Abstract
This case report considers an iatrogenic pseudoaneurysm (PSA), a rare event occurring in fewer than 2% of patients, after a core-needle biopsy of the breast in a 46-year-old with stage IIIC triple negative invasive breast carcinoma on preoperative chemoimmunotherapy with the PD-1 inhibitor pembrolizumab. A post-biopsy MRI (magnetic resonance imaging) demonstrated a well-defined, contrast-enhancing lesion with pulsatile flow consistent with PSA. Multidisciplinary discussions addressed PSA management and timing of immunotherapy, given the potential association of PD-1/PD-L1 inhibitors with the progression of vascular anomalies. Although breast PSA is uncommon, this complication warrants investigation in light of the increasing application of immunotherapies, which may mediate vascular inflammation.
Keywords
Pseudoaneurysm, Breast, Ultrasound, Pembrolizumab, Biopsy
Introduction
Pseudoaneurysm (PSA) of the breast is a rare entity, with only 24 cases reported in the literature. While existing literature indicates that iatrogenic PSA after diagnostic or therapeutic interventions occurs in <2% of patients [1], incidence rates after breast-specific procedures are unknown. Risk factors for PSA development include advanced age, female sex, underlying atherosclerosis, pregnancy, lactation, the use of anticoagulant or antiplatelet therapy, cancer, surgery, or percutaneous procedures, especially those requiring large-gauge needles and/or multiple puncture attempts during CNB [2]. Pathogenesis involves arterial wall disruption, which facilitates blood leaking into the extravascular space. Although this leak is initially contained by perivascular tissue, it later forms a structurally weak wall composed of fibrin and platelets. The tenuous fibrin and platelet barrier is at risk for rupture [1].
Immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway, such as pembrolizumab, are associated with hematologic and vascular complications [3]. Reported adverse vascular events include venous thromboembolism, arterial thrombosis, vasculitis, aneurysm formation, and hemorrhagic events [4,5]. Although direct causality with PSA formation remains unproven, proposed mechanisms involve dysregulation of vascular integrity and coagulation pathways during PD-1/PD-L1 inhibition [3,6]. PD-1 is broadly expressed on hematopoietic and non-hematopoietic cells, while its ligand PD-L1 is found on vascular endothelial cells and on certain cancer cells. The expression of PD-L1 on vascular endothelial cells has been shown to be a regulator of vasoconstriction and vasodilation, vessel permeability, and platelet aggregation [7]. Experimental models have shown that, in an inflamed state, loss of PD-L1 can lead to compromised vascular integrity due to enhanced CD8+ T cell-mediated attacks [8].
Here, we present a case report of a breast PSA following CNB in a patient undergoing chemoimmunotherapy with PD-1 blockade (KEYNOTE-522 regimen) for triple-negative breast cancer. Given that preclinical studies suggest that immune checkpoints, namely PD-1, may play a role in the progression of vascular anomalies [9], this case report highlights PSA as a salient consideration in oncology patients receiving PD-1/PD-L1 inhibitors (e.g., pembrolizumab). Oncology patients who experience PSA are at risk for hemorrhage and thrombosis, may require anticoagulation, chemotherapy adjustments, delays in surgical management, and additional imaging. The purpose of this case report is to highlight these and other challenges of breast PSA in patients undergoing immune checkpoint therapy and to guide the timing of appropriate therapies.
