Abstract
IL-6 concentrations rise with the onset of COVID-19 infection and is detected in 68% of patients on admission but is expected to reduce after the acute phase. IL-6 concentrations at time points: (2-7 days), (6-11 days), (11-15 days) and (13-20 days) after intensive care unit (ICU) admission showed the highest level of IL-6 concentrations at time point 2-7 days, we decided to characterize IL-6 concentrations in serum samples collected in the sera of COVID-19 convalescent patients (40 – 93 days) post onset of symptoms. The estimated IL- 6 concentrations detected ranged between 2.65 pg/ml and 29.01 pg/ml, with a median of 9.18 pg/ml. Nine out of the 28 (32.14%) patients had normal IL-6 levels (<7 pg/ml) and 19 of the 28 patients (68%) had IL-6 elevated concentrations (>7 pg/ml). The IL-6 concentrations were grouped normal (<7 pg.ml, n= 9), mildly elevated (7 – 10 pg/ml, n = 10) and elevated (>10 pg/ml n = 9). There was no relationship between IL-6 concentrations with gender, days from onset of symptoms, days from subsiding symptoms and COVID-19 IgG antibodies. There was some relationship with age, as the younger patient cohort produced much higher IL-6 concentrations than the older patient cohort. This study describes an increase in blood IL-6 concentrations in the sera of COVID-19 convalescent patients (40 – 93 days) post onset of symptoms like that seen in COVID patients on admission and higher levels seen in the younger patient cohort indicating that “cytokine storms” still occur in the recovery phase.
Keywords
Cell signaling pathways, COVID-19, IL-6 concentrations, Immunoassay
Introduction
There are a number of cytokines such as interleukin-1β (IL-1β), IL-RA, IL-2, IL-6, IL-7, IL-8, IL-9, IL-10, IL-17, IL-18, tumor necrosis factor (TNF-α), interferon-gamma (IFN-gamma), granulocyte colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), macrophage inflammatory protein 1 (MIP-1alpha/CCL3), monocyte chemoattractant protein-1 (MCP-1/CCL2), interferon gamma-induced protein 10 (IP-10/CXCL10) and fibroblast growth factor (FGF) [1]. IL-6 concentrations have emerged as a potential prognostic marker in COVID-19 patients [2-4]. Elevated IL-6 levels have been associated with an increased risk of ICU admission, mechanical ventilation, and mortality [5-11]. Results showed that COVID-19 patients have higher serum level of cytokines (TNF-α, IFN-γ, IL-2, IL-4, IL-6, and IL-10) and CRP than control individuals. Within COVID-19 patients, serum IL-6 and IL-10 levels are significantly higher in critical group (n = 17) than in moderate (n = 42) and severe (n = 43) group. It was concluded that IL-6 could be used as a predictor for fast diagnosis of patients with a higher risk of disease deterioration [9]. Elevated levels of IL-6 in serum were associated with the development of critical disease [12]. A meta-analysis that included 11 studies with 1,357 patients with COVID-19 infection showed higher levels of IL-6 in patients with more severe disease than those with milder disease [13].
The longitudinal kinetics of IL-6 in COVID patients has shown that IL-6 has a short duration and peaks around 7 – 10 days after the onset of symptoms and tends to return to normal after 10 days post onset of symptoms [14]. We demonstrated the kinetics of IL-6 concentrations in the blood of a subject with mild COVID-19 infection, showing it rising on day 1 post onset of symptoms and returning to normal by day 8 post onset of symptoms [15]. In a study, estimating IL-6 concentrations at time points: (2-7 days), (6-11 days), (11-15 days) and (13-20 days) after intensive care unit (ICU) admission. The median IL-6 concentrations for the time points were 314 pg/ml, 188 pg/ml, 133 pg/ml, and 133 pg/ml, respectively. The highest median IL-6 concentrations were observed within the 2–7-day time point [16].
Immunoassays have been used to measure IL-6, recently an alternative to a fully automated large laboratory method, iChroma 50 system a fluorescent immunoassay was described [17]. We have recently used an iChroma II immunoassay method to measure the IL-6 concentrations in COVID-19 patient [15]. It will be important to characterize IL-6 in the blood of COVID-19 patients after the initial acute period. There we decided to characterize IL-6 concentrations in serum samples collected in the sera of COVID-19 convalescent patients (40 – 93 days) post onset of symptoms.
