Abstract
Imaging flow cytometry (IFC) is a form of high-throughput microscopy which combines the advantages of fluorescent microscopy with the quantitative capabilities of traditional flow cytometry. This platform has recently been standardized for quantitatively analyzing the cellular uptake of extracellular vesicles (EVs), which are nanosized lipid bilayer-bound vesicles that are naturally secreted from most cell types as a communication mechanism to deliver proteins, lipids, and genetic material. Due to their innate characteristics and low immunogenicity and toxicity, EVs are used as therapeutic delivery shuttles when loaded with a cargo. However, there are limited in vitro systems with quantitative methods for use in selecting optimal EV source(s) or how loaded cargo and other modifications alter cellular uptake efficiency and target specificity. Here we further explore IFC as a platform for characterizing cellular uptake of neural stem cell-derived extracellular vesicles (NSCEVs) by neurological disease-relevant in vitro cell systems, including human mature neurons and a neurovascular cell line mimicking the blood brain barrier (BBB) which prevents the entry of majority of therapeutics into the central nervous system. We found that NSCEVs are efficiently internalized by both neural cells in vitro. The quantified results from IFC suggest that NSCEVs were internalized at a higher quantity by neural cells comparing to HEK293T cells. These data support the use of NSCEVs as a promising therapeutic shuttle to overcome the BBB and enhance the drug delivery and target efficiency for neurological disorders. The IFC-based method we developed to quantitatively characterize the cellular uptake and specificity of nanodelivery shuttles in a high throughput manner will help the selection and optimization of EVs for specific diseases and facilitate EV-based therapeutics.
Keywords
VExtracellular vesicles, Cellular uptake, Neurological disorders, Imaging flow cytometry, Therapeutic delivery
Introduction
Nanomedicine, which includes nanoparticles and other nanomaterials for diagnosis and therapeutic delivery to treat numerous diseases, is making advances [1]. Utilizing engineered nanomaterials as delivery shuttles for therapeutics provides an opportunity to increase targeting specificity for treatments of certain disorders, as well as provide intrinsic benefits to cell systems of interest [2,3]. However, it is important to understand the functional properties of materials in pre-clinical studies. Proper selection of nanomaterials could be enhanced by recent studies in the field of cellular uptake of nanomaterials.
The study of EVs is an expanding field within nanomedicine and are one such example of nanomaterials as a drug delivery shuttle. EVs are lipid-bound vesicles ranging in size from 40-1000 nm that mediate intercellular communication through shuttling nucleic acids, protein and lipids between cells. Once thought to be the secreted waste of cells, EVs have been engineered as drug delivery vectors due to multiple intrinsic benefits such as biocompatibility of genetic materials, therapeutic potentials, low immunogenicity and toxicity, ability to cross bio-barriers and for repeated dosing, various routes of administration [4-6]. Previous work from our group and others indicates that the therapeutic potential and targeting specificity of EVs highly depend on the origin cellular sources. The BBB penetration and neuroprotective properties of neural stem cell-derived EVs (NSCEVs) make them promising drug delivery vectors for devastating neurological disorders without effective treatment mainly due to the complex pathogenesis and the poor drug delivery efficiency to disease-relevant cells in the central nervous system (CNS). To optimize EV-based therapy, it is imperative to develop an in vitro platform to screen for the best fit donor cells and validate the delivery and targeting efficiency of EVs to disease-relevant cells. Recently, a highthroughput platform utilizing imaging flow cytometry (IFC), known as the ImageStreamX MKII, was optimized by our group to measure kinetic extracellular vesicle (EV) uptake and internalization features for in vitro cell systems and provides the opportunity for the comparison of therapeutic delivery shuttles in disease relevant cells [7]. This platform can assist researchers and clinicians in selecting a more efficient and beneficial mode of delivering therapeutics for specific pathological disorders.
