Abstract
Liver fibrosis resulting from chronic liver injury can progress to cirrhosis and liver failure. Current treatments are limited, creating an urgent need for novel antifibrotic therapies. Multiple emerging approaches have shown preclinical promise in inhibiting liver fibrogenesis or stimulating regeneration, including artificial liver support, stem cell therapy, cell/gene therapy, nanomedicines, immunotherapy, and herbal medicines. Artificial liver support provides detoxification but has shown inconsistent transplant bridging benefits. Stem cell transplantation demonstrates antifibrotic paracrine effects and differentiation potential. Cell therapy with hepatocytes or modulatory immune cells, as well as genetic engineering approaches, aims to replace damaged cells or suppress inflammation. Nanoparticles enable targeted delivery of antifibrotic drugs and genes. Immunotherapy using checkpoint inhibitors, vaccines, or engineered cells can attenuate fibrogenesis-related inflammation. Some traditional Chinese herbal formulas and compounds exhibit antifibrotic, anti-inflammatory, and regenerative mechanisms. Despite encouraging preclinical data, most novel antifibrotic therapies have yet to achieve clinical translation, limited by challenges in safety, delivery, and efficacy. Combination treatment regimens may provide maximal therapeutic impact. Ongoing optimization and rigorous clinical evaluation are needed to develop effective new antifibrotic therapies for patients with chronic liver disease.
Keywords
Liver fibrosis, Antifibrotic therapy, Artificial liver, Cell therapy, Nanomedicine, Immunotherapy
Background
Liver fibrosis is characterized by excessive accumulation of extracellular matrix proteins including collagen, resulting in distortion of the normal liver architecture and impairment of liver function. Advanced liver fibrosis leads to cirrhosis, which is a major cause of morbidity and mortality worldwide. The main causes of liver fibrosis include chronic viral hepatitis, alcoholic liver disease, and nonalcoholic fatty liver disease [1]. Currently, the only effective treatment for end-stage cirrhosis is liver transplantation. However, transplantation is limited by donor organ shortage, high costs, and the need for lifelong immunosuppression. There is an urgent need for alternative therapies that can halt or reverse fibrosis progression in patients with chronic liver disease [2]. In recent years, progress has been made in understanding the cellular and molecular mechanisms driving liver fibrogenesis. Hepatic stellate cells are the major effector cells responsible for extracellular matrix deposition in the fibrotic liver. In response to liver injury, stellate cells undergo activation and transdifferentiation into myofibroblast-like cells, acquiring contractile, pro-inflammatory, and profibrogenic phenotypes. Activated stellate cells proliferate, migrate, and produce excessive amounts of collagen and other ECM components. They also release pro-fibrogenic mediators such as transforming growth factor beta (TGFβ) and platelet-derived growth factor (PDGF) that act in autocrine and paracrine fashions to perpetuate fibrosis. Immune cells including macrophages and lymphocytes also mediate fibrogenesis through the release of cytokines, chemokines, and growth factors [3]. Based on improved understanding of fibrosis pathogenesis, several potential therapeutic approaches have emerged to directly target the activated stellate cells, modulate the immune response, or stimulate matrix degradation and liver regeneration. Artificial liver support technologies can physically remove circulating fibrogenic mediators in patients with advanced cirrhosis as a bridge to transplantation. Cell therapies using hepatocytes, stem cells, or genetically engineered cells aim to replace damaged tissue and suppress inflammation. Nanomedicine approaches leverage targeted drug delivery systems to inhibit fibrogenic signaling pathways. Immunomodulatory therapies such as therapeutic vaccines and checkpoint inhibitors restore normal immune homeostasis. Despite promising preclinical data, most emerging antifibrotic therapies have yet to achieve clinical translation due to limitations in safety, delivery, and efficacy. Further optimization and combination strategies are needed to successfully develop novel antifibrotic treatments [4].
Liver Transplantation
Liver transplantation is the definitive therapy for patients with end-stage liver failure due to chronic diseases such as cirrhosis or acute liver failure. The procedure involves surgical removal of the diseased liver and replacing it with a healthy graft from a deceased or living donor. Liver transplant has evolved to become the standard of care for patients with liver failure and complications such as recurrent variceal bleeding, hepatic encephalopathy, or hepatocellular carcinoma. Improvements in surgical techniques, organ preservation, immunosuppression, and post-transplant management over the past decades have led to excellent outcomes. The early pioneers of liver transplant in the 1960s demonstrated the technical feasibility of the procedure but were limited by poor patient survival. The introduction of cyclosporine in the 1980s represented a major breakthrough, improving 1-year survival from less than 30% to over 80%. Additional immunosuppressive agents such as tacrolimus, mycophenolate mofetil, and monoclonal antibodies have further reduced acute rejection rates. Advances in diagnostics, anesthesiology, intensive care, infection control, and post-transplant monitoring also significantly enhanced transplant outcomes [5]. Today, 1-year patient survival rates exceed 90% for liver transplant recipients in the US and Europe. Five-year survival is also over 75% for adults and 80% for pediatric patients. However, about 10-15% of patients still develop graft failure or death in the first month post-transplant, highlighting opportunities for continued improvements. Long-term outcomes are also affected by consequences of lifelong immunosuppression such as infections, renal dysfunction, metabolic disorders, and malignancies. There is a need for tolerogenic strategies to minimize immunosuppression requirements.
