Thesis
Charcot joint disease is a condition of degradation in bones and joints that is severely debilitating to the foot and ankle structure and function. The breakdown in bony architecture causes predictable foot ulcers, such as at the plantar aspect of the fifth or first metatarsal heads. These ulcers may progress to osteomyelitis, which combined with loss of foot structure, makes below-knee amputation an option, albeit a suboptimal one. The goal of rearfoot reconstruction, therefore, is to rebuild the architecture of the foot so it may once again proceed through the gait cycle with low risk of osteomyelitis.
Introduction
Charcot joint disease refers to the structural breakdown of the joints in the presence of neuropathy [1]. It has multiple pseudonyms but one of the most descriptive names of this condition is “Diabetic Neuroarthropathy.” The first cases studied by Jean-Marie Charcot however were not due to diabetic peripheral neuropathy but syphilis-related peripheral neuropathy [2]. As diabetes mellitus has become more prevalent, Charcot Joint Disease has become synonymous with diabetic peripheral neuropathy. This however should not confound surgeons and clinicians; any condition that causes neuropathy in the lower extremity can cause neuroarthropathy [3]. For example, syringomyelia or lumbar impingement radiculopathy can cause Charcot Joint Disease. The pathophysiology is exactly the same, but the cause of neuropathy can be different.
Nervous Control and Mechanical Function
Sensory, autonomic and sympathetic nervous function impairment reduces the capacity of the foot to adapt against vectors of force from ground during the gait cycle [4]. The loss of protective threshold prevents the normal reflexive feedback the body needs to avoid repetitive injury. In combination, the inability of normal sympathetic tone control causes of demineralization of bone [5]. In summary, the combination of weakened bone and inability to feel plantar pressure is the current prevailing pathophysiological explanation of diabetic neuroarthropathy.
During the regular gait cycle, the plantar posterior heel strikes against the ground with the heel in a varus position. The vector of forces from the ground against the foot, also called ground reactive forces, change direction from the plantar posterior heel to proceed along the lateral column of the foot. The ground reactive force vector proceeds through the cuboid to the fifth metatarsal. The ground reactive force transfers then from “heel strike” to begin the “forefoot loading” phase. The gait cycle proceeds from the lateral column forefoot loading of the foot toward the medial column forefoot loading at the first metatarsal head. The inability of the diabetic foot to withstand these forces during weightbearing causes structural failure [6].
From heel strike to forefoot loading to heel lift, the interplay between the subtalar, talo-navicular, calcaneocuboid and the remaining midfoot-metatarsal joints is complex and interdependent. This allows for the greatest possible adaptation by the foot versus uneven surfaces to prevent loss of balance. These interactions are sequence dependent and any missing step negatively affects all subsequent steps. The presence of various lesions in the foot is going to depend upon where the deficit in the gait cycle occurred [7].
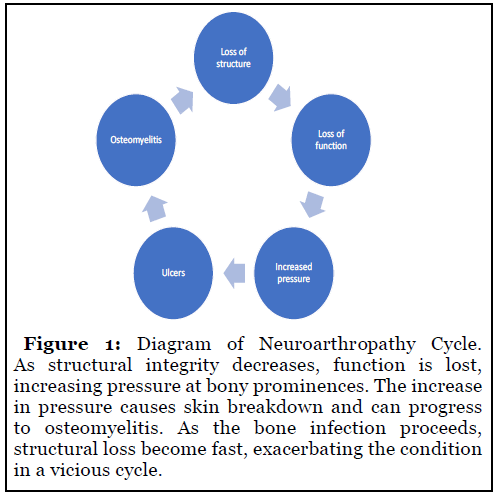
The loss of inability of the foot to withstand ground reactive forces during weightbearing causes structural failure. Total structural failure of the foot causes increased pressure at bony prominences in either the foot or the ankle. Increased pressure leads to ulceration. Eventually, the surface of the ulcer will erode to the depth of bone, at which point there is likely osteomyelitis. Progressive osteomyelitis further reduces the structural integrity of the foot and ankle. This vicious cycle of neuroarthropathy negatively impacts quality of life and severely degrades their capacity to perform regular activities of daily living. The presence of osteomyelitis, structure loss and a myriad of comorbidities such as end-stage renal disease makes successful reconstruction difficult [8]. Although reconstructive surgery in the setting Charcot joint disease is challenging to the patient and the surgeon, the mortality rates after diabetic foot amputations justify the effort [9]. The goal of reconstruction is to return the previous structural rigidity before prolonged cycling of pathological foot mechanics. This is achieved via a combination of osteotomy, osseous fusion, placement of internal hardware beams or a combination of all three to interrupt the pathophysiological cycle of Charcot Joint Disease.