Diagnosis
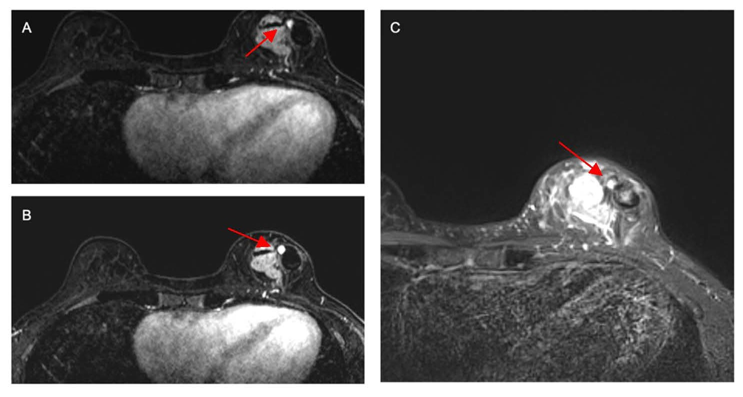
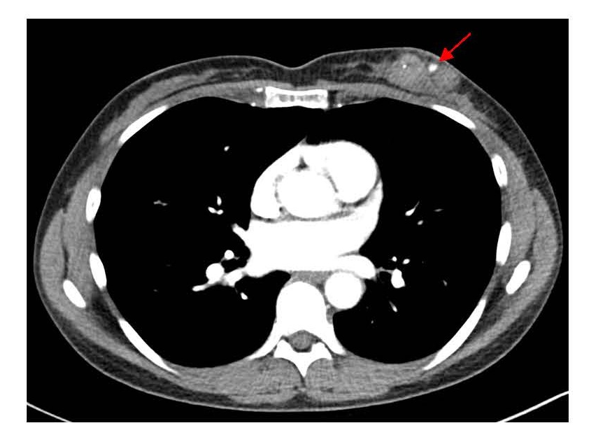
A 46-year-old woman with a past medical history of back pain, hypercholesterolemia, hypertension, migraines, and heart palpitations received a bilateral diagnostic mammography after self-palpating a left breast mass in January 2025. A new and suspicious 2–3 cm mass was visualized in the lower inner quadrant of the left breast, which corresponded to the palpable finding. Breast ultrasound confirmed the 2.6 cm irregular mass at the 7:00 position of the left breast, 2 cm from the nipple, and identified a left axillary lymph node with cortical thickening and hilar effacement suspicious for metastatic involvement. An ultrasound-guided CNB was performed 3 weeks after the patient initially palpated her breast mass. This was performed with three passes of a 9-gauge needle for histologic evaluation. Pathology demonstrated grade 3 invasive ductal carcinoma, triple-negative subtype (Estrogen Receptor (ER) 0%, Progesterone Receptor (PR) 0%, HER2- (IHC 1+; FISH ratio 1.2; HER2/cell 2.7), Ki-67 high, CK-19+, E-cadherin+). Further workup to delineate extent of disease included a breast MRI (magnetic resonance imaging), performed 1 week after her CNB, which estimated the biopsy-proven malignancy to be 4 cm and revealed multiple abnormal level 1 and 2 lymph nodes, and the presence of a PSA (Figure 1). Staging scans, including computerized tomography (CT) of the chest, abdomen, and pelvis (Figure 2), along with a bone scan, showed no evidence of distant metastatic disease. Preoperative chemoimmunotherapy with pembrolizumab per the KEYNOTE-522 regimen was recommended with breast ultrasound planned at 6 weeks to evaluate for PSA progression.
Figure 1. Axial T1-weighted contrast-enhanced subtracted breast MRI (A, B, and C) demonstrating medial enhancing left breast cancer,adjacent non-enhancing hematoma laterally, and central 6×6×5 mm round, homogeneously enhancing PSA. Key findings show a narrowneckedcommunication and signal intensity correlating to a stable hematoma within the PSA. MRI: Magnetic Resonance Imaging; PSA:Pseudoaneurysm.
Figure 2. Axial contrast-enhanced chest CT showing enhancement with the left breast PSA. Focal hyper-density within the known carcinomamedial to the PSA corresponds with the biopsy marker. Key findings include outpouching of contrast from connected parented vessel and clear delineation of surrounding structures. CT: Computerized Tomography; PSA: Pseudoaneurysm.
Multidisciplinary discussions ensued regarding the appropriate timing of neoadjuvant chemoimmunotherapy in the context of the newly diagnosed PSA; management decisions carefully weighed the urgency of initiating treatment against the procedural risks and potential delays associated with embolization. Prompt embolization with minimal disruption to systemic therapy would have been a reasonable approach to reduce hemorrhagic risk; however, there were concerns about the ability to arrange this in a timely manner with minimal disruption to the treatment schedule. Therefore, a follow-up ultrasound of the left breast was performed to assess for PSA stability and to guide course of action prior to the initiation of therapy. No internal vascularity was noted on this ultrasound, suggesting complete thrombosis of the PSA. Given the apparent stability of the PSA on imaging and the absence of high-risk features, the multidisciplinary team elected for continued observation. A six-week follow-up ultrasound was scheduled to monitor for thrombus stability, revascularization, and enlargement. Had ultrasound evaluation been insufficient, MRI would have been pursued to ensure comprehensive characterization and surveillance.