Worldwide, tens of thousands of liver transplants are now performed annually, mostly living donor procedures in Asia and deceased donors in Western countries. However, the number of patients added to transplant waiting lists continues to exceed available organs. The median time on the waitlist before transplantation is over 6 months in the US and greater than 1 year in parts of Europe. Up to 20% of waitlisted patients die before receiving a suitable graft, emphasizing the major organ shortage. Strategies to expand the donor organ pool include donation after cardiac death, split liver grafts, and living donation. Donation after cardiac death involves recovering organs from donors after withdrawal of life support. Metabolic support and perfusion technologies help optimize and assess graft function prior to transplant. Splitting a deceased donor liver enables transplantation into two recipients and doubles usage. Adult-to-adult living donor transplantation was pioneered in Asia and has become widely adopted to increase organ availability. However, living donation carries risks to the donor and is still limited by size matching constraints. To improve outcomes, liver allocation policies aim to prioritize patients with greatest medical urgency and predicted transplant benefit while balancing waitlist mortality. The Model for End-Stage Liver Disease (MELD) scoring system is widely used to prioritize allocation based on risk of short-term mortality on the waitlist. Geographic sharing of livers for highly urgent status patients also aims to alleviate regional disparities. Efforts to project longer-term post-transplant survival are still ongoing to optimize organ allocation [6].
Liver Xenotransplantation
Liver transplantation has become the standard life-saving therapy for patients with end-stage liver disease. However, its wider application is severely limited by the shortage of deceased human donor organs. Xenotransplantation, the transplantation of animal organs into humans, has long been proposed as a potential solution to address the organ shortage crisis. While the majority of research has focused on pig-to-human xenotransplantation, multiple challenges remain before it can become a clinical reality. The pig is considered the most suitable candidate donor animal for humans based on comparable anatomy and physiology. Pigs also breed rapidly with large litters, are readily available, and pose less ethical concerns compared to primates. Advances in genetic engineering have enabled the creation of pigs with multiple genetic modifications to avoid rejection and improve compatibility. However, the formidable immunological barriers posed by xenotransplantation remain the biggest obstacle [7].
Hyperacute rejection was the first key barrier identified, occurring within minutes to hours after transplantation. Preformed antibodies in human recipients recognize the galactosyl-α(1,3)galactose (αGal) antigen on pig cells and activate complement leading to graft destruction. Alpha 1,3 galactosyltransferase knockout pigs lacking αGal were generated using genetic engineering, overcoming hyperacute rejection. However, subsequent studies found that other preformed and elicited antibodies against non-Gal pig antigens can still drive severe rejection. The complement system is also activated by the alternate pathway against pig cells independent of preformed antibodies. Coagulation dysregulation due to molecular incompatibilities between pig and human coagulation factors contributes to disruption of the microvasculature in the liver graft. Innate immune cells including macrophages, NK cells, neutrophils, and platelets are additionally activated against the xenograft, releasing inflammatory cytokines, and recruiting adaptive immune cells. Cellular rejection remains mediated primarily by T cells against major histocompatibility complex (MHC) molecules on pig cells, though lower in intensity compared to allografts due to evolutionary distance. B cell activation by xenoantigens leads to elicitation of anti-pig antibodies which further drive humoral rejection. Overall, xenografts confront a multifaceted immune response involving both innate and adaptive components as well as coagulation dysregulation [8].
Intense immunosuppressive regimens including costimulation blockade, T cell depletion, and anticoagulants have enabled pig liver graft survival for up to 3-4 weeks in non-human primates. Genetic engineering approaches to overexpress human complement and coagulation regulatory proteins like CD46, CD55, and thrombomodulin in pigs also help ameliorate rejection. Combination treatment with cellular immunosuppression, antibody depletion, coagulation control, and transgenic organs achieved a record survival beyond 6 months in one baboon. In clinical xenotransplantation, temporary evidence of liver function was reported after transplant of pig livers into 4 patients in the 1990s. However, survival was only up to 24 hours. Recently, researchers at NYU Langone transplanted genetically engineered pig livers into 2 brain-dead patients on ventilator support whose families consented to the procedure. The grafts functioned with signs of coagulation compatibility for up to 72 hours before planned removal. Despite incremental progress, survival beyond a few weeks has not yet been achieved, and risks of complications from long-term immunosuppression remain unclear [9].
