Abstract
This manuscript summarizes recent hypotheses to explain the distribution of regenerative abilities among animals. The hypothesis is based on evolutionary considerations pointing out that while organ or even body regeneration is present in marine animals, limited regeneration or no regeneration is manifested in terrestrial animals. This loss derives from the terrestrial conditions for life, primarily including shortage of water, high UV and ROS exposition. These conditions are incompatible with regeneration after injury, a process that requires formation of soft embryonic-like tissues. While marine animals include variably complex larval forms and metamorphosis in their biological cycles, these characteristics were lost in terrestrial animals that evolved a direct development. Regeneration was lost during land colonization, initiated in the Paleozoic Period. Therefore, developmental gene pathways governing metamorphosis and regeneration in water could no longer be expressed on land. During the water to land transition the changing environment characteristics determined modification in the genomes of marine animals through epigenetic mechanisms that modified developmental gene pathways, including those operating for organ regeneration. Broad regenerative ability is still present in numerous fish and amphibians that live in submerged or amphibious environments and possess larval forms and metamorphosis, while amniotes, including humans rapidly form scars after organ injury.
Keywords
Animals, Human, Healing, Regeneration, Biological evolution
Introduction to Regeneration in Extant Animals and Humans
After extensive injury or even loss of organs or body appendages, different animals recover with variable ability, from a large regeneration to a limited recovery healing to scarring [1–7]. Generally, simple or more complex marine animals (jellyfish, flatworms, starfish, marine annelids, sea squirts, many fishes etc.) or semi-aquatic (some snails and earthworms, various amphibians) can regenerate their damaged tissues or organs broadly with respect to terrestrial animals (insects, spiders, scorpions, reptiles, birds and mammals). The latter instead can only variably heal or forms scars (Figure 1) [8–12]. The simple and common question that from over 300 years comes to human minds is: why some animals can regenerate successfully while others, including humans, cannot. In particular, referring to the failure of organ regeneration in humans, from long time a tentative answer has been that "the highly regenerating animals" possess cell and special molecular mechanisms that we do not have. Therefore, if we learn from their cellular regenerative mechanisms, we can apply this information and improve our own ability to regenerate. However, although also mammals and humans possess most of genes present and utilized in regenerative competent animals [4,8,11,13], their genome and transcriptome are however insufficient to allow a high regeneration. The studies so far conducted have shown that genes activated during development only in part are re-activated during regeneration [14–16]. In fact, while development initiates from a single cell that multiplies and interacts with other cells that are formed in the embryo, instead regeneration initiates from a body made from billions of somatic cells. Therefore, regeneration cannot repeat the same activation of gene sequences and intensity operating in the embryo.
It may be possible that the molecules coded from the genome of various animals and their interactions that determine the ability to regenerate will become known in some future. These molecular and cellular details will eventually disclose how these regenerative competent animals, hydra, planaria, marine worms of different types, starfish, fish and caudate amphibians, can variably but efficiently regenerate organs and tissues after injury (Figure 1). However, the knowledge of developmental genes utilized also for regeneration is still far away from building an integrative and general explanation of differences in regeneration among animals [4,14,15]. When molecular details become known, we may think to use this information for ameliorate our own poor regenerative ability, translating developmental genes involved in regeneration into our body or even integrating these genes in our genome. However, aside from the fundamental molecular details sustaining regeneration, the consideration of the biological evolution of different animals has indicated some reasonable doubts on the feasibility of this application in regenerative medicine. The following discussion is based on previous theoretical studies on the regenerative ability of different animals from a zoological, cellular and molecular point of view, and details can be found in these cited papers [5–7,12,13].
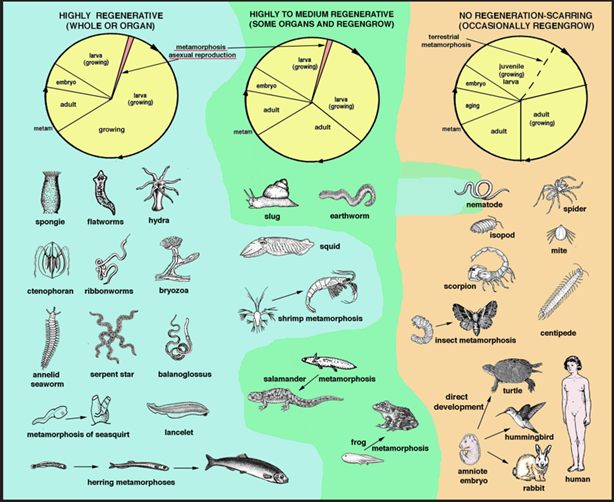
Figure 1. Schematic and generalized representation on the distribution of regeneration among invertebrates and vertebrates. The yellow circles represent main life cycles (not completely valid for all species of these animals) of invertebrates and vertebrates with high, medium-low and low to absent regenerative ability. On the left, highly regenerating animals live in water (blue background) and often present metamorphosis and/or asexual reproduction. Their genomes contain genes for these developmental programs. Crustaceans and cephalopods, living in the sea, have however more limited regenerative ability. In the middle, are shown the animals with some ability to regenerate organs, appendages or even body parts, but that live in freshwater or humid environments (green background). Slug, earthworms, crustaceans, mollusks, fish and amphibians often continue to grow/restructure their tissues during their entire life. On the right, are shown the generalized biological cycle for terrestrial adapted animals (the hatched line only refers to arthropods). Nematodes cannot regenerate because they have a fixed number of cells. Some insects possess terrestrial-adapted larvae without regenerative ability that can regengrow appendages only during molting. For more details see the indicated references [5–7,12,13].