This approach prioritized the timely initiation of dose-dense chemotherapy, critical for optimizing oncologic outcomes, while maintaining vigilant monitoring for vascular complications. Should there have been evidence of PSA progression or imminent rupture, prompt embolization would have been advocated despite the potential impact on treatment timelines.
Discussion
Breast PSA is an uncommon yet clinically significant complication, particularly in the context of oncologic treatments that may intensify the risk for rupture. As in our case, asymptomatic PSA may be detected incidentally during imaging obtained for other conditions or on follow-up imaging after a benign biopsy. Ultrasound is the imaging modality of choice for PSA diagnosis; it is readily available in resource-limited settings, involves no ionizing radiation, and has high specificity, with Color and Spectral Doppler showing characteristic ‘yin-yang’ sign and ‘to-and-fro’ pattern, respectively [9]. The differential diagnosis for breast PSA includes hematoma, abscess, oil cyst, and true aneurysm, which may appear similar on grayscale imaging but lack the turbulent arterial waveform seen in PSA [10]. The pathogenesis is typically iatrogenic [1]. Patient-level factors that may have increased risk for PSA included female sex and the technical aspects of the biopsy procedure itself: a large-gauge needle and multiple puncture attempts during CNB. Furthermore, the planned treatment with pembrolizumab introduced an additional concern given the theoretical association between PD-1 blockade and the progression of vascular anomalies, and evidence for the role of immune checkpoint inhibition in vascular inflammation and endothelial dysfunction from preclinical studies [3,6,11,12]. Thus, the combination of procedural vascular injury and the pro-inflammatory effects of chemoimmunotherapy for TNBC may increase the risk of PSA progression or rupture, highlighting the importance of careful multidisciplinary planning.
Treatment strategies for PSA include observation for stable, asymptomatic cases; or ultrasound-guided thrombin injection; or embolization for enlarged or symptomatic PSA [13]. The variable natural history of PSA complicates the management of asymptomatic PSA. As spontaneous thrombosis may occur, observation for small, asymptomatic PSA may be appropriate [14]. PSA stability or thrombosis may need to be documented before initiating or continuing immunotherapy. Patients should be educated with respect to signs and symptoms of rupture. However, since the epidemiology and likelihood of progression or complication for breast-specific PSA remain poorly characterized, its occurrence in oncology patients, particularly those receiving immunotherapy, warrants further investigation. Additionally, further research is needed to understand the true risk of immune-mediated vascular injury in PD-1 blockade to determine whether PSA instability or progression warrants modification of systemic therapy.
Although the rarity of PSA precludes our ability to accurately assess the rate at which downstream sequelae occur, the presence of PSA in this setting has meaningful implications for both systemic and locoregional treatment planning [7,11,15,16]. Active vascular lesions pose a risk for hemorrhagic complications during neoadjuvant systemic therapy [17], especially when these regimens include agents that modulate immune and inflammatory responses [5]. In particular, pembrolizumab, a PD-1 inhibitor, was approved for use as part of a preoperative chemoimmunotherapy regimen for patients with stage II-III TNBC [18]. The improvement in pathologic complete response rate and survival among patients with TNBC receiving the KEYNOTE-522 regimen compared to those treated with standard cytotoxic chemotherapy has garnered interest in expanding the use of PD-1/PDL-1 inhibitors in breast cancer, including KEYNOTE-756, which considers a role for pembrolizumab in patients with estrogen responsive breast tumors [13]. As PD-1/PD-L1 engagement normally dampens cytotoxic CD8+ T cell responses, loss of this checkpoint can lead to unchecked T cell activation, increased inflammatory cytokine production, and direct endothelial damage [8,15,16]. Its inhibition has been associated with increased vascular inflammation and aneurysm progression in animal models [18]. The increased use of these medications advocates for improved understanding of how comorbid and adverse events such as PSA impact multidisciplinary treatment decisions, especially in light of preclinical studies that have implicated the PD-1 pathway not only in immune regulation but also in vascular homeostasis [11].