Conservative Treatment
The goal of any reconstructive surgery for treatment of foot and ankle neuroarthropathy is to create a plantigrade foot and limb that can bear weight upon the ground, that can fit inside a shoe or bracing system, that is pain free and has even distribution of ground reactive forces. Before attempting any surgical therapy, conservative treatment must be exhausted. It is imperative that the surgeon set expectations early in the treatment calendar [10]. Patients must understand the difficulty of bone healing when the rest of the host is not idealized. Therefore, it is recommended to start treatment with conservative therapy for the bony deformities.
Conservative treatment begins with appropriate clinical exam and medical history. Neuroarthropathy begins as an acute inflammation in the foot with increased erythema, edema and skin temperature that is often mistaken for cellulitis [11]. Pain may or may not be present. Thermography can aid clinicians in assessing a patient’s perfusion to the affected limb and in narrowing the differential diagnosis [12].
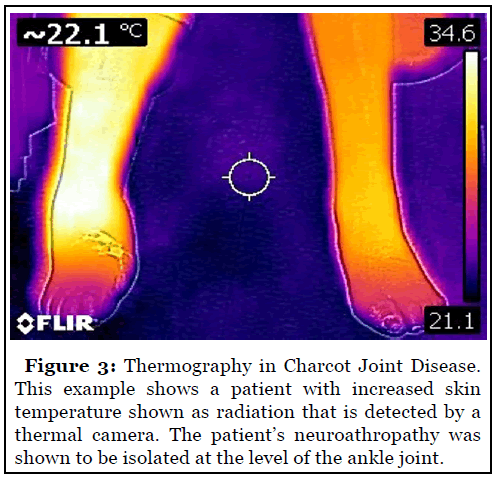
Following the initial visit, imaging studies should be performed. Neuroarthropathy progresses from the acute inflammatory phase to a chronic destructive phase and then to the final remodeling phase. A combination of x-ray, CT and MRI studies are vital for pre-operative planning and intra-operative decision making. Serial X-rays taken over time should be included with any neuroarthropathy patient’s records to evaluate structural change over time. MRI studies can be too sensitive a treatment modality in the setting of acute phases of neuroarthropathy but are good for evaluation of concomitant soft tissue disease [13]. CT is much more valuable for three-dimensional appreciation of foot structure and shows bone pathology X-ray is not sensitive enough to detect.
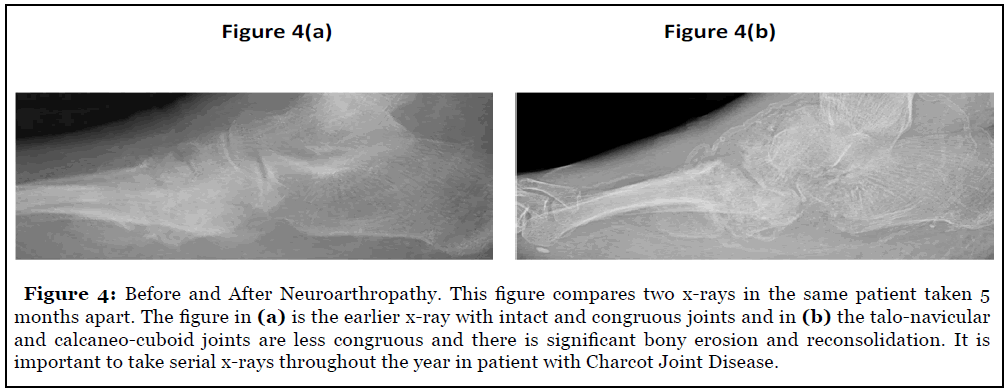
As the bone itself is weakened in Charcot joint disease, off-loading the affected limb is paramount. This can be achieved through crutches, walkers and even wheelchairs as needed. The surgeon should coordinate with physical and occupational therapists to train patients how to ambulate without weightbearing on affected limbs if possible. The choice of casting versus using controlled ankle motion walkers is usually up to the surgeon. Per the literature, the benefit of casting, especially total contact casting technique, is increased patient compliance and satisfaction versus walking boots [14]. It is highly recommended to prevent all weightbearing upon all limbs in the acute inflammatory to prevent as much bone destruction and loss. Care should be taken to avoid surgery until the signs of erythema, edema and elevated skin temperature are resolved. The best time to perform reconstructive surgery, early in the acute phase or later after consolidation, is still an area of research [15].