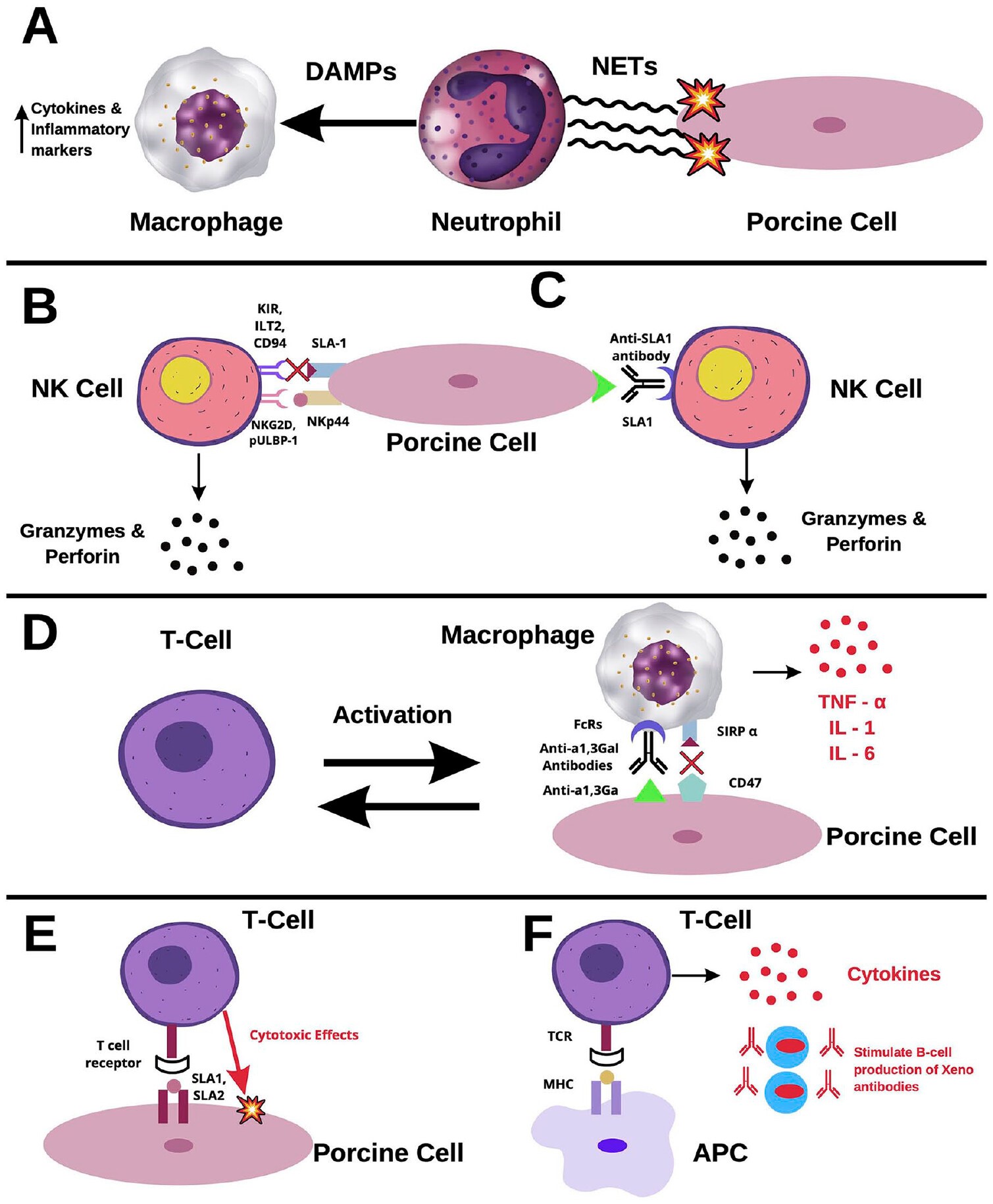
Beyond immunological rejection, physiological incompatibilities between pig and human tissues raise concerns. For instance, human coagulation factors and complement may not regulate well on pig endothelium, increasing risks of thrombosis, inflammation, and graft dysfunction. Discordant metabolic pathways, signaling mechanisms, and hormonal interactions between pig livers and humans could impair graft function over the long-term. However, genome editing tools like CRISPR may enable addressing some molecular incompatibilities by humanizing key genes involved in regulation of coagulation, immunity, inflammation, and cellular growth. The potential risk of zoonotic infections also remains a concern with xenotransplantation. Porcine endogenous retroviruses (PERVs) embedded in the pig genome could theoretically recombine to infect human cells. However, no PERV transmission occurred in recent clinical trials, even with prolonged immunosuppression. Comprehensive screening and selection of PERV-free donor animals can mitigate zoonosis risks. Ongoing screening will also be needed to prevent transmission of any novel pig microorganisms to humans. Figure 1 illustrates the various cellular players and pathways involved in rejection of xenotransplants. Neutrophil activation leads to release of neutrophil extracellular traps (NETs) containing reactive oxygen species and damage signals that activate recipient macrophage infiltration. Natural killer (NK) cells are activated by stimulatory receptors like NKG2D and pULBP-1 binding xenograft cells, while inhibitory receptors like KIR and CD94 are less effective at dampening NK cell activity due to poor recognition of swine leukocyte antigens (SLA). NK cells can also mediate antibody-dependent cellular cytotoxicity (ADCC) when their Fc receptors bind xenoantibodies like anti-SLA1. Macrophages activated through Fc receptors similarly release inflammatory cytokines like TNF-α, IL-1, and IL-6, with ineffective inhibition by CD47 interactions. Direct T cell rejection involves recognition of xenograft SLA by recipient T cell receptors. Indirect pathways activate T cells through recipient antigen presenting cells that process xenoantigens [10].
Figure 1. Rejection of xenotransplants.
Beyond medical challenges, xenotransplantation raises ethical concerns around animal welfare, personal autonomy, and dignity in the consent process, and equitable access to such a limited resource. Public distrust represents another barrier, amplified by memories of the HIV epidemic originating from non-human primate tissues. Thoughtful public engagement and regulated oversight would be imperative for responsible implementation if xenotransplantation becomes clinically feasible.
