Abstract
The aim of this study was to investigate whether post exercise carbohydrate intake improves training effect in the first postoperative days, in patients who have had a primary total knee or hip arthroplasty. Subjects were primary total hip (THA) (n=18), and total knee arthroplasties (TKA)(n=25) patients randomized to consume 30 g maltodextrin or placebo within 15 minutes after each training session as a supplement to the standard diet. The outcome measures were six minute walk test (6-MWT), number of completed training sessions, training intensity, and duration. There was no difference in the 6-MWT, number of completed training sessions, training intensity and duration between the intervention vs control group. When stratified for operation procedure, THA patients in the intervention group trained longer (26.7 ± 5.8 vs 15.2 ± 4.1 min, P = 0.017), and at an overall higher intensity (scale 1-5) (2.4 ± 1.0 vs 1.75 ± 0.8, P = 0.021) and during the training sessions with the physiotherapist (2.5 ± 1.1 vs and 1.8 ± 0.9, P = 0.044) compared with controls. In contrary, the duration of self-training was longer in the TKA control group compared with the intervention group (19.7 ± 7.0 vs 13.8 ± 3.3 min, P = 0.021). In conclusion, minor positive effects of maltodextrin were observed after hip arthroplasties, while minor negative effects of maltodextrin were observed after knee arthroplasties. It is unknown whether these findings are due to the low dose of maltodextrin, small number of study participants, differences in overall nutritional status, or lack of effect.
Keywords
Recovery, Nutrition, Hip Arthroplasties, Knee arthroplasties, Physical training rehabilitation
Introduction
Immediately after surgery a metabolic stress response occurs, which involves peripheral insulin resistance that leads to reduced muscle cell glucose uptake due to GLUT-4 (glucose transporter type 4) translocation defects, and thereby less glucose availability in skeletal muscle cells [1]. Glucose is the key substrate for the brain and central nervous system as well as a versatile muscular substrate during exercise due to anaerobic and aerobic metabolic pathways [2]. In healthy subjects, an increased muscular glucose uptake and glycogen synthesis have been demonstrated in the period after training [3,4]. Low levels of carbohydrates increase muscle protein breakdown, and reduce the net protein balance, especially in conditions of low muscle glycogen compared with high glycogen availability [5]. Ingestion of approximately 1–1.2 g carbohydrate per kg body weight per hour for the first four hour after exercise have been demonstrated to increase the rate of glycogen restoration improving the premises for short intervals between training sessions [2,4]. However, the effect of postoperative stress metabolism on glycogen restoration is unknown. The aim of the present study was therefore to investigate whether intake of rapidly absorbed carbohydrates immediately after exercise sessions in the first postoperative days following primary THA or TKA can improve rehabilitation, assessed by a 6 minute walk test (6 MWT), the number of completed training sessions, training intensity, and duration.
Methods
This trial was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Regional Committee for Ethics in Science (H-1-2013-128) as well as the National Data Controlling Authorities.
Study design
A randomized controlled double blinded study design was used, and participants were allocated for either intervention (carbohydrates) or control (placebo) during the hospitalization period of 2-5 days.
Participants
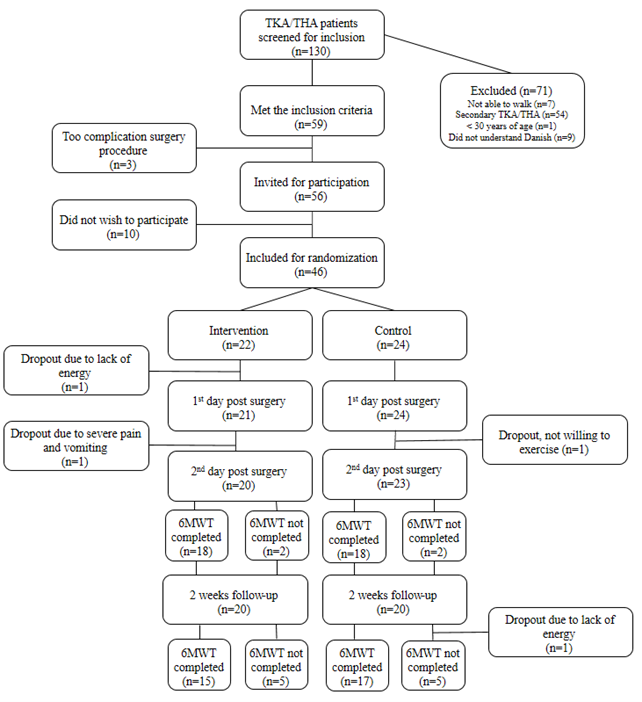
Subjects were recruited from the Orthopedic Surgery Division at Bispebjerg Hospital, Copenhagen, Denmark during pre-operative seminars providing both oral and written information about the study. Subjects included were >30 years of age with primary insertion of a total hip or knee joint prosthesis. Exclusion criteria were inability to walk, missing a limb, earlier TKA/THA and not speaking Danish. Initially 130 patients were considered for inclusion of which 46 subjects were randomized for either intervention (n=22) or control (n=24) (Figure 1). Very few patients are staying in hospital for more than two days postoperatively. Accordingly, the testing was limited to two days. None of the patients was screened at nutritional risk (NRS-2002).
