Abstract
Proton minibeam radiotherapy (pMBRT) is an external beam radiotherapy method with reduced side effects by taking advantage of spatial fractionation in the normal tissue. Due to scattering, the delivered small beams widen in the tissue ensuring a homogeneous dose distribution in the tumor. In this review, the physical and biological principles regarding dose distribution and healing effects are explained. In the last decade, several preclinical studies have been conducted addressing normal tissue sparing and tumor control in-vitro and in-vivo, using human skin tissue and mouse or rat models. The major results acquired in these studies are summarized. A further newly emerging therapy method is FLASH radiotherapy, i.e. the treatment using ultra-high dose rates. The possibility of combining these methods in proton minibeam FLASH therapy (pMB FLASH) is worked out. Additionally, technical feasibility and limitations will be discussed by looking at simulations as well as preclinical studies and also pointing out new ways of delivering the desired tumor dose, such as interlacing. We will also highlight the opportunities that emerge regarding high dose radiation, hypofractionation and the combination with immunotherapy.
Keywords
Proton minibeam therapy, pMBRT, FLASH therapy, Hypofractionation, High dose, pMB FLASH, Radiotherapy.
Introduction
Radiotherapy (RT) is besides chemotherapy, surgery and immunotherapy, one of the four pillars of cancer treatment. Approximately 50% of all cancer patients worldwide are treated using radiotherapy. The application of radiation from the outside of the body (external beam, EBRT) is the major way of applying radiotherapy. The most frequently used method worldwide is intensity modulated radiotherapy (IMRT) using x-rays. Radiation affects tissue by damaging DNA in the cells. Therefore, healthy tissue is also damaged in the beam path in front (proximal) and behind (distal) the tumor, which limits the dose that can be applied to the tumor. The main aim of modern radiotherapy is to widen the therapeutic window, i.e. either by enhancing the efficiency of tumor control or by reducing the risk of side effects. Already in 1946, the idea of using protons for radiotherapy was introduced by R.R. Wilson [1]. Due to the unique depth dose distribution of particles following the Bragg curve, no radiation is applied to tissue behind the tumor, as particles are stopped in the Bragg peak [2]. This clearly reduces the chances of side effects, although healthy tissue in front of the tumor is still exposed to radiation and therefore damaged.
A method to reduce normal tissue damage in front of the tumor is spatial fractionation, introduced in 1909 by Alban Köhler via the use of grids in x-ray therapy [3]. Spatial fractionation - as opposed to time/dose fractionation - reduces radiation damage in normal tissue by simply sparing (large) parts of it from radiation. Continuous research from the 1950s led to the development of microbeam radiotherapy (MRT) using dedicated research beamlines at synchrotron radiation sources in Brookhaven National Laboratory (USA) [4] and ESRF in France in the 1990s [5], investigating the benefits and constraints of the MRT method for patient treatment. In MRT, radiation is applied in a grid like pattern with high doses in the radiation channels and low doses in the valleys in between. Using photons for this method, the tumor is irradiated with the same beam pattern with peaks and valleys as the normal tissue. Typical dimensions of the microplanar beams for pre-clinical studies are 25-100 μm with a spacing of several hundred μm. Entrance doses of several hundred Grays and valley doses of approx. 10-30 Gy are conventionally used in this method [6-8]. Although promising results in rat and mouse brains [8-10] give the opportunity to push this method further to clinical trials, several disadvantages and problems remain.
First of all, valley doses are very high regarding the normal tissues but at the same time low in the tumor: healthy tissue between the microbeams is still receiving non-negligible doses which limit the beneficial effects of MRT, while tumor cells in the valleys might not be killed by doses lower than in conventional RT, thus endangering efficient tumor control. Furthermore, the problem of irradiating tissue behind the tumor remains using photon beams. Finally, the production of a beam pattern with such small sizes for clinical use is technically challenging.