Artificial Liver Support Systems
Artificial liver support systems were developed to temporarily take over the metabolic and detoxification functions of the liver in patients with acute or acute-on-chronic liver failure. The most commonly used systems are based on albumin dialysis, which aims to remove circulating albumin-bound toxins without loss of essential compounds. The Molecular Adsorbent Recycling System (MARS) is the best characterized albumin dialysis system and has been evaluated in multiple clinical trials for liver failure. Meta-analyses suggest that MARS therapy improves hepatic encephalopathy and hemodynamics in patients with advanced liver disease awaiting transplant, with variable effects on survival. The mechanism of action likely involves removal of bilirubin, bile acids, and vasoactive mediators that promote portal hypertension and systemic inflammation [11].
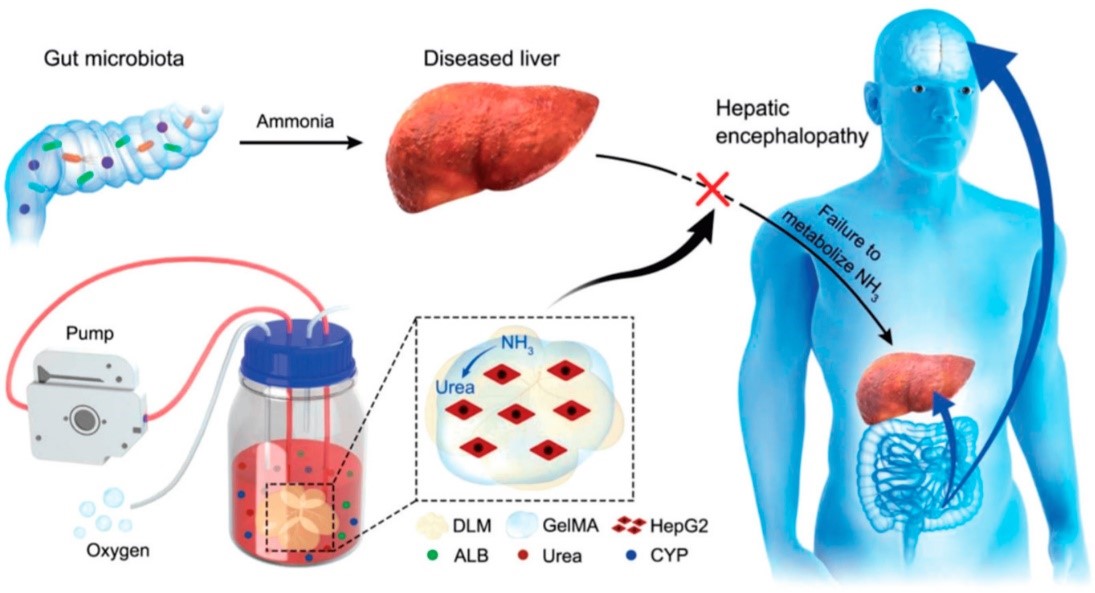
More complex bioartificial liver support systems contain human liver-derived cells within hollow fiber bioreactors to provide both detoxification and synthetic functions. HepatAssist and ELAD consist of cryopreserved human hepatocytes, while the AMC-BAL uses a hepatoblastoma cell line. Clinical trials found that bioartificial livers improve hepatic encephalopathy, but effects on bridging to transplant have been inconsistent. Limitations include poor hepatocyte viability and insufficient metabolic activity. A promising approach is the use of immortalized human hepatocytes engineered to maintain phenotypic stability and function. Extracorporeal cellular therapies based on human mesenchymal stem cells can also modulate systemic inflammation and immune responses. While artificial liver support therapies have not shown definitive survival benefit, they provide transient support and are still being optimized as a bridge to transplant or recovery in acute liver failure. In 2020, Wu Guohua and his team engineered an advanced bio-artificial liver apparatus to tackle the insufficient detoxification capabilities of hepatocytes. The innovative Bio-Artificial Liver Support System (BALSS) utilizes a bioengineered liver constructed from Decellularized Liver Matrix/Gelatin Methacryloyl (DLM/GelMA), demonstrated in Figure 2, with the intent to prevent Hepatic Encephalopathy (HE). Patients suffering from liver failure struggle to convert intestinal ammonia due to impaired hepatocytes, leading to an excess of ammonia in the brain via the blood-brain barrier, causing HE. By integrating GelMA and HepG2 cells into a DLM under continuous culture in an oxygen-rich bioreactor, a full bioengineered liver is produced. This DLM/GelMA-based cell bioengineered liver exhibits superior biosynthesis and biotransformation abilities, presenting promising potential in treating liver failure patients and preventing the onset of HE [12].
Figure 2. Artificial liver support therapies.