In this study, NSCEVs were isolated, fluorescently labeled, and co-cultured with recipient cell lines including HEK293Ts, human pluripotent stem cell derived neurons and neurovascular endothelial cells with the goal of validating the IFC-based platform we established before in evaluating the targeting specificity of EVs to neurological disorder-relevant cells in vitro. NSCEVs are specifically of interest due to their anti-inflammatory, neural regeneration and neuroprotective properties which improve recovery post-stroke and traumatic brain injury by decreasing lesion size, mitigating inflammation and swelling, and increasing motor and behavioral activities in murine and porcine disease models [8-11]. Among the recipient cells, neurovascular endothelial cells are initially encountered by EVs as they cross the blood brain barrier (BBB), a significant barrier limiting the penetration of drugs and complicating the treatment of CNS disorders. The human mature neurons represent target cells of therapeutics for neurological disorders. Here, we provide data quantifying the process of NSCEV internalization into relevant neural cells using the established and standardized ImageStreamX MKII IFC platform. This IFC-based analysis will assist in the selection and pre-validation of nanodelivery shuttles targeting specific diseases.
Materials and Methods
Cell culture
Human embryonic kidney cells (HEK293T) were purchased from ATCC and cultured in DMEM containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/ mL streptomycin. The blood brain barrier hCMEC/D3 neurovascular cell line was purchased from Millipore Sigma (Massachusetts, US) and cultured in EndoGROMV Complete Culture Media. Human mature neurons (Neuronets) were acquired from ArunA Biomedical (Athens, GA, US) and cultured in AB2 basal neural media containing 2% neural media supplement, 2 mM L-gluatmine, 100 U/mL penicillin, and leukemia inhibitory factor. HEK293T cells, human mature neurons, and neurovascular cells were all cultured under standard conditions at 37°C, 5% CO2 prior to extracellular vesicle uptake assays.
EV isolation/acquirement and labeling
Neural stem cell-derived extracellular vesicles were obtained from ArunA Biomedical post-isolation via filter ultracentrifugation. Concentration and size distributions were measured on Nanosight NS300 by the manufacturer’s protocol (Malvern, UK). To fluorescently label EVs, carboxyfluorescein succinimidyl ester (CFSE) was added to 1 mL of isolated NSCEVs at a concentration of 5 mM and set to incubate for 2 hrs at 37°C. The reaction was stopped with 1 mL 0.1% BSA solution and subsequently washed via ultrafiltration using a 100-kDa regenerated cellulose Amicon centrifugal filter units. EVs were then concentrated via ultracentrifugation at 100,000 ×G for 1 hr and resuspended in 1 mL PBS -/-.
Cellular uptake assays
Recipient HEK293T, mature neurons, and neurovascular endothelial cells were seeded at 60% confluency in a 6-well plate for 24 hrs under standard culture conditions at 37°C. Standard media were changed to fetal bovine serum-free (FBS−) media prior to extracellular vesicle co-culture where applicable. CFSE-labeled extracellular vesicles were administered to cells and co-cultured for 4 hrs. After co-culture, cells were resuspended in 0.05% trypsin and concentrated to approximately one million cells per 50 μL for flow cytometry. 37°C is the standard for EV uptake experiments in our assays as it has been the standard used for both cell culture and in vitro EV uptake platforms [7].
ImageStream
Acquisition was performed on the ImageStreamX Mark II Imaging Flow Cytometer (Luminex Corporation, Seattle, Washington) using the INSPIRE software. A minimum of 5000–10,000 cell events were acquired. Each biological sample was replicated in three technical replicate wells and individually acquired on the ISx. Bright field images were collected on channel one and side scatter (785 nm) on channel six. CFSE was excited by 488 nm argon laser at 150 mW, and fluorescence was collected on channel two (480-560 nm). A magnification of 60× was used on every sample along with a low acquisition rate for high sensitivity. Three parameters standardized in our previous study were used to quantify the uptake of EVs in these recipient cell lines: spot count, maximum pixel count, and mean fluorescent intensity [7].
Statistics
Statistical analyses were performed on all quantitative data using GraphPad Prism 9.1.1 (San Diego, California). All data was analyzed in triplicates and presented as mean ± standard error of the mean (SEM). Statistical significance was determined using a one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test compared with controls when appropriate. p<0.05 was considered significant.