Another quite new exploration is FLASH radiotherapy, i.e. radiotherapy using ultra-high dose rates ≥ 40 Gy/s [11], which has been shown to reduce radiation toxicity in normal healthy tissues with similar tumor control efficiency as conventional dose rate irradiation in preclinical models. The reduction in normal tissue toxicity was first discovered in the last century in murine gut and skin models [12,13] and confirmed in mice tail using high instead of conventional dose rates [14]. In 2014, this phenomenon has been “re-discovered” by Favaudon et al. [11], yielding sparing of normal tissues together with a comparable tumor effect as in conventional RT, examined with electrons, photons and protons in various normal tissue and tumor models [11,15-17]. Already a first melanoma patient was treated using FLASH therapy [18]. Studies using pulsed proton beams with FLASH dose rates have also been performed in cells [19-24], tissues [24] and mice [25] at the ion microbeam SNAKE (superconducting nanoprobe for applied nuclear (kern-) physics experiments, Garching Germany) [26,27], showing the same normal tissue and tumor effects. The difference in dose application time between FLASH and conventional RT (28,29]. Furthermore, a lower fraction of circulating blood cells is irradiated, however with (much) higher doses, potentially affecting the response of the immune system [30].
Proton minibeam radiotherapy (pMBRT) and especially combining it with FLASH radiotherapy (pMB FLASH) can give solutions to all the challenges of x-ray MRT and MBRT, while including all advantages of proton and FLASH radiotherapy. In this article, we will give an overview on the various performed preclinical studies on pMBRT as well as on possibilities for future studies in both pMBRT and pMB FLASH. Finally, we will give a perspective on the implementation of pMBRT into clinics and its potential to revolutionize radiotherapy regarding hypofractionation and by its combination with FLASH.
Proton Minibeam Radiotherapy Principle
Physical background
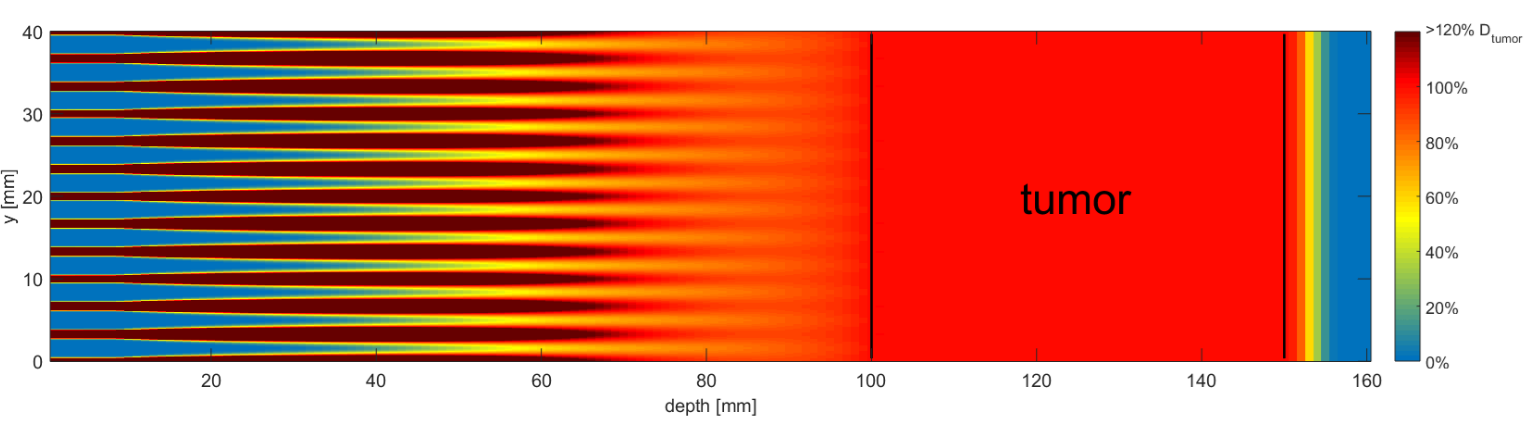
Proton minibeam radiotherapy [31,32] exploits the large angular straggling (Multiple Coulomb scattering) of the protons to merge the widened minibeams to a homogeneous broad beam in the tumor and achieve a conformal dose distribution like in conventional RT (Figure 1). Minibeam irradiation is similar to conventional pencil beam scanning techniques, where a pencil beam of size σ (or FWHM = 2.35 σ, typically a few mm) is scanned on a rectangular grid covering the target area. In standard proton RT, the inter-beam or center-to-center distance (ctc) between two adjacent points on the grid is typically chosen smaller than 1.5 – 2 times the pencil beam width (ctc ≤ 2σ), resulting in a nearly homogeneous dose distribution in the tumor, but also already in the skin of the patient. In micro- or minibeam irradiations, the proton beam width is much smaller, in the micrometer or at least sub-millimeter range , while the inter-beam distances remain in the millimeter range. Due to small angle scattering of the protons, together with initial beam divergence, the minibeams spread laterally while passing through normal tissue and merge together at the depth of the tumor. The inter-beam distance is optimized for a dose coverage of the target between 95% and 107% of the prescribed tumor dose according to ICRU [33]. The ctc can be further enlarged when introducing additional beam divergence or when dropping the upper dose constraint and allowing heterogeneous tumor doses with only a limit for the minimum prescribed dose. The number of protons per minibeam is the same as in the case of mm-sized pencil beams applied on the same grid, and thus results in a homogeneous tumor irradiation with the same average dose that is reached with a scanned pencil beam or broad beam.