Stem Cell Therapy
Stem cell-based therapies have shown promising antifibrotic effects in preclinical models through multiple mechanisms: 1) differentiation into functional hepatocytes to replace damaged epithelium, 2) modulation of inflammatory responses by paracrine signaling, and 3) stimulation of endogenous regenerative pathways. A variety of stem and progenitor cells have been investigated for potential to inhibit fibrosis progression and stimulate liver regeneration. Mesenchymal stem cells (MSCs) can be isolated from bone marrow, adipose tissue, umbilical cord, and other sources. MSCs secrete a variety of paracrine factors that suppress hepatic stellate cell activation, promote extracellular matrix degradation, and reduce inflammation. In rodent models of liver fibrosis, MSC transplantation decreased collagen deposition, improved liver function, and reduced mortality. Multipotent stellate cells isolated from human livers could differentiate into hepatocyte-like cells in vitro, motivating their therapeutic potential. Hematopoietic stem cells (HSCs) home to injured tissues and secrete paracrine factors that modulate immune responses. HSC transplantation in fibrotic rodent liver increased hepatic progenitor cell expansion and reduced fibrosis. Early-phase clinical trials found hematopoietic stem cell therapy to be safe and feasible in patients with cirrhosis. However, effects on clinical outcomes have been modest to date. Induced pluripotent stem cells (iPSCs) can be generated from adult somatic cells and differentiated into functional hepatocyte-like cells. Transplantation of iPSC-derived hepatocytes inhibited cirrhosis progression in animal models. However, clinical translation of iPSC therapy faces challenges related to maturation status, risk of tumorigenicity, and low repopulation efficiency. Ongoing research aims to improve stem cell homing, engraftment, and hepatocyte differentiation efficiency to maximize antifibrotic benefits [13].
Cell Therapy
Hepatocyte transplantation is a cell therapy approach aimed at replacing damaged parenchymal cells in end-stage liver disease. Hepatocytes can be obtained from donor livers unsuitable for whole organ transplant or derived from stem cell sources. Hepatocyte infusion via the portal vein aims to integrate cells into the native liver parenchyma. Several early trials found metabolic improvement and bridging to transplant in acute liver failure patients receiving primary human hepatocytes. However, wider application is limited by restricted cell availability and poor long-term engraftment. To overcome these challenges, emerging strategies include cotransplantation with mesenchymal stem cells to improve hepatocyte engraftment and engineering of hepatocytes or progenitor cells to enhance proliferative capacity.
Cell therapy can also modulate liver inflammation to attenuate fibrosis progression. Regulatory macrophages can be generated ex vivo and infused to repopulate the liver with antifibrotic macrophages that produce lower levels of inflammatory cytokines. Regulatory T cell therapy also aims to suppress effector T cell responses and inflammation in fibrotic liver disease. Genetic engineering approaches are being applied to modify therapeutic cells prior to transplant. For example, macrophages can be transduced with genes encoding antifibrotic factors such as hepatocyte growth factor (HGF) or bone morphogenetic protein 7 (BMP-7). While cell therapies offer promise for fibrosis treatment, clinical translation is still limited by technical challenges related to cell sourcing, expansion, cryopreservation, and uncertain long-term safety [14].
Nanomedicine
Nanomedicine leverages engineered nanoparticles to precisely deliver drugs and genes to the injured liver while reducing off-target effects. Nanoparticles are typically designed to be biodegradable and nonimmunogenic, allowing for safe intravenous administration and biodistribution to the liver. Surface functionalization with targeting ligands can further enhance specific uptake by hepatic stellate cells and inflammatory cells that mediate fibrosis progression. A wide range of antifibrotic drugs, siRNAs, microRNAs, and CRISPR gene editing constructs have been successfully encapsulated and delivered using nanoparticles. In rodent liver fibrosis models, nanomedicine approaches demonstrated targeted delivery to hepatic stellate cells and macrophages, leading to reduced collagen deposition and inflammation. Polymer-based nanoparticles loaded with 2-deoxy-D-glucose or gliotoxin to inhibit stellate cell activation showed potent antifibrotic effects. Liposomal nanoparticles delivering siRNA against heat shock protein 47 suppressed collagen production. Solid lipid nanoparticles containing curcumin or adipose-derived stem cells exerted antifibrotic and regenerative effects. Although still early in development, clinical trials are underway to evaluate the safety and efficacy of liposomal nanoparticles co-delivering siRNAs that silence pro-fibrotic genes in the liver. While showing promise, nanomedicine therapies face challenges in scale-up of manufacturing, uncertainties in long-term toxicity, and need for repeated dosing to sustain antifibrotic effects [15].
Immunotherapy
The close link between liver inflammation and fibrosis progression has motivated immunotherapy approaches to treat patients with chronic liver disease. Therapeutic vaccines aim to restore immune tolerance to liver-specific antigens and suppress chronic hepatic inflammation. A number of vaccine candidates based on targeting autoantigens, modulating dendritic cells, or attenuating TLR signaling have shown efficacy in reducing liver fibrosis in preclinical studies. However, only a few vaccines have progressed to early clinical testing in patients. Further optimization of antigen selection, delivery methods, and synergistic combination therapies may help maximize clinical benefits. Checkpoint inhibitor immunotherapy is emerging as a promising approach to attenuate fibrosis by blocking inhibitory receptors on T cells and natural killer cells. Antibodies blocking PD-1, CTLA-4, TIM-3 and other immune checkpoints alleviated fibrosis in mouse models while enhancing antiviral T cell responses. Phase I/II trials of checkpoint blockade are underway in patients with hepatitis B and C-related cirrhosis. Inhibitors of cytokines such as TGF-β and TNF-α that drive liver inflammation and matrix deposition could also have therapeutic potential. However, clinical translation of cytokine blockade has been limited by lack of liver-targeted delivery and potential safety risks of systemic immunosuppression. Adoptive transfer of engineered regulatory T cells or mesenchymal stem cells genetically modified to overexpress immunomodulatory cytokines is also under investigation as a cell-based immunotherapy approach. Overall, while immunotherapy strategies hold promise, the balance between attenuating liver inflammation versus impairment of needed immune responses against chronic pathogens remains a key challenge in clinical development. Rational combination therapies and targeting approaches may help provide sufficient therapeutic windows [16].
