. Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Annals of Oncology. 2005 Aug 1;16(8):1311-9.
19. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al . Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. The Lancet. 2018 Mar 24;391(10126):1163-73.
20. Chuma M, Uojima H, Hiraoka A, Kobayashi S, Toyoda H, Tada T, et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: a multicenter analysis. Hepatology Research. 2020 Dec 3.
21. Sho T, Suda G, Ogawa K, Shigesawa T, Suzuki K, Nakamura A, et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatology Research. 2020 Aug;50(8):966-77.
22. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. Journal of Clinical Oncology. 2020 Jul 27.
23. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. The lancet Gastroenterology & Hepatology. 2018 Jun 1;3(6):424-32.
24. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New England Journal of Medicine. 2020 May 14;382(20):1894-905.
25. Dubbelman AC, Rosing H, Nijenhuis C, Huitema AD, Mergui-Roelvink M, Gupta A, et al. Pharmacokinetics and excretion of 14 C-lenvatinib in patients with advanced solid tumors or lymphomas. Investigational New Drugs. 2015 Feb 1;33(1):233-40.
26. Beppu T, Ohara C, Yamaguchi Y, Ichihara T, Yamanaka T, Katafuchi S, et al. A new approach to chemoembolization for unresectable hepatocellular carcinoma using aclarubicin microspheres in combination with cisplatin suspended in iodized oil. Cancer. 1991 Dec 15;68(12):2555-60.
27. Terashima T, Yamashita T, Takata N, Toyama T, Shimakami T, Takatori H, et al. Comparative analysis of liver functional reserve during lenvatinib and sorafenib for advanced hepatocellular carcinoma. Hepatology Research. 2020 Jul 15;50(7):871–84.
28. Maesaka K, Sakamori R, Yamada R, Urabe A, Tahata Y, Oshita M, et al. Therapeutic efficacy of lenvatinib in hepatocellular carcinoma patients with portal hypertension. Hepatology Research. 2020 Sep;50(9):1091- 100.
29. Hidaka H, Uojima H, Nakazawa T, Xue S, Hara Y, Iwasaki S, et al. Portal hemodynamic effects of lenvatinib in patients with advanced hepatocellular carcinoma: a prospective cohort study. Hepatology Research. 2020 Sep 27;50(9):1083–90.