Materials
Patients
A twenty-eight COVID-19 unique donor panel was obtained from Cambridge Biosciences who obtain fresh human blood service in partnership with London-based Research Donors. Research Donors is a HTA licenced and ISO 9001 2015 certified company with Research Ethics (REC) approval as a Research Tissue bank, as well as participating in the UK NEQAS QA scheme. The COVID-19 positivity of the donors was confirmed by positive PCR or MD diagnosis and serum samples collected in the sera of COVID-19 convalescent patients (40 – 93 days) post onset of symptoms.
COVID-19 IgG antibodies had been estimated using the Diasorin Liaison method detecting IgG antibody Concentration - A negative result (< 15.0 AU/mL) may indicate absence or level of IgG antibodies to COVID-19 below the limit of detection of this test. A positive result (≥ 15.0) indicates the presence of IgG antibodies to SARS-CoV-2 and generally indicates exposure to COVID-19. All donors were positive for COVID-19 IgG antibodies.
|
Subject # |
Age |
Gender |
Days from onset of symptoms |
Days from subsiding symptoms |
Liaison Concentration (AU/mL) |
IL-6 concentration pg/ml |
|
1 |
38 |
F |
73 |
57 |
254.00 |
12.24 |
|
2 |
45 |
F |
66 |
54 |
23.80 |
7.30 |
|
3 |
44 |
F |
80 |
49 |
79.30 |
11.46 |
|
4 |
48 |
F |
49 |
36 |
121.00 |
6.43 |
|
5 |
55 |
F |
74 |
67 |
29.50 |
3.59 |
|
6 |
65 |
F |
87 |
72 |
116.00 |
2.65 |
|
7 |
40 |
F |
76 |
73 |
108.00 |
5.22 |
|
8 |
39 |
M |
77 |
62 |
109.00 |
3.13 |
|
9 |
34 |
F |
58 |
46 |
56.70 |
12.06 |
|
10 |
46 |
F |
72 |
54 |
133.00 |
9.69 |
|
11 |
30 |
M |
65 |
52 |
18.20 |
4.77 |
|
12 |
47 |
F |
75 |
49 |
57.80 |
10.46 |
|
13 |
57 |
F |
80 |
66 |
24.20 |
6.75 |
|
14 |
36 |
M |
73 |
67 |
147.00 |
15.97 |
|
15 |
30 |
F |
67 |
51 |
346.00 |
11.75 |
|
16 |
60 |
F |
66 |
57 |
94.00 |
5.65 |
|
17 |
30 |
M |
48 |
37 |
59.60 |
10.00 |
|
18 |
50 |
F |
68 |
51 |
62.90 |
6.09 |
|
19 |
35 |
F |
80 |
68 |
36.30 |
29.01 |
|
20 |
47 |
F |
80 |
52 |
120.00 |
9.83 |
|
21 |
45 |
M |
61 |
51 |
114.00 |
8.67 |
|
22 |
42 |
F |
48 |
44 |
38.70 |
8.52 |
|
23 |
66 |
M |
63 |
61 |
25.60 |
8.60 |
|
24 |
53 |
F |
93 |
79 |
83.90 |
11.43 |
|
25 |
50 |
M |
73 |
58 |
111.00 |
7.23 |
|
26 |
25 |
F |
78 |
39 |
116.00 |
8.29 |
|
27 |
63 |
M |
40 |
35 |
355.00 |
9.10 |
|
28 |
30 |
F |
76 |
61 |
154.00 |
11.26 |
Methods
This test uses a sandwich immunodetection method; the detector antibodies in buffer bind to antigens in the sample, forming antigen-antibody complexes and migrate onto nitrocellulose matrix to be captured by the other immobilized-antibodies on test strip. More antigens in the sample will form more antigen antibody complexes which lead to stronger fluorescence signal by detector antibodies, which is processed by instrument for ichroma™ tests to show IL-6 concentration in the sample.
Assay test procedure
1) Transfer 150 µL of the detector diluent using a pipette to a detector tube containing granules. When the granule form is completely dissolved in the tube, it becomes detection buffer.
2) Transfer sample 35 µL (human whole blood/serum/ plasma/control) using a pipette to a detector tube. If you use a capillary tube (35 μL), put it into the detector tube after collecting sample.
3) Close the lid of the detector tube and mix the sample thoroughly by shaking it about 20 times.
4) Pipette out 75 µL of a sample mixture and load it into the sample well on the cartridge.