Results
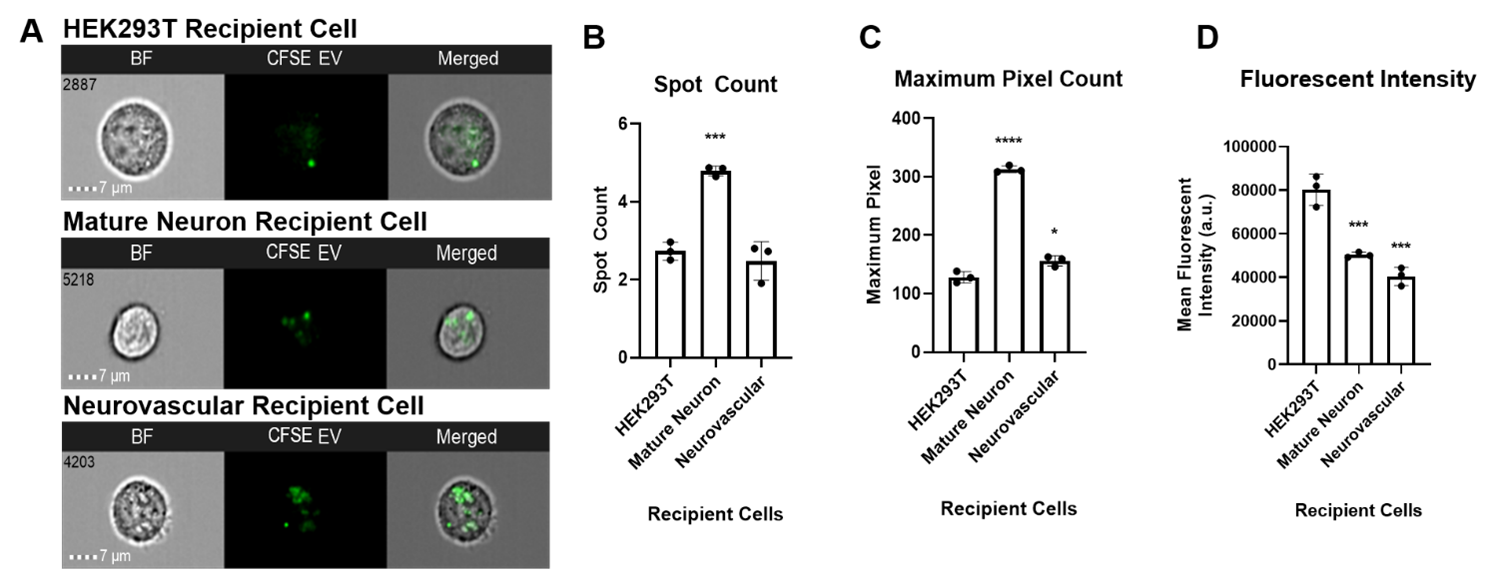
Active uptake of neural stem cell-derived extracellular vesicles by disease-relevant cells
To determine internalization efficiency of NSCEVs by recipient cells, NSCEVs were labeled with CFSE and cocultured with recipient cell lines to measure their uptake in disease-relevant cells, including human mature neurons and human neurovascular cells with HEK293T cells as a control (Figure 1A). Compared to HEK293T cells, human mature neurons had a significantly higher spot count and maximum pixel count (p=0.0004, p<0.0001) (Figures 1B and 1C); neurovascular cells had a significantly higher maximum pixel count (p=0.0112) (Figure 1C). However, HEK293T cells showed a significantly higher mean fluorescent intensity than human mature neurons (p=0.0005) and neurovascular cells (p=0.0001) (Figure 1D). These results suggest that NSCEVs are efficiently taken up by neural stem cells in vitro and NSCEVs could serve as a promising therapeutic delivery shuttle for neurological disorders.
Discussion
The kinetic and selective uptake of extracellular vesicles was elucidated by our group in a recent study to show the influence of EV uptake efficiency by EV dosage and time of co-culture of EVs with recipient cells [7]. The use of IFC to quantify cellular uptake efficiency and selectivity offers a standardized method to compare various nanomaterials as therapeutic shuttles to aid in the selection and prevalidation of nanodelivery shuttles targeting specific cells. As neurological disorders and brain tumors present significant challenges for the delivery of therapeutics due to obstacles like the BBB, we addressed the use of NSCEVs as a potential delivery shuttle in disease-relevant cell systems, including mature neurons and a neurovascular cell line which are part of the BBB.