Pre-Operative Planning
Conservative therapies should be first attempted in arthropathy patients. If patient can keep a functional lower limb that is pain free, able to bear weight, tolerate bracing and does not have a reduction in quality of life or inability to perform activities of daily living, then conservative treatment should be considered a success. The criteria of a successful surgery to reconstruct the foot is exactly the same; a pain-free plantigrade foot that can bear weight, be braced, and allow for activities of daily living. Failure to meet that criteria implies urgent need for reconstruction to prevent the vicious cycle of ulcer, osteomyelitis and bone loss.
The first and most important factor for a successful reconstruction is adequate perfusion to the affected limb. It is not recommended to attempt any surgery before confirmation the patient has sufficient blood supply to the operative limb. Coordination with vascular specialists is important pre-operative planning. The patient’s pulse volume recordings, TcPO2 at the operative site, ankle and toe brachial indices and pulse plethysmography at the toes is highly recommended [16].
The patient’s social structure, including family assistance at home and profession, should be assessed prior to surgery. Charcot reconstruction patients will need assistance during their convalescence and appropriate planning must be undertaken prior to surgery. Often, admittance to a hospital for in-patient care and treatment coordination is recommended, as case management coordinators can aid surgeons in post-operative care.
Surgical Options
The chief goal of reconstructive surgery for neuroarthropathy is to reduce the deformities present in all three-dimensional planes as to create a foot that is plantigrade to the ground and fits within a brace [17]. There are multiple classification schemes, such as by Sanders and Frykberg et al., that list the common zones of structural breakdown [18]. For example, a common area of structural failure is over the calcaneal-cuboid joint. Once the cuboid dislocates from its articulation with the calcaneus, it will form an apex of pressure that increases risk of ulceration and infection. Imaging studies, especially CT imaging with 3D renderings, are vital to appreciate where exactly the rearfoot complex is deformed. The general concept is still the same regardless of the location of the apex of deformity; reduction of deformity in each plane as to create a plantigrade and braceable foot [17]. In the example of a calcaneal-cuboid joint dislocation, the forefoot and midfoot area are typically abducted post-dislocation with the center of rotational axis (CORA) at the that joint. Also, the calcaneus will be plantarflexed due to the force of Achilles tendon at its posterior calcaneus insertion. This malposition of the calcaneus deforms the normal architecture of the subtalar joint, in turn causing the talus to adduct and increasing abductory, plantarflexory and valgus rotational pressure at the forefoot and midfoot. Abduction of the forefoot and midfoot will also increase the amount of the radiographic talo-calcaneal joint angle, known as Kite’s angle on anterior-posterior x-ray view of the foot [19]. In summary, in this hypothetical example, the patient has a flatfoot deformity secondary to a neuroarthropathy-related structural collapse at the calcaneal-cuboid joint. Treatment then is to adduct the forefoot and midfoot, dorsiflex the calcaneus by reducing the effect of the Achilles tendon and internally rotate the foot at the subtalar joint. Percutaneous Achilles tendon lengthening is a powerful procedure itself for reduction of sagittal plane deformity and can be an adjunct technique in multiple settings [20]. The degree of surgical deformity reduction should only be enough to reduce the sagittal, frontal and transverse plane angular abnormalities to perpendicular, and not necessarily to increase medial foot arch height.