Evolution Explain Animal Changes Including Regeneration
If we reflect on the typical reasoning "why some animals regenerate and we, humans, do not", it appears that we consider simple or complex highly regenerative animals living today in different ecological conditions, as equivalent in terms of general cellular mechanisms or body functions, to non-regenerative animals, including humans. We generally forget or even neglect to consider that each animal species followed a specific evolution and environment that lasted millions of years, giving rise to different animals with their specific biological characteristics, including their regenerative ability. This evolution operated on the genomes of these species during the adaptation to their specific environments and life cycles [1,6,7]. Since life initiated in the sea over three billion years ago and regeneration is common in simple or complex marine animals from at least 1 billion years (protozoa, sponges, jellyfish, etc.), it is likely that regeneration is a basic and ancestral ability of animals, a basal property of cellular life. It is also likely that, for different ecological reasons, and led by epigenetic changes derived from specific environmental adaptations, regeneration was lost in some evolutionary lineages. Since most animals unable to regenerate their organs are terrestrial, it is likely that the later adaptation from the water to the land that occurred during the late Paleozoic Period (300-340 million years ago) is involved in the loss of regeneration in these terrestrial animals, including humans. Therefore, comparing a human with regenerative competent animals "why we can't while they can", is inappropriate from a biological point of view because this interrogation only considers extant animals without their history, namely their environmental-related evolution.
Some fundamental differences between aquatic (mainly marine) animals and the terrestrial are here only listed (see details in [5–7,12,13]). Also, derived from their life in water, aquatic organisms possess biological cycles that include larvae stages and metamorphic transition before becoming adults. This means that their genomes possess genes and developmental programs that determine the transformation from the embryo into a larva, sometimes from a larva to another larval stage, and finally from the last larval stage to the adult body (Figure 1). During these metamorphoses numerous organs or even the entire shape of the animal is changed, with an initial destructive phase of some organs and a following constructive (regenerative) phase of these organs, from larval into adult organs and relative functions [13,15]. Terrestrial adaptation has largely eliminated larval forms and metamorphosis, and consequently also the developmental gene programs involved, often erasing regeneration. Terrestrial adaptation means to resist new conditions that were absent in the marine environment. These mainly include dryness that makes it impossible forming embryonic tissue for regeneration, higher damaging UV exposure, higher ROS species determining a manifest aging, higher microbial load requiring a more sophisticated immune system, higher mechanical and neural muscular requirements to move on grounds under higher gravity, direct development with "solid" larvae for moving on the higher terrestrial gravity.
Based on this reasoning, independently from what molecular developmental biology will instruct us on the mechanisms of regeneration in regenerative competent animals, we cannot neglect that once some animals undertook a certain evolutionary direction during which they erased regeneration, this process was/is hardly reversible. In fact, restituting regeneration would likely determine alteration of other anatomical and physiological functions, with teratogenic or deadly consequences [5]. From the above discussion we can now consider whether regeneration is possible in amniotes, and humans in particular.
Amniote Regeneration Ability
As indicated above, in rare cases few amniotes manifest extensive recovering healing abilities or regengrow (regeneration and somatic growth occurring at the same time, [5–7,12]) to justify the term of regeneration. In fact, most of recovery healing of amniotes (reptiles, birds, and mammals) after an injury or organ loss is scarring [7,9,10,12]. One case of broad but heteromorphic regeneration is represented from numerous lizards that can regenerate the tail, and another case, recently discovered, is represented from some species of rodents of the genus Acomys, the spiny mouse [13,17]. Numerous studies have revealed in both lizards and spiny mice that many tissue and even part of some organs can extensively regenerate after injury or large volumetric losses. The transcriptome analyses for skin, heart, and tail regeneration have determined in these two cases the activation of some of the genes that were utilized in these species during their embryonic development [16,18,19]. These two exceptional examples of broad recovery and regeneration in lizards and rodents indicate that, despite the limitation of healing imposed from the terrestrial conditions, these two types of amniotes were able to evolve alternative developmental gene pathways capable to reactivate some genes previously utilized during development without incurring in incompatible developmental and physiological problems, including death, in their adult bodies. Furthermore, the evolution of high "recovery healing or regeneration" was so well integrated in their life to be a successful biological achievement for survival [5–7,17,18]. Continuing research on these exceptional amniote species and discovering other amniote species with such outstanding healing abilities will be essential to derive potential regenerative therapies for humans.