Imaging flow cytometry addresses the limitations of traditional flow cytometry and other low-throughput methods of microscopy. IFC combines fluorescent imaging with high-throughput quantitative capabilities of traditional flow cytometry. Optimization of the platform will advance drug nanodelivery technologies [12]. EVs in our study were labeled with CFSE. In previous studies, it has been determined the lipophilic dyes can give rise to artefacts and background fluorescence [13]. CFSE and the maleimide-thiol coupling of dyes have been used as alternatives to these dyes to remove the risk of artefacts [13,14]. Further optimization for fluorescent tagging, such as the use of GFP markers, would eliminate the potential risk for artefacts and background noise in IFC microscopy to provide more accurate fluorescent intensity and pixel count calculations.
We chose to collect data with specific features in the software with this platform, including fluorescent intensity, maximum pixel count, and spot count based on our previous study. Fluorescent intensity measures the relative fluorescence between cells and within different regions of the same cell, whereas maximum pixel count offers more sensitive readouts for identifying true positive events. Spot count is a measure of connected fluorescent components within an image and provides information on EV internalization in this study. The higher fluorescent intensity seen in HEK293Ts could result from their larger size and transformed qualities in comparison to the neural recipient cells, while the higher maximum pixel and spot counts in mature neurons suggests greater true positive events of EV internalization.
With this platform, the ability to compare potential therapeutic shuttles within more accessible in vitro cells will aid in the selection of the best fit drug delivery vectors. We have shown data on the internalization of NSCEVs by CNS neural cell lines as an extension on the research performed by Jurgielewicz et al., which developed the standardized use of IFC for the study of EV kinetics and selectivity [7]. The application of IFC assays in this study set an example for efficiency evaluation of the drug delivery vectors for specific diseases, which can also be used for the comparison of different nanomaterials as therapeutic shuttles.
References
2. Kim BY, Rutka JT, Chan WC. Nanomedicine. New England Journal of Medicine. 2010 Dec 16; 363(25):2434-43.
3. Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems.Annual Review of Biomedical Engineering. 2012 Aug 15; 14:1-6.
4. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology. 2013 Feb 18; 200(4):373-83.
5. Reed MB, Wang R, Shillington AM, Clapp JD, Lange JE (2007)The relationship between alcohol use and cigarette smoking in a sample of undergraduate college students. Addictive Behaviors 32: 449-464.
6. Zaborowski MP, Balaj L, Breakefield XO, Lai CP. Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience. 2015 Aug 1; 65(8):783-97.
7. Jurgielewicz BJ, Yao Y, Stice SL. Kinetics and specificity of HEK293T extracellular vesicle uptake using imaging flow cytometry. Nanoscale Research Letters. 2020 Dec; 15(1):1-1.
8. Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, et al. Human neural stem cell extracellular vesicles improve recovery in a porcine model of ischemic stroke. Stroke. 2018 May; 49(5):1248-56.
9. Rong Y, Liu W, Lv C, Wang J, Luo Y, Jiang D, et al. Neural stem cell small extracellular vesicle-based delivery of 14-3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1. Aging (Albany NY). 2019 Sep 30; 11(18):7723.
10. Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Translational Stroke Research. 2018 Oct; 9(5):530-9.
11. Sun MK, Passaro AP, Latchoumane CF, Spellicy SE, Bowler M, Goeden M, et al. Extracellular vesicles mediate neuroprotection and functional recovery after traumatic brain injury. Journal of Neurotrauma. 2020 Jun 1; 37(11):1358-69.
12. Barteneva NS, Fasler-Kan E, Vorobjev IA. Imaging flow cytometry: coping with heterogeneity in biological systems. Journal of Histochemistry & Cytochemistry. 2012 Oct; 60(10):723-33.
13. Roberts-Dalton HD, Cocks A, Falcon-Perez JM, Sayers EJ, Webber JP, Watson P, et al. Fluorescence labelling of extracellular vesicles using a novel thiol-based strategy for quantitative analysis of cellular delivery and intracellular traffic. Nanoscale. 2017; 9(36):13693-706.
14. Russell AE, Sneider A, Witwer KW, Bergese P, Bhattacharyya SN, Cocks A, et al. Biological membranes in EV biogenesis, stability, uptake, and cargo transfer: an ISEV position paper arising from the ISEV membranes and EVs workshop. Journal of Extracellular Vesicles. 2019 Dec 1; 8(1):1684862.