5) Leave the Cartridge at room temperature for 12 minutes before inserting the device into the holder. Scan the sample-loaded cartridge immediately when the incubation time is over. If not, it will cause inaccurate test result.
6) To scan the sample-loaded cartridge, insert it into the cartridge holder of the instrument for ichroma™ tests. Ensure proper orientation of the cartridge before pushing it all the way inside the cartridge holder. An arrow is marked on the cartridge especially for this purpose.
7) Tap the ‘START’ button on the instrument for ichroma™ tests to start the scanning process.
8) The instrument for ichroma™ tests will start scanning the sample-loaded cartridge immediately.
9) Read the test result on the display screen of the instrument for ichroma™ tests.
Results
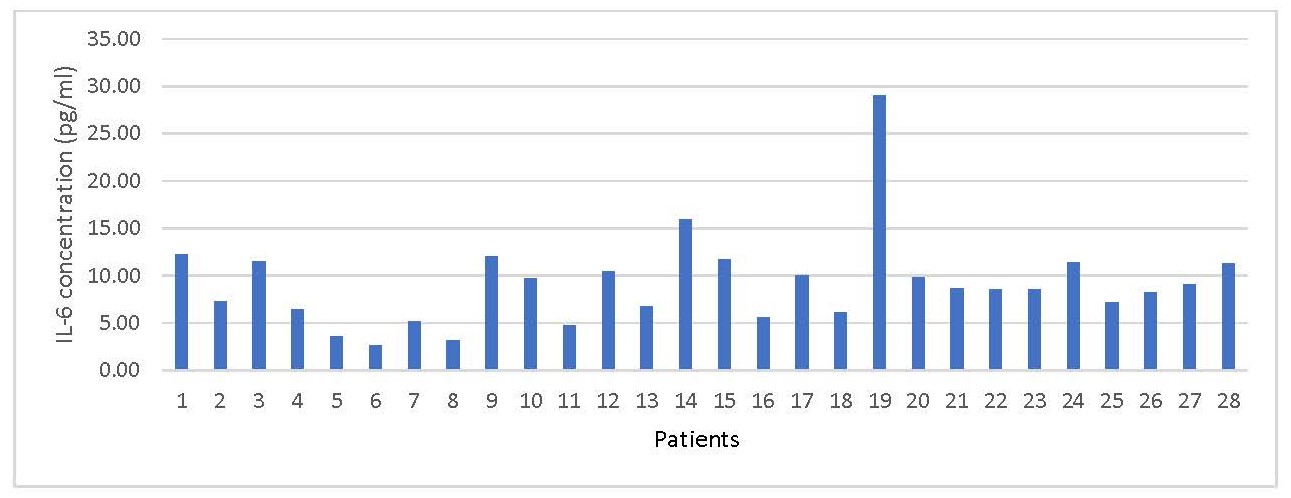
The IL- 6 concentrations ranged between 2.65 pg/ml and 29.01 pg/ml, with a median of 8.63 pg/ml. The normal reference range is < 7.0 pg/ml (Figure 1).
Figure 1. Showing IL-6 concentrations in 28 patients recovering from COVID-19 infection.
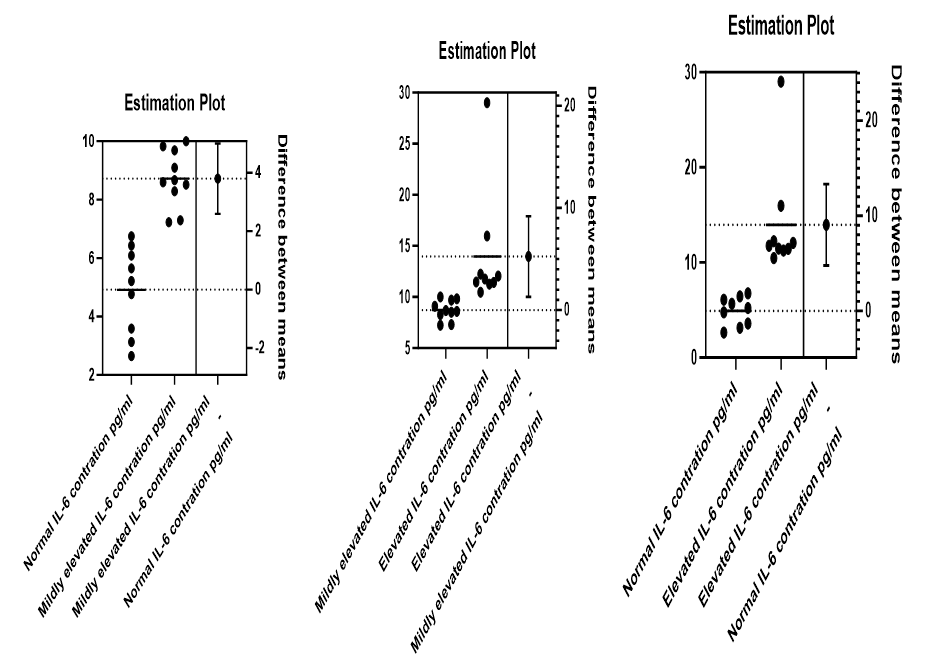
In order to classify the IL-6 concentrations, 9 out of the 28 (32.1%) estimates <7 pg/ml were grouped as normal, 10 out the 28 (35.7%) were between 7 – 10 pg/ml are grouped as mildly elevated and 9 out of 28 (32.1%) were >10 pg/ml and grouped as elevated. Using the unpaired t test, there was significant difference between normal and mildly elevated (p<0001), normal and elevated (p=0.0004), mildly elevated and elevated (p=0.0124), see Figure 2.