Herbal and Chinese Traditional Medicine
Liver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular matrix proteins, which occurs in most types of chronic liver diseases. Advanced liver fibrosis results in cirrhosis, portal hypertension, and liver failure. In Western medicine, no effective antifibrotic therapies are currently available apart from treating the underlying liver disease. In contrast, traditional Chinese medicine (TCM) and other herbal medicinal systems have accumulated extensive knowledge on plant-based therapies for treating liver disease. In recent years, modern scientific research has started to investigate the potential mechanisms and clinical evidence on using Chinese herbs and traditional formulas for alleviating liver fibrosis. TCM describes fibrosis as arising from blood stasis and disturbance in the flow of qi or vital energy. Herbal treatments based on different TCM formulas are prescribed to promote blood circulation, clear stagnation, and restore homeostasis. Common herbs used include Artemisia capillaris (Yin Chen Hao), Salvia miltiorrhiza (Dan Shen), Astragalus membranaceus (Huang Qi), and Cynanchum wilfordii (Ge Shu Yu). Many of these herbs contain active compounds with demonstrated antifibrotic properties. For example, salvianolic acid from Salvia miltiorrhiza inhibited activation of hepatic stellate cells in vitro and reduced collagen deposition in rodent models. Tetrandrine from Stephania tetrandra (Han Fang Ji) interferes with TGF-β signaling and myofibroblast differentiation. Curcumin from Curcuma longa (Jiang Huang) exhibits broad anti-inflammatory and antioxidant effects that ameliorate fibrosis progression [17].
Multiple clinical studies, particularly from China, have evaluated the benefits of herbal medicine in patients with chronic hepatitis B and liver cirrhosis. Randomized trials found that astragalus-based formulas improved liver function, reduced fibrosis markers, and delayed disease progression compared to placebo. Combination therapy of salvia, astragalus, and schisandra berries improved clinical symptoms and liver histology compared to colchicine in one trial. Other studies reported antifibrotic benefits of formulas containing Artemisia capillaris, Curcuma longa, or mixtures of numerous herbs. However, variability in trial design, patient characteristics, and quality control of herbal preparations makes it difficult to systematically evaluate efficacy across studies. While traditional formulas rely on synergistic effects from multiple herbs, modern approaches also purify and concentrate bioactive components from herbs as targeted antifibrotic therapies. For example, capsules containing high-purity tetrandrine from Stephania tetrandra roots demonstrated antifibrotic and anti-inflammatory activity in clinical studies. An emulsion formulation of artemisinin from Artemisia annua showed efficacy against liver fibrosis in a phase 2 trial. However, challenges with cost, sourcing, purity, and standardization need to be overcome for developing standardized phytotherapies [18].
Chinese herbal medicines and their bioactive components demonstrate antifibrotic activity through multiple proposed mechanisms of action [19]. These include:
1) Inhibition of hepatic stellate cell activation and proliferation by blockade of pro-fibrogenic signaling pathways mediated by TGF-β, PDGF, and VEGF, as well as induction of stellate cell apoptosis and senescence.
2) Promotion of extracellular matrix degradation through increased activity and expression of matrix metalloproteinases and downregulation of their tissue inhibitors.
Anti-inflammatory effects by reducing inflammatory cell infiltration, cytokine release, and inhibiting NF-κB and JAK/STAT signaling pathways.
4) Antioxidant effects via scavenging of free radicals and enhancing antioxidant defenses to alleviate oxidative stress.
5) Improved microcirculation and reduced portal hypertension through vasodilation, decreased intrahepatic vascular resistance, and reduced endothelial dysfunction.
6) Stimulation of liver regeneration by activation of regenerative growth factors and signaling pathways leading to proliferation of hepatocytes and stem/progenitor cells.
7) Modulation of gut microbiota to restore intestinal barrier integrity, decrease endotoxemia, and exert anti-inflammatory effects on dysbiosis. Elucidation of the mechanisms underlying the antifibrotic benefits of Chinese herbal medicines may enable better standardization and therapeutic application.
Notably, many herbs exhibit multiple mechanisms of action rather than a single specific target. However, the molecular targets and effects of many traditional Chinese herbs remain to be fully elucidated using modern scientific methods. Challenges are also faced in consistently achieving sufficient concentrations of bioactive constituents in vivo through oral administration. Ongoing studies are exploring improved delivery methods for Chinese herbal extracts using nanoparticles, liposomes, and phospholipid complexes [20].