In conclusion, while molecular biology may eventually explain the mechanism of regeneration in typical simple or complex invertebrate and vertebrate models of regeneration, evolutionary consideration already explains the general picture for why some animals broadly regenerate while others, mainly terrestrial including humans, cannot regenerate. These theoretical considerations based on zoological knowledge, however, need specific molecular information on the developmental gene pathways activated during regeneration. A list of invertebrate and vertebrate models of regeneration representing main phyla, where numerous molecular details are known, is reported in [4,11,13–15,20]. Since one of the principal and immediate obstacles to organ regeneration is the lack of water on land, possible therapies stimulating regeneration may evaluate conducting the treatments for appendages recovery in immersion conditions.
Acknowledgments
Study self-supported with no Institutional support.
Conflict of Interest
The author declares no conflict of interest in the MS.
References
2. Tiozzo S, Copley RR. Reconsidering regeneration in metazoans: an evo-devo approach. Frontiers in Ecology and Evolution. 2015 Jun 23; 3:67.
3. Elchaninov A, Sukhikh G, Fatkhudinov T. Evolution of regeneration in animals: A tangled story. Frontiers in Ecology and Evolution. 2021 Mar 5; 9:621686.
4. Srivastava M. Beyond casual resemblance: rigorous frameworks for comparing regeneration across species. Annual Review of Cell and Developmental Biology. 2021 Oct 6; 37(1):415–40.
5. Alibardi L. Regeneration or scarring derive from specific evolutionary environmental adaptations of the life cycles in different animals. Biology. 2023 May 17; 12(5):733.
6. Alibardi L. Regeneration among animals: An evolutionary hypothesis related to aquatic versus terrestrial environment. Developmental Biology. 2023 Sep 1; 501:74–80.
7. Alibardi L. Progressive modifications during evolution involving epigenetic changes have determined loss of regeneration mainly in terrestrial animals: A hypothesis. Developmental Biology. 2024 Nov 1; 515:169–77.
8. Iismaa SE, Kaidonis X, Nicks AM, Bogush N, Kikuchi K, Naqvi N, et al. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regenerative medicine. 2018 Feb 23; 3(1):6.
9. Maden M. The evolution of regeneration-where does that leave mammals?. The International journal of developmental biology. 2018;62(6-7-8):369–72.
10. Durant F, Whited JL. Finding solutions for fibrosis: understanding the innate mechanisms used by super-regenerator vertebrates to combat scarring. Advanced Science. 2021 Aug;8(15):2100407.
11. Zaraisky AG, Araslanova KR, Shitikov AD, Tereshina MB. Loss of the ability to regenerate body appendages in vertebrates: from side effects of evolutionary innovations to gene loss. Biological Reviews. 2024 Oct;99(5):1868–88.
12. Alibardi L. Regeneration, Regengrow and Tissue Repair in Animals: Evolution Indicates That No Regeneration Occurs in Terrestrial Environments but Only Recovery Healing. Journal of Developmental Biology. 2025; 13(1):2.
13. Alibardi L. Appendage regeneration in anamniotes utilizes genes active during larval‐metamorphic stages that have been lost or altered in amniotes: The case for studying lizard tail regeneration. Journal of morphology. 2020 Nov; 281(11):1358–81.
14. Goldman JA, Poss KD. Gene regulatory programmes of tissue regeneration. Nature Reviews Genetics. 2020 Sep; 21(9):511–25.
15. Warner J, Amiel A, Johnston H, Röttinger E. Regeneration is a partial redeployment of the embryonic gene network. 2020; Hal-03021512. 2020.
16. Nurhidayat L, Benes V, Blom S, Gomes I, Firdausi N, de Bakker MA, et al. Tokay gecko tail regeneration involves temporally collinear expression of HOXC genes and early expression of satellite cell markers. BMC biology. 2025 Jan 8; 23(1): 6.
17. Maden M, Varholick JA. Model systems for regeneration: the spiny mouse, Acomys cahirinus. Development. 2020 Feb 15; 147(4):dev167718.
18. Brant JO, Boatwright JL, Davenport R, Sandoval AG, Maden M, Barbazuk WB. Comparative transcriptomic analysis of dermal wound healing reveals de novo skeletal muscle regeneration in Acomys cahirinus. PloS one. 2019 May 29; 14(5):e0216228.
19. Koopmans T, van Beijnum H, Roovers EF, Tomasso A, Malhotra D, Boeter J, et al. Ischemic tolerance and cardiac repair in the spiny mouse (Acomys). NPJ Regenerative medicine. 2021 Nov 17; 6(1):78.
20. Alibardi L. Speculations on the loss of regeneration derived from developmental modifications during land adaptation in some evolutionary lineages of animals. Acta Zoologica. 2024 Oct; 105(4):387–402.