Figure 1. Flow chart for recruitment, inclusion, exclusion, and drop outs of study participants. THA: Primary Total Hip Arthroplasties; TKA: Primary Total Knee Arthroplasties; 6MWT: Six Minute Walk Test.
Training
A physiotherapist provided a daily individualized training session during the 2-5 days admission period initially focusing on mobilization and walking practices progressing to strength training, stability of the operated leg and coordination practices, and finally climbing stairs, and recorded the duration and intensity of each session. Furthermore, subjects were encouraged to train as frequently and for as long as possible on their own focusing on strength, coordination and balance practices performed according to the physiotherapist’s instructions. The patients recorded the duration and intensity of each self-training session and evaluated the sessions together with a research group member on a daily basis. A self-training session was defined as ≥ 10 min of either walking, ergometer cycling or step machine (only THA) with a self-estimated intensity from 1 to 5 (1: No breathlessness at all, 2: slight breathlessness, 3: moderate breathlessness, 4: severe breathlessness, 5: very severe breathlessness).
Nutritional support and intervention drink
In the department no particular dietetic strategy exists for fast track patients. Diet is free, but all patients are recommended commercially produced oral nutritional supplements in addition to food being the standard in surgical departments [6]. All participants received sweetened fluid as a supplement after each training session (self-training and with physiotherapist). The intervention drink contained 30 g maltodextrin (Energi-Plus, Toftcare) with an absorption rate similar to glucose, but with low osmolality to support gastric emptying and reduce gastrointestinal stress [7,8], and 29 mg stevia glycosides to cover the taste, mixed in 158 mL water. The placebo drink contained 58 mg stevia glycosides mixed in 175 mL water providing an equal volume of fluid. Breakfast, lunch, and dinner were served following the department dietary routine, and patients were offered a supplementary nutrient drink twice a day.
The 6 Minute Walk Test
To assess aerobic capacity and endurance, a 6-MWT was performed on the day of recruitment, the second day post-surgery, and before discharge. Patients were asked to walk as far as possible during 6 min on a 22 m lane without walking aids. The patients were instructed not to talk or run, and to take a break if they experienced breathlessness, dizziness, or other types of discomfort but without pausing the timekeeping.
Pain score
Immediately after the 6-MWT on the second postoperative day, patients scored their pain on a scale from 0 to 10, with 10 representing the worst imaginable pain.
Statistics
All statistical analysis were performed using STATA, version12 (StataCorp, Texas, USA). For comparison of medians between patient groups Wilcoxon Rank-Sum test was used. For estimates of changes from pre-operation to discharge Wilcoxon Signed-Rank test was used. To test whether there was a difference between the two kinds of classification Fisher’s exact test was applied. To measure the degree of positive or negative association between continuous outcomes, Spearman’s correlation coefficient was calculated. The statistical significance level was defined as P<0.05. Continuous variables are presented as mean ± SD. Categorical variables are presented as median and interquartile range (25-75).
Results
There were no differences in age, weight, BMI (Table 1), and not in pain scores between the intervention and the control groups (2.7 ± 1.5 vs 3.4 ± 1.4, P = 0.193). There were no differences in baseline walking distance between the intervention and the control groups (Table 1), nor post-surgery when stratified for type of surgery or between the different test-days within groups (Table 2). Including all subjects, walking distance was negatively associated with pain score (r = -0.38, P = 0.034). In the THA group, the intervention group trained longer, and with an over-all higher intensity compared with the control group, while in the TKA group the mean duration of self-training sessions was longer in the control group compared with the intervention group (Table 3).