Future Directions and Outlook for Potential Therapies for Liver Fibrosis
While emerging antifibrotic approaches have shown promise in preclinical studies and early clinical testing, realization of their full therapeutic potential will require advancing the optimization and clinical translation of these novel therapies. Each innovative strategy has its own unique set of challenges and opportunities for improvement to enhance delivery, efficacy, and safety. For artificial liver support technologies, research priorities include engineering more metabolically active and durable cell-based systems, as well as developing new biomimetic constructs that can replicate a wider range of hepatic functions. Improving biochemical compatibility and vascular integration remain key goals for the field of xenotransplantation. Stem cell therapy would benefit from strategies to increase engraftment and differentiation efficiency of transplanted cells. Progress in cell and gene therapy will necessitate resolving technical hurdles related to cell sourcing, expansion, and genetic engineering. Priority goals for nanomedicine include scale-up of nanoparticle production, prolonged delivery, and assessment of long-term toxicity. Immunotherapy faces challenges in mitigating side effects of systemic immune activation and requires liver-targeted delivery systems. Further standardization and validation of bioactive compounds are needed to translate traditional herbal medicines into clinical practice [21]. Beyond refinements to individual therapies, an emerging paradigm is that of combinatorial antifibrotic therapies that can modulate multiple targets and pathways simultaneously. Potential synergies exist between strategies that directly attenuate fibrogenic mechanisms and approaches that stimulate regeneration and repair [22]. For instance, co-administration of stellate cell-targeted nanoparticles with stem cell infusion may simultaneously inhibit fibrosis drivers while enhancing liver cell regeneration. Combining checkpoint blockade immunotherapy with liver-directed gene therapy offers possibilities to attenuate inflammation alongside replacement of damaged cells. Artificial liver support in conjunction with herbal medicines could provide detoxification benefits alongside antifibrotic effects from natural compounds [23].
In the future, antifibrotic regimens tailored to individual patients based on fibrosis stage, etiology, and dominance of particular pathways could be designed by selecting compatible therapies from the growing repertoire of novel treatments. However, significant work remains in elucidating mechanisms of synergy versus antagonism between different therapeutic approaches. Carefully designed preclinical studies and clinical trials will be imperative to test combination regimens and define optimal treatment strategies that provide maximal antifibrotic effects for patients with chronic liver disease.
Etiology of Liver Fibrosis and Prevention
Liver fibrosis can arise from diverse etiologies that cause prolonged inflammation and injury to hepatocytes. Major causes include viral infections such as chronic hepatitis B and C, metabolic diseases like non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH), excessive alcohol consumption leading to alcoholic liver disease, autoimmune disorders, cholestatic liver diseases, and toxin/drug induced liver injury. Preventing the underlying causes of liver damage can help reduce the risk of developing fibrosis. For viral hepatitis, key prevention strategies include vaccination, safe injection practices, and antiviral treatment. NAFLD and NASH can be prevented by maintaining a healthy body weight through diet and exercise. Avoiding excessive alcohol intake and managing alcohol use disorders are critical to prevent alcoholic liver disease. Caution with certain prescription medications and environmental toxins may help prevent drug/toxin induced fibrosis. Treatment of metabolic disorders and autoimmune conditions as well as avoiding triggering toxins and drugs can also lower risk. Protecting the liver from insults and promoting general health is important for prevention as illustrated in Table 1.
|
Etiology |
Prevention Strategies |
|
Chronic viral hepatitis B and C |
Vaccination, safe injection practices, antiviral treatment |
|
NAFLD/NASH |
Weight loss through diet and exercise |
|
Alcoholic liver disease |
Avoiding excessive alcohol, managing alcohol disorders |
|
Autoimmune hepatitis |
Avoiding triggering medications/toxins |
|
Cholestatic diseases |
Treatment of underlying disorders |
|
Toxin/drug induced |
Avoiding toxins, limiting medications |
|
Metabolic disorders |
Diet, treatment of underlying conditions |
Conclusions
While current treatment options for advanced liver fibrosis and cirrhosis remain limited, an array of innovative emerging approaches have shown preclinical potential to inhibit fibrogenesis or stimulate regeneration. However, despite encouraging preliminary data, most novel therapies including artificial livers, stem cells, gene therapy, nanomedicines, immunotherapy, and herbal medicines have not yet achieved successful clinical translation. Ongoing optimization to improve delivery, efficacy, and safety as well as rigorous clinical evaluation in patients will be crucial to develop effective antifibrotic treatments. Combination regimens leveraging synergies between therapies targeting different mechanisms may ultimately provide the most benefit in halting progression of liver fibrosis.
List of Abbreviations
ECM: Extracellular Matrix; TGFβ: Transforming Growth Factor Beta; PDGF: Platelet-Derived Growth Factor; MELD: Model for End-Stage Liver Disease; MSCs: Mesenchymal Stem Cells; HSCs: Hematopoietic Stem Cells; iPSCs: Induced Pluripotent Stem Cells; MARS: Molecular Adsorbent Recycling System; ELAD: Extracorporeal Liver Assist Device; AMC-BAL: Academic Medical Center Bioartificial Liver; siRNA: Small interfering RNA; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; TLR: Toll-Like Receptor; TNF-α: Tumor Necrosis Factor Alpha; NK: Natural Killer; KIR: Killer cell Immunoglobulin-like Receptor; ILT2: Immunoglobulin-Like Transcript 2; SLA: Swine Leukocyte Antigen; MHC: Major Histocompatibility Complex; αGal: Galactosyl-α(1,3)galactose; PERV: Porcine Endogenous Retrovirus
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
All data is available, and sharing is available as well as publication.
Competing interests
The authors hereby declare that they have no competing interests.
Funding
The corresponding author supplied all study materials. There was no further funding for this study.
Authors' contributions
The authors completed the study protocol and were the primary organizers of data collection and the manuscript's draft and revision process. Tamer A. Addissouky wrote the article and ensured its accuracy. All authors contributed to the discussion, assisted in designing the study and protocol and engaged in critical discussions of the draft manuscript. Lastly, the authors (TA, MA, IE, YW) reviewed and confirmed the final version of the manuscript.