Figure 2. Showing estimation plots of IL-6 concentrations in normal (<7 pg/ml), mildly elevated (7 – 10 pg/ml), and elevated (>10 pg/ml).
There was no significant difference with regards to gender between normal, mildly elevated, and elevated IL-6 concentrations. There was no significant difference with regards to days from subsiding symptoms between normal, mildly elevated, and elevated IL-6 concentrations. There was no significant difference with regards to days from onset of symptoms between normal, mildly elevated, and elevated IL-6 concentrations. There was no significant difference with regards to COVID-19 antibodies between normal, mildly elevated, and elevated IL-6 concentrations.
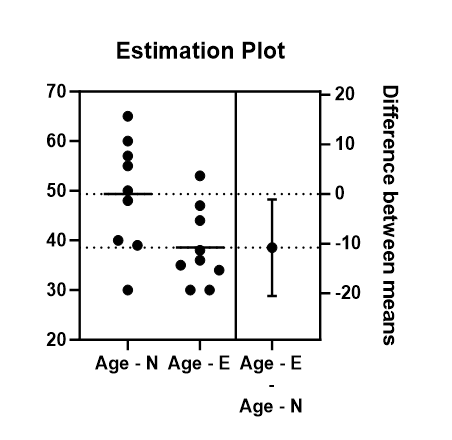
There was a significant difference with regards to age between normal and elevated IL-6 concentrations (p=0.0320). The younger patients had higher levels of IL-6 concentrations. In the normal IL-6 group, the minimum age was 30 years, maximum age of 65 years, with a mean age of 49.3 years. In the elevated IL-6 group, the minimum age was 30 years, maximum age of 55 years, with a mean age of 38.5 years see Figure 3.
Figure 3. Showing estimation plot between ages of patients with normal IL-6 concentrations and elevated IL-6 concentrations.
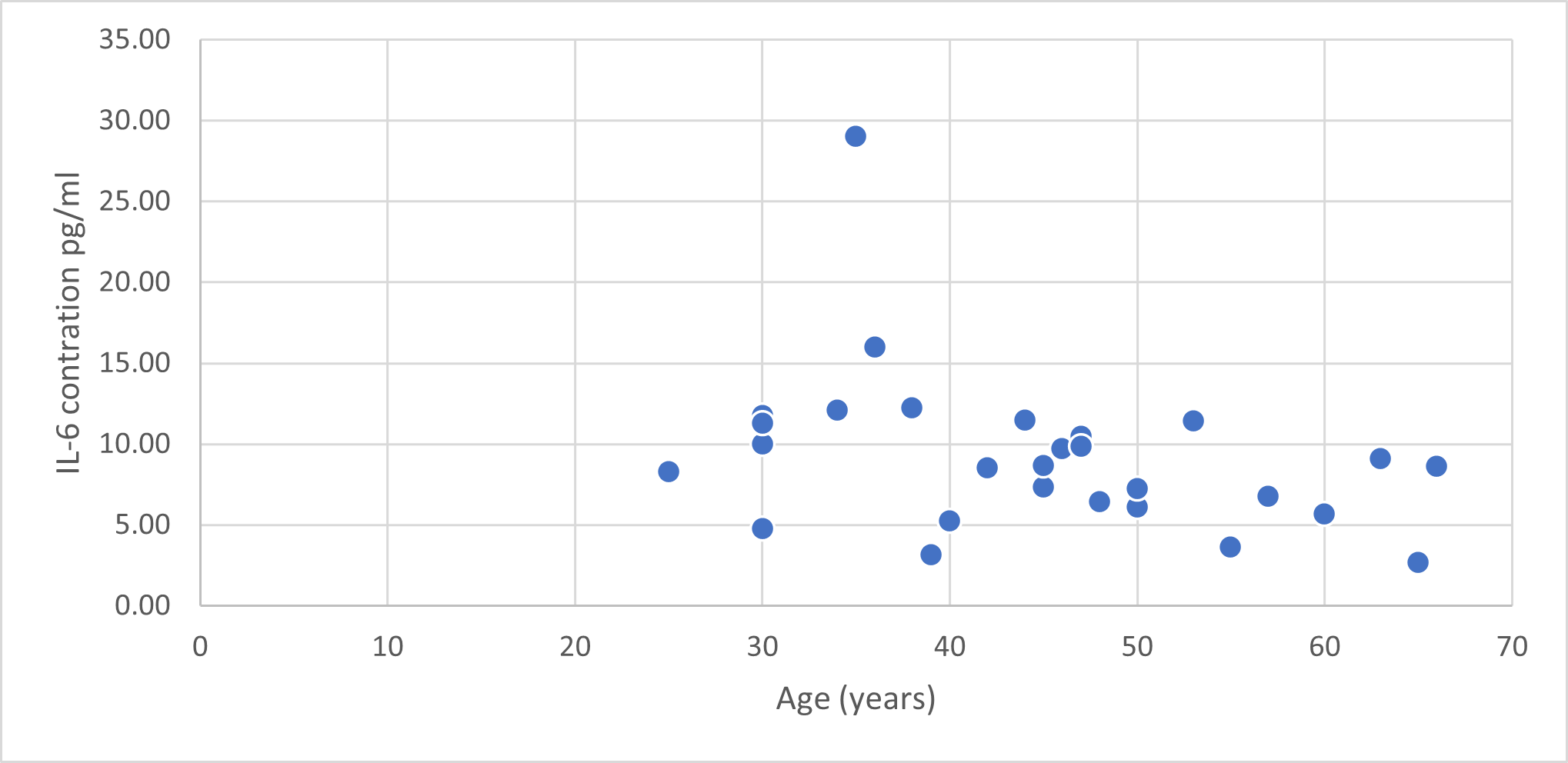
In Figure 4, we can see that most of the younger patients had higher levels of IL-6 in their sera compared to the older patients.
Figure 4. Showing IL-6 concentrations (pg/ml) by age (years) of patients.
Discussion
Monitoring IL-6 concentrations in the blood and serum of COVID-19 patients provides valuable insights into the immunological response and disease progression. Elevated IL-6 levels have been associated with severe disease, poor prognosis, and increased mortality. Utilizing IL-6 as a prognostic marker can aid in risk stratification and guide therapeutic interventions. Targeting IL-6 signaling represents a promising therapeutic approach, although careful consideration is required to balance immunomodulation with potential adverse effects.
The IL-6 kinetics show it peaks around 7 – 10 days after the onset of symptoms and tends to return to normal after 10 days post onset of symptoms and has been shown generally to remain low over the subsequent 20 days [16]. There is very little data showing what happens to IL-6 in the blood of patients post 20 days of onset of symptoms. In this short study, elevated levels of IL-6 were found in the sera of 68% of COVID-19 convalescent patients (40 – 93 days) post onset of symptoms, this figure is comparable with the increased levels of IL-6 detected in 67.9% of COVID-19 patients on admission [14]. This suggests that after the acute cytokine storm, there could be more exacerbations of cytokine storms occurring in the recovery period.