|
|
TKA (n = 25) |
THA (n = 18) |
||
|
Carbohydrate (n=13) |
Placebo (n=12) |
Carbohydrate (n=7) |
Placebo (n=11) |
|
|
Gender |
6 F/7M |
6 F/6M |
5 F/2M |
6 F/5M |
|
Age (years) |
64.0 ± 8.5 |
68.2 ± 10.1 |
62.9 ± 6.7 |
63.0 ± 9.0 |
|
Body weight (kg) |
89.8 ± 24.9 |
86.4 ± 14.0 |
76.9 ± 12.8 |
83.4 ± 17.4 |
|
BMI (kg/m2) |
29.4 ± 6.9 |
28.2 ± 3.4 |
26.2 ± 3.2 |
27.5 ± 5.3 |
|
6 MWT (m) |
429.3 ± 112.3 |
424.1 ± 95.7 |
460.3 ± 85.7 |
377.3 ± 121.2 |
|
Data are presented as mean ± SD. BMI: Body Mass Index; F: Females; M: Males; THA: Total Hip Arthroplasties; TKA: Total Knee Arthroplasties; 6 MWT: Six Minute Walk Test |
||||
|
|
TKA |
THA |
|||||
|
|
Carbohydrate |
Placebo |
p-value |
Carbohydrate |
Placebo |
p-value |
|
|
Pre op. 6MWT (m) |
429.3 ± 112.3 |
424.1 ± 95.7 |
0.957 |
460.3 ± 85.7 |
377.3 ± 121.2 |
0.135 |
|
|
1st post op. 6MWT (m) |
148.3 ± 63.0 |
152.8 ± 48.8 |
0.664 |
173.8 ± 104.5 |
175.2 ± 99.8 |
0.808 |
|
|
2nd post op. 6MWT (m) |
309.0 ± 105.4 |
306.8 ± 78.2 |
0.713 |
380.0 ± 135.2 |
285.4 ± 134.3 |
0.178 |
|
|
No. of participants |
|||||||
|
Pre op. 6MWT |
13 |
12 |
7 |
11 |
|||
|
1st post op. 6MWT |
13 |
8 |
5 |
7 |
|||
|
2nd post op. 6MWT |
10 |
7 |
5 |
10 |
|||
|
No. of days pre/post-operative |
|||||||
|
Pre op. 6MWT |
11.1 ± 8.1 |
8.2 ± 5.9 |
0.322 |
9.3 ± 3.3 |
9.1 ± 6.3 |
0.962 |
|
|
1st post op. 6MWT |
2.3 ± 0.6 |
2.3 ± 0.5 |
1.000 |
2.4 ± 0.5 |
2.1 ± 0.4 |
0.332 |
|
|
2nd post op. 6MWT |
13.9 ± 1.2 |
14.4 ± 0.8 |
0.250 |
13.4 ± 1.3 |
13.8 ± 0.6 |
0.884 |
|
|
Pain score 1st post op. 6MWT (0-10) |
|||||||
|
n |
13 |
7 |
|
5 |
7 |
|
|
|
2 [1-5] 2.6 ± 1.6 |
4 [2-5] 3.7 ± 1.1 |
0.114 |
3 [2-5] 3.0 ± 1.2 |
3 [1-6] 3.0 ±1.6 |
0.933 |
||
|
Data are presented as mean ± SD. Abbreviations: 6 MWT: Six Minute Walk Test; Op: Operation; THA: Total Hip Arthroplasties, TKA: Total Knee Arthroplasties. |
|||||||
|
|
TKA (n = 25) |
THA (n = 18) |
||||
|
Carbohydrate (n=13) |
Placebo (n=12) |
P-value |
Carbohydrate (n=7) |
Placebo (n=11) |
P-value |
|
|
Mean duration of self-training (min) |
13.8 ± 3.3 |
19.7 ± 7.0 |
0.020 |
26.7 ± 5.8 |
15.2 ± 4.1 |
0.021 |
|
Training intensity, overall (1-5) |
2 [1-4] 2.2 ± 0.7 |
2 [1-5] 2.6 ± 1.3 |
0.419 |
2 [1-4] 2.4 ± 1.0 |
2 [1-3] 1.8 ± 0.8 |
0.017 |
|
Training intensity, with physiotherapist (1-5) |
2 [1-4] 2.4 ± 0.7 |
2.5 [1-5] 2.7 ± 1.4 |
0.539 |
2.5 [1-4] 2.5 ± 1.1 |
1.5 [1-3] 1.8 ± 0.9 |
0.044 |
|
Self-training is presented as mean ± SD. Training intensity is presented as mean [group range]. Abbreviations: THA: Total Hip Arthroplasties; TKA: Total Knee Arthroplasties. |
||||||
Discussion
In the era of fast track surgery, the optimization of every step of rehabilitation is crucial. Patients are often hospitalized only for a few days and need to learn how to continuously self-improve the rehabilitation process after discharge. None of the patients were malnourished, and all patients received the standard nutritional program for surgical departments including ONS shown to be beneficial at least after hip fractures [6].