Acknowledgements
Authors thank all the researchers who have made great efforts on their studies. Moreover, we are grateful to the editors, reviewers, and readers of this journal.
References
2. Addissouky TA, Wang Y, Megahed FA, El Agroudy AE, El Sayed IE, El-Torgoman AM. Novel biomarkers assist in detection of liver fibrosis in HCV patients. Egyptian Liver Journal. 2021 Dec;11(1):1-5.
3. Hora S, Wuestefeld T. Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives. Cells. 2023 Aug 22;12(17):2129.
4. Pei Q, Yi Q, Tang L. Liver Fibrosis Resolution: From Molecular Mechanisms to Therapeutic Opportunities. International Journal of Molecular Sciences. 2023 Jun 2;24(11):9671.
5. Goffaux A, Delorme A, Dahlqvist G, Lanthier N. Improving the prognosis before and after liver transplantation: Is muscle a game changer?. World Journal of Gastroenterology. 2022 Oct 10;28(40):5807-17.
6. James L, LaSala VR, Hill F, Ngai JY, Reyentovich A, Hussain ST, et al. Donation after circulatory death heart transplantation using normothermic regional perfusion: The NYU Protocol. JTCVS Techniques. 2023 Feb 1;17:111-20.
7. Lamm V, Ekser B, Vagefi PA, Cooper DKC. Bridging to Allotransplantation-Is Pig Liver Xenotransplantation the Best Option? Transplantation. 2022 Jan 1;106(1):26-36.
8. Battistella S, D'Arcangelo F, Grasso M, Zanetto A, Gambato M, Germani G, et al. Liver transplantation for non-alcoholic fatty liver disease: indications and post-transplant management. Clin Mol Hepatol. 2023 Feb;29(Suppl):S286-S301.
9. Senoner T, Breitkopf R, Treml B, Rajsic S. Invasive Fungal Infections after Liver Transplantation. J Clin Med. 2023 Apr 30;12(9):3238.
10. Arabi TZ, Sabbah BN, Lerman A, Zhu XY, Lerman LO. Xenotransplantation: Current Challenges and Emerging Solutions. Cell Transplant. 2023 Jan-Dec;32:9636897221148771.
11. Chen Y, Han T, Duan Z; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Clinical application of artificial liver and blood purification: expert consensus recommendations. Hepatol Int. 2023 Feb;17(1):4-17.
12. Jasirwan CO, Muradi A, Antarianto RD. Bio-Artificial Liver Support System: A Prospective Future Therapy. Livers. 2023 Feb 9;3(1):65-75.
13. Huai Q, Zhu C, Zhang X, Dai H, Li X, Wang H. Mesenchymal stromal/stem cells and their extracellular vesicles in liver diseases: insights on their immunomodulatory roles and clinical applications. Cell & Bioscience. 2023 Dec;13(1):1-23.
14. Cardinale V, Lanthier N, Baptista PM, Carpino G, Carnevale G, Orlando G, et al. Cell transplantation-based regenerative medicine in liver diseases. Stem Cell Reports. 2023 Aug 8;18(8):1555-72.
15. Chen L, Wang Y. Interdisciplinary advances reshape the delivery tools for effective NASH treatment. Mol Metab. 2023 Jul;73:101730.
16. Shao M, Wang Y, Dong H, Wang L, Zhang X, Han X, et al. From liver fibrosis to hepatocarcinogenesis: Role of excessive liver H2O2 and targeting nanotherapeutics. Bioactive Materials. 2023 May 1;23:187-205.
17. Tan Y, Zhang F, Fan X, Lu S, Liu Y, Wu Z, et al. Exploring the effect of Yinzhihuang granules on alcoholic liver disease based on pharmacodynamics, network pharmacology and molecular docking. Chin Med. 2023 May 11;18(1):52.
18. Zhou Z, Zhang J, You L, Wang T, Wang K, Wang L, et al. Application of herbs and active ingredients ameliorate non-alcoholic fatty liver disease under the guidance of traditional Chinese Medicine. Front Endocrinol (Lausanne). 2022 Sep 20;13:1000727.
19. Foghis M, Bungau SG, Bungau AF, Vesa CM, Purza AL, Tarce AG, et al. Plants-based medicine implication in the evolution of chronic liver diseases. Biomed Pharmacother. 2023 Feb;158:114207.
20. Munteanu C, Schwartz B. The Effect of Bioactive Aliment Compounds and Micronutrients on Non-Alcoholic Fatty Liver Disease. Antioxidants (Basel). 2023 Apr 10;12(4):903.
21. Suresh D, Srinivas AN, Prashant A, Harikumar KB, Kumar DP. Therapeutic options in hepatocellular carcinoma: a comprehensive review. Clinical and Experimental Medicine. 2023 Feb 13:1-6.
22. Lou S, Cao Z, Chi W, Wang X, Feng M, Lin L, et al. The safety concerns regarding immune checkpoint inhibitors in liver cancer patients rising mainly from CHB. Frontiers in Pharmacology. 2023 Apr 24;14:1164309.
23. Charles J, Vrionis A, Mansur A, Mathias T, Shaikh J, Ciner A, et al. Potential Immunotherapy Targets for Liver-Directed Therapies, and the Current Scope of Immunotherapeutics for Liver-Related Malignancies. Cancers. 2023 May 5;15(9):2624.