The choice of osteotomy technique should be robust enough to address the angular deformities in multiple planes, such as a wedge osteotomy with the apex at the lateral aspect of the foot, and through navicular or cuneiform bones. To increase the amount of frontal plane deformity correction, such as with a valgus forefoot or rearfoot, the plantar surface of the osteotomy can be made wider versus the dorsal surface. This is done by adjusting the position of the osteotome or sagittal saw during the osteotomy. As in an Evans procedure for pes planus reconstruction, this aggressive wedge osteotomy increases the length of the lateral column relative to the medial column.
In the previous example of calcaneal-cuboid joint deformity, the neuroarthropathy structural failure is addressed with concepts from pes planus reconstruction. In the presence of Charcot neuroarthropathy at the subtalar or ankle joints, the technique should be triple arthrodesis to stabilize the calcaneal-cuboid, talonavicular and talo-calcaneal joints [21]. If the ankle joint is also involved, this joint should be fused as well, as in the case of a pantalar joint fusion. As in all joint fusions, the joint preparation in this setting involves atraumatic technique that respects local blood supply, removal of all joint cartilage and fixation to maintain the correction. The amount of angular correction can be adjusted via osteotomy prior to fixation. For example, in the setting of significant ankle joint varus, the apex of the osteotomy can reposition the talus perpendicular to the long axis of the tibia so weightbearing is more efficient.
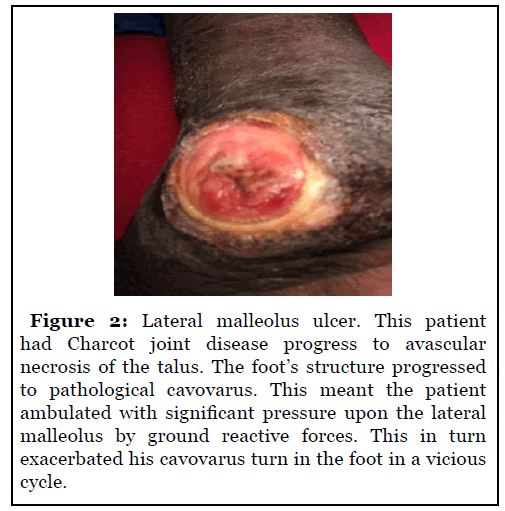
A significant number of Charcot joint disease with the ankle and subtalar joint involvement progress to avascular necrosis and reabsorption of the talus [22]. These patients clinically present with a significant amount of ankle, heel and forefoot varus [23]. As the foot is no longer underneath the tibia during weightbearing, the lateral aspect of the foot and/or the lateral malleolus bear more of the ground reactive force and become the location of ulcers. Since the talus is no longer viable for weightbearing, a strategy of fusion of the calcaneus underneath the tibia can be attempted [24].
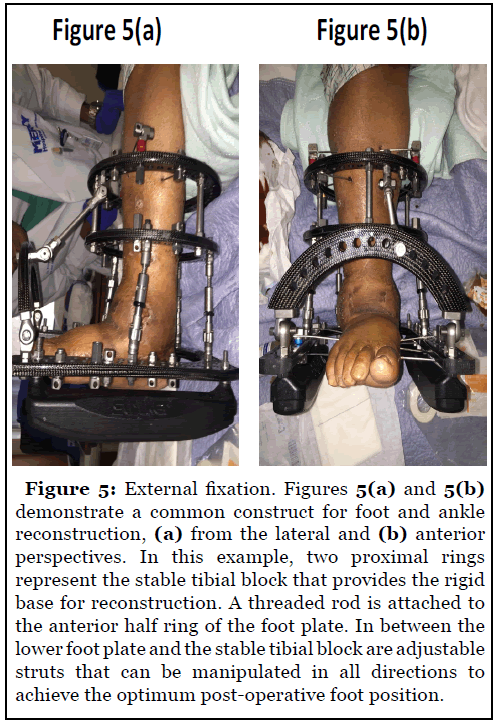
Following appropriate dissection, joint preparation, and deformity correction in the affected joints, the correction must be maintained. The best amongst current strategies is to utilize a “superconstruct” of both internal and external fixation to maintain the correction [25]. This technique was discussed in a landmark paper by Sammarco in 2009.