In addition, there was no relationship between IL-6 concentrations and gender, days from onset of symptoms, days from subsiding symptoms and COVID-19 IgG antibodies. However, there was some relationship with age as the younger patients had higher levels of IL-6 concentrations. This is contrary to the data in literature where higher serum levels of IL-6 and IL-10 were associated with the severity of the disease and a higher comorbidity index among adults aged 65 and over with COVID-19 [13]. However, in this study the oldest patient was 66 years of age and therefore it would not be advisable to conclude that these results are contrary to what is described in the literature. In this study, of the 4 patients over the age of 60 years, 2 of them (one male and female) had mildly elevated IL-6 levels with the remaining two normal IL-6 levels. It has been postulated that patients that continuously show increased IL-6 concentrations may be the ones unable to dampen the inflammation and result in complications and worsening of symptoms suggesting that the presence of IL-6 after the acute phase could be a marker to show the individuals who are likely to progress and develop complications or more severe disease especially in the elderly. The elevated IL-6 concentrations in the sera of the younger patient in this study could be an indication of the occurrence of cytokine storms because of their immune systems still being triggered even in the recovery phase of COVID-19 infection. The monitoring of IL-6 levels over time can provide valuable insight regarding disease progression and response to therapy, especially with regards to long COVID. Recently, increased IL-6 levels were associated with patients with long COVID-19 infection [18].
In conclusion, this short study shows that elevated IL-6 levels were seen in the sera of COVID-19 convalescent patients (40 – 93 days) post onset of symptoms and these were much higher in the younger age population although the oldest patient in this study was 66 years of age and there was not a similar number of >65-year-olds in this study. These elevated IL-6 levels could be consistent with activation of the immune system and could indicate long COVID, thereby requiring the need to further study the kinetics of IL-6 in patients with long COVID-19 infection.
Declaration of Interest
JB manages the distribution of the iCHROMA device and reagents in the UK and TKK works for Boditech Medi Inc, the manufacturers of the iCHROMA device and reagents.
References
2. Jones SA, Hunter CA. Is IL-6 a key cytokine target for therapy in COVID-19?. Nat Rev Immunol. 2021;21:337-39.
3. Uciechowski P, Dempke WCM. Interleukin-6: A Masterplayer in the Cytokine Network. Oncology. 2020;98(3):131-137.
4. Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev Med Virol. 2020 Nov;30(6):1-9.
5. Yin JX, Agbana YL, Sun ZS, Fei SW, Zhao HQ, Zhou XN, et al. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty. 2023 Apr 24;12(1):43.
6. Brasen CL, Christensen H, Olsen DA, Kahns S, Andersen RF, Madsen JB, et al. Daily monitoring of viral load measured as SARS-CoV-2 antigen and RNA in blood, IL-6, CRP and complement C3d predicts outcome in patients hospitalized with COVID-19. Clin Chem Lab Med. 2021 Aug 30;59(12):1988-97.
7. Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020 Jul;146(1):128-136.e4.
8. Sun H, Guo P, Zhang L, Wang F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China, in January and February 2020. Med Sci Monit. 2020;26:e926941.
9. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123-30.
10. Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020 Jun;127:104370.
11. Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020 Jul 7;12(7):e12421.
12. van Riel D, Embregts CWE, Sips GJ, van den Akker JPC, Endeman H, van Nood E, et al. Temporal kinetics of RNAemia and associated systemic cytokines in hospitalized COVID-19 patients. mSphere. 2021;6:e0031121.
13. Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe COVID-19: a systematic review and meta-analysis. Eur Cytokine Netw. 2020 Jun 1;31(2):44-49.
14. Santa Cruz A, Mendes-Frias A, Oliveira AI, Dias L, Matos AR, Carvalho A, et al. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front Immunol. 2021 Feb 18;12:613422.
15. Bolodeoku J, Bass M, Anyaeche C, Retnasingham V. A Mild Case of COVID-19 Infection: An Observational Longitudinal Study 27 Days Post Symptom of Antigen, Antibodies (IgM & IgG), IL-6 and D-Dimer. A Review. Am J Biomed Sci & Res. 2021;11(6):AJBSR. MS.ID.001686.
16. Luporini RL, Rodolpho JMA, Kubota LT, Martin ACBM, Cominetti MR, Anibal FF, et al. IL-6 and IL-10 are associated with disease severity and higher comorbidity in adults with COVID-19. Cytokine. 2021 Jul;143:155507.
17. Dierks S, Wiederhold M, Schanz J, Fischer A, Schnelle M. Evaluation of a novel, stand-alone system to measure interleukin-6. Clin Chem Lab Med. 2023 Jan 27;61(7):e128-e130.
18. Yin JX, Agbana YL, Sun ZS, Fei SW, Zhao HQ, Zhou XN, Chen JH, Kassegne K. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect Dis Poverty. 2023 Apr 24;12(1):43.