While the association between immune checkpoint inhibitors and thrombotic or vascular complications has been increasingly recognized, no studies to date have specifically examined outcomes in patients with pre-existing vascular anomalies, such as PSA, who are receiving immune checkpoint inhibitors versus chemotherapy. Early identification and appropriate management of vascular complications such as PSA are essential to prevent hemorrhagic sequelae and ensure uninterrupted cancer treatment.
Conflict of Interests
There are no conflicts of interest to disclose.
Ethics Statement
Institutional review board (IRB) approval was not required for a single-patient case report according to our institution’s guidelines. All efforts were made to protect patient confidentiality.
Consent to Participate Declaration
Informed consent was obtained from the patient for participation in this case report and for the publication of relevant clinical information and images. The patient was informed that all identifying details would be removed to ensure anonymity.
Funding
Sara Myers is funded by Conquer Cancer Career Development Award and The American College of Surgeons Faculty Research Foundation. These awards are unrelated to the content of this submission.
References
2. Swain B, Castelhano R, Litton K, Chaudhry A. Core needle biopsy causing a pseudoaneurysm in the breast. Ann R Coll Surg Engl. 2022 Jan;104(1):e21–4.
3. Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022 Nov 7; 43(42):4458–68.
4. Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019 Sep; 16(9):563–80.
5. Calabretta R, Hoeller C, Pichler V, Mitterhauser M, Karanikas G, Haug A, et al. Immune Checkpoint Inhibitor Therapy Induces Inflammatory Activity in Large Arteries. Circulation. 2020 Dec 15;142(24):2396–8.
6. Zarifa A, Kim JW, Lopez-Mattei J, Palaskas N, Iliescu C, Kim PY. Cardiac Toxicities Associated with Immune Checkpoints Inhibitors: Mechanisms, Manifestations and Management. Korean Circ J. 2021 Jul; 51(7):579–97.
7. Veluswamy P, Wacker M, Scherner M, Wippermann J. Delicate Role of PD-L1/PD-1 Axis in Blood Vessel Inflammatory Diseases: Current Insight and Future Significance. Int J Mol Sci. 2020 Oct 31; 21(21):8159.
8. Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med. 2012 Dec 17; 209(13):2485–99.
9. Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005 Oct; 25 Suppl 1:S173–89.
10. de Souza Chamadoira JP, de Carvalho Figueiredo C, D'Ávila GO, de Carvalho Miranda Rosati Rocha AP, Endo É. Pseudoaneurysm after an ultrasound-guided breast core needle biopsy in a lactating woman. J Radiol Case Rep. 2021 Oct 1; 15(10):10–9.
11. Chen RY, Zhu Y, Shen YY, Xu QY, Tang HY, Cui NX, et al. The role of PD-1 signaling in health and immune-related diseases. Front Immunol. 2023 May 16; 14:1163633.
12. Sun P, Zhang L, Gu Y, Wei S, Wang Z, Li M, et al. Immune checkpoint programmed death-1 mediates abdominal aortic aneurysm and pseudoaneurysm progression. Biomed Pharmacother. 2021 Oct; 142:111955.
13. Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014 Apr; 202(4):W390–9.
14. El Khoury M, Mesurolle B, Kao E, Mujoomdar A, Tremblay F. Spontaneous thrombosis of pseudoaneurysm of the breast related to core biopsy. AJR Am J Roentgenol. 2007 Dec; 189(6):W309–11.
15. He T, Zhang M, Qin J, Wang Y, Li S, Du C, et al. Endothelial PD-1 Regulates Vascular Homeostasis and Oligodendrogenesis during Brain Development. Adv Sci (Weinh). 2025 Apr; 12(16):e2417410.
16. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New England journal of medicine. 2012 Jun 28; 366(26):2443–54.
17. Yang XJ, Wu YJ, Wang JZ, Zheng YL, Li XQ. Pulmonary artery pseudoaneurysm-induced massive hemoptysis after chemotherapy combined with tislelizumab for lung squamous cell carcinoma: a case report. Front Med (Lausanne). 2025 Apr 3; 12:1524248.
18. Schmid P, Cortes J, Dent R, McArthur H, Pusztai L, Kümmel S, et al. Overall Survival with Pembrolizumab in Early-Stage Triple-Negative Breast Cancer. N Engl J Med. 2024 Nov 28; 391(21):1981–91.