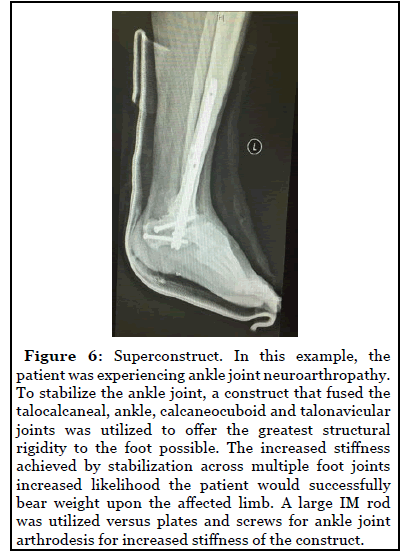
The goal of superconstruct techniques is to fuse multiple joints across an area of neuroarthropathy structural collapse [26]. External fixation can be useful to correct the deformity by transferring all deforming ground reactive forces away from the foot and toward the tibia.
Conclusion
Charcot joint neuroarthropathy is a very challenging condition for both the patient and the surgeon. These patients often have significant comorbidities that have to be concomitantly addressed with the orthopedic factors. Foot function is complex and any abnormality in the normal process of the gait cycle causes cascading negative effects. No bone surgery can survive lack of sufficient perfusion for bone healing. A multi-disciplinary approach that utilizes concepts from areas such as vascular surgery and infectious disease is the best way to bring these patients back to normal function. Conservative treatment has value for these patients because the extent of surgery required, the risk for repeat procedures and the risk of poor outcomes is high. High quality imaging studies are vital to identify the extent of osseous and joint deformity in these patients. The choice of osteotomy is based upon the amount of deformity rotation in all three dimensions. The goal is to create a plantigrade foot that can tolerate bracing and allow for weightbearing with a reduced risk of ulceration. Internal and external fixation combination concepts address all of the factors that deformed the normal architecture of the foot. These patients need more research in this field so the decision making can be easier and great outcomes more easily reproducible.
References
2. Kaynak G, Birsel O, Fatih Güven M, Öğüt T. An overview of the Charcot foot pathophysiology. Diabetic Foot & Ankle. 2013 Jan 1;4(1):21117.
3. Nacir B, Cebeci SA, Cetinkaya E, Karagoz A, Erdem HR. Neuropathic arthropathy progressing with multiple joint involvement in the upper extremity due to syringomyelia and type I Arnold-Chiari malformation. Rheumatology International. 2010 May 1;30(7):979-83.
4. Fernando M, Crowther R, Lazzarini P, Sangla K, Cunningham M, Buttner P, et al. Biomechanical characteristics of peripheral diabetic neuropathy: a systematic review and meta-analysis of findings from the gait cycle, muscle activity and dynamic barefoot plantar pressure. Clinical Biomechanics. 2013 Oct 1;28(8):831- 45.
5. Petrova NL, Shanahan CM. Neuropathy and the vascular-bone axis in diabetes: lessons from Charcot osteoarthropathy. Osteoporosis International. 2014 Apr 1;25(4):1197-207.
6. Sawacha Z, Gabriella G, Cristoferi G, Guiotto A, Avogaro A, Cobelli C. Diabetic gait and posture abnormalities: a biomechanical investigation through three dimensional gait analysis. Clinical Biomechanics. 2009 Nov 1;24(9):722-8.
7. Alam U, Riley DR, Jugdey RS, Azmi S, Rajbhandari S, D’Août K, et al. Diabetic neuropathy and gait: a review. Diabetes Therapy. 2017 Dec 1;8(6):1253-64.
8. Jeyaraman K, Berhane T, Hamilton M, Chandra AP, Falhammar H. Mortality in patients with diabetic foot ulcer: a retrospective study of 513 cases from a single Centre in the Northern Territory of Australia. BMC Endocrine Disorders. 2019 Dec 1;19(1):1.
9. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. Journal of Foot and Ankle Research. 2020 Dec;13(1):1-4.
10. Eschler A, Gradl G, Wussow A, Mittlmeier T. Late corrective arthrodesis in nonplantigrade diabetic Charcot midfoot disease is associated with high complication and reoperation rates. Journal of diabetes research. 2015;2015.