In well-trained individuals carbohydrate intake after training with limited recovery time between training sessions is recommended to ensure optimal glycogen storage and thereby performance [2,4]. This is however not necessarily true for elderly untrained post-operative patients, as our results indicate in this first study investigating the effect of carbohydrate intake immediately after training in post-operative patients. Besides the small number of patients, there are several shortcomings in this study that could explain the non-convincing result. In contrast, the ingestion of maltodextrin preoperatively to reduce the postoperative insulin resistance is well described [9].
In healthy individuals glycogen depletion by 70-120 min exercise at >62% of VO2-max leads to an increased glycogen synthesis-rate due to insulin and exercise stimulated non-insulin dependent glucose uptake related to the GLUT-4 protein content of the plasma membrane, with the highest glucose uptake immediately at the end of glycogen depletion [10,11]. When time to recover between training sessions is limited, 1 g per kg body mass is therefore recommended immediately after training [3,4]. The patients in this study are not directly comparable to the healthy individuals reported. They are mainly untrained for many years, and in addition kept in a state of low mobility due to the severe arthrosis leading to operation. The estimated training intensity in the present study was probably >62% of VO2-max corresponding to slightly strenuous or strenuous exercise, while the mean duration of training sessions was only 19 minutes, and therefore not sufficient to ensure glycogen depletion. In the early postoperative phase patients have individual degrees of insulin resistance that declines over weeks [3], and the non-insulin dependent increase of muscle cell glucose transport after training could therefore potentially be even more relevant for postoperative patients compared to healthy individuals. Furthermore, although the optimal carbohydrate intake after training in post-surgery patients is unknown the ~0.4 g/kg provided to the patients in the intervention groups in the present study might have been too small.
The 6-MWT used in this study was not optimal, as patients experienced walking difficulties not only due to the reduced muscle function, but also due to the postoperative pain. Although training intensity may be a relevant factor to the degree of improvement, the physiotherapeutic assessment is associated with difficulties. Therefore, more sensitive, validated, and objective tests are needed to assess the degree of improvement-rate of rehabilitation in these patients.
This study is the first to focus on nutritional support in the early postoperative training period to optimize rehabilitation in patients with TKA and THA. Larger studies investigating the effect of different dosage of carbohydrate combined with protein intake are needed. Furthermore, it is recommended to include objective assessment of the training intensity, biomarkers of protein synthesis, the degree of stress metabolism, and insulin resistance in future studies.
Acknowledgements
Physiotherapist Amanda Sjunnesson is acknowledged for her support in construction of the training programs. The study was supported by an educational grant from the Department of Nutrition, Exercise and Sports, University of Copenhagen.
Statement of Authorship
The protocol was designed by JRA, CFW, MS, KH, and JB. The measurements were performed by CFW and MNN. Calculations were performed by JRA, CFW, and MS. ILF wrote the first draft. All authors have read and approved the final submitted manuscript.
Conflicts of Interest
None declared.
References
2. Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J Acad Nutr Diet. 2016;166:501-28.
3. Jentjens R, Jeukendrup AE. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003;33:117-44.
4. Price TB, Rothman DL, Taylor R, Avison MJ, Shulman GI, Shulman RG, et al. Human muscle glycogen resynthesis after exercise: insulin-dependent and -independent phases. J Appl Physiol. 1994;76:104-11.
5. Howarth KR, Phillips SM, MacDonald MJ, Richards D, Moreau NA, Gibala MJ. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J Appl Physiol. 2010;109:431-8.
6. Chen B, Z hang JH, Duckworth AD, Clement ND. Effect of oral nutritional supplementation on outcomes in older adults with hip fractures and factors influencing compliance. A systematic review and meta-analysis of randomized controlled trials. Bone Joint J. 2023;105:1149-58.
7. Hofman DL, van Buul VJ, Brouns FJ. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit Rev Food Sci Nutr. 2016;56:2091-100.
8. Piehl Aulin K, Söderlund K, Hultman E. Muscle glycogen resynthesis rate in humans after supplementation of drinks containing carbohydrates with low and high molecular masses. Eur J Appl Physiol. 2000;81:346-51.
9. Gümüs K, Pirhan Y, Aydin G, Keloglan S, Tasova V, Kahveci M. The effect of preoperative oral intake of liquid carbohydrate on postoperative stress parameters in patients undergoing laparoscopic cholecystectomy: An experimental study. J Perianest Nurs. 2021;36:526-31.
10. Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. 2011;29:S17-27.
11. Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. J Appl Physiol. 1988;64:1480-5.