11. Thewjitcharoen Y, Sripatpong J, Parksook W, Krittiyawong S, Porramatikul S, Srikummoon T, et al. Salient features and outcomes of Charcot foot–An oftenoverlooked diabetic complication: A 17-year-experience at a diabetic center in Bangkok. Journal of Clinical & Translational Endocrinology. 2018 Mar 1;11:1-6.
12. Xue EY, Chandler LK, Viviano SL, Keith JD. Use of FLIR ONE smartphone thermography in burn wound assessment. Annals of Plastic Surgery. 2018 Apr 1;80(4):S236-8.
13. Thorning C, Gedroyc WM, Tyler PA, Dick EA, Hui E, Valabhji J. Midfoot and hindfoot bone marrow edema identified by magnetic resonance imaging in feet of subjects with diabetes and neuropathic ulceration is common but of unknown clinical significance. Diabetes Care. 2010 Jul 1;33(7):1602-3.
14. Health Quality Ontario. Fibreglass total contact casting, removable cast walkers, and irremovable cast walkers to treat diabetic neuropathic foot ulcers: a health technology assessment. Ontario Health Technology Assessment Series. 2017;17(12):1.
15. Schneekloth BJ, Lowery NJ, Wukich DK. Charcot neuroarthropathy in patients with diabetes: an updated systematic review of surgical management. The Journal of Foot and Ankle Surgery. 2016 May 1;55(3):586-90.
16. Rajagopalan C, Viswanathan V, Rajsekar S, Selvaraj B, Daniel L. Diabetic foot ulcers—comparison of performance of ankle-brachial index and transcutaneous partial oxygen pressure in predicting outcome. International Journal of Diabetes in Developing Countries. 2018 Apr 1;38(2):179-84.
17. Mittlmeier T, Klaue K, Haar P, Beck M. Should one consider primary surgical reconstruction in Charcot arthropathy of the feet?. Clinical Orthopaedics and Related Research®. 2010 Apr 1;468(4):1002-11.
18. Rosskopf AB, Loupatatzis C, Pfirrmann CW, Böni T, Berli MC. The Charcot foot: a pictorial review. Insights into imaging. 2019 Dec 1;10(1):77.
19. Wukich DK, Raspovic KM, Hobizal KB, Rosario B. Radiographic analysis of diabetic midfoot Charcot neuroarthropathy with and without midfoot ulceration. Foot & Ankle International. 2014 Nov;35(11):1108-15.
20. Ramanujam CL, Zgonis T. Surgical Correction of the Achilles Tendon for Diabetic Foot Ulcerations and Charcot Neuroarthropathy. Clinics in Podiatric Medicine and Surgery. 2017 Apr 1;34(2):275-80.
21. Schade VL, Andersen CA. A literature-based guide to the conservative and surgical management of the acute Charcot foot and ankle. Diabetic Foot & Ankle. 2015 Jan 1;6(1):26627.
22. Stapleton JJ, Zgonis T. Concomitant osteomyelitis and avascular necrosis of the talus treated with talectomy and tibiocalcaneal arthrodesis. Clinics in Podiatric Medicine and Surgery. 2013 Apr 1;30(2):251-6.
23. Aikawa T, Watanabe K, Matsubara H, Nomura I, Tsuchiya H. Tibiocalcaneal fusion for Charcot ankle with severe talar body loss: case report and a review of the surgical literature. The Journal of Foot and Ankle Surgery. 2016 Mar 1;55(2):247-51.
24. Wukich DK, Raspovic KM, Hobizal KB, Sadoskas D. Surgical management of Charcot neuroarthropathy of the ankle and hindfoot in patients with diabetes. Diabetes/ Metabolism Research and Reviews. 2016 Jan;32:292-6.
25. Alrashidi Y, Hügle T, Wiewiorski M, Herrera-Perez M, Valderrabano V. Surgical treatment options for the diabetic Charcot midfoot deformity. Clinics in Podiatric Medicine and Surgery. 2017 Jan 1;34(1):43-51.
26. Sammarco VJ. Superconstructs in the treatment of Charcot foot deformity: plantar plating, locked plating, and axial screw fixation. Foot and Ankle Clinics. 2009 Sep 1;14(3):393-407.