The dose profile with peaks and valleys is often characterized by the peak-to-valley dose ratio (PVDR), which is the quotient of the dose in the minibeams and the dose between the beams. Large PVDR ratios at the beam entrance are desired for efficient radiotherapy with low normal tissue damage, meaning peak doses that are lethal to most dividing cells and valley doses that are low enough to allow most normal cells to survive the radiation.
Biological background
A higher tolerance for spatially segmented than for continuous radiation beams is attributed to undamaged, migratory cells adjacent to the radiation-damaged areas [4,5], but the biological mechanisms are still not completely understood. In minibeam or grid therapy, small non-confluent areas of the skin and subcutaneous normal tissues have been shown to tolerate large radiation doses without significant acute or late normal tissue damage [34-36]. This is attributed to the “dose-volume effect” [37], which means that the maximum tolerable doses increase as irradiated tissue volumes are made smaller. The cells in the path of the minibeams receive very high doses causing high cell death rates in these regions, but migrating viable cells from the unirradiated regions adjacent to the irradiated site can infiltrate the damaged tissue and thus reduce tissue necrosism [4,38]. Another possible explanation is the “microscopic prompt tissue repair effect” for submillimeter beam diameters, describing the fast repair of capillary blood vessels in the direct path of the minibeams via regeneration of angiogenic cells from the undamaged regions in-between within days or even hours, which then can support and enhance the repair of other normal tissues in the minibeam paths [39-41]. Further effects that might play a role in minibeam radiotherapy are the so-called radiation-induced bystander effects in cells not directly hit by radiation [42,43].
Preclinical studies with pMBRT
The investigation of the effects of proton minibeam radiotherapy was started with skin tissue irradiations at the ion microbeam SNAKE [31,34]. These studies showed enhanced tissue viability, less expression of inflammatory parameters and enhanced conservation of genetic stability in a human skin model using proton microbeams of 10 μm, 50 μm and 180 μm. The promising results in this tissue studies initiated a variety of preclinical animal studies.
In the first in-vivo study at SNAKE, the right ear of BALB/c mice was chosen as a more authentic and complex skin model with vasculature and all naturally occurring cell types, together with a working immune system. Irradiations using a grid of 4 × 4 minibeams with a size of 180 μm (square) and a ctc distance of 1.8 mm were compared to a homogeneous field of 7.2 mm × 7.2 mm [44]. The mean dose in both cases was 60 Gy, which means a minibeam dose of 6000 Gy (peak dose) and a valley dose of 0 Gy in between the minibeams. Minibeams were generated by scanning a focused microbeam (i.e. beam size of ~ 1 μm) to form a quadratic spot of the desired sub-millimeter dimensions. Despite the low proton energy of only 20 MeV, the found effects on the mouse ear are still transferrable to clinics, as the difference in dose deposition and scattering is not relevant for the proof of concept. In the 90 days follow-up, the authors show that the acute side effects, represented by ear swelling and inflammation scoring, were absent after minibeam irradiation, whereas ear swelling of up to 4 times and clear erythema, desquamation, changes in ear morphology as well as hair loss was monitored in the homogeneously irradiated ears. Additionally, histologic findings 90 days after irradiation showed loss of sebaceous glands, inflammation, fibrosis and enlargement of epidermis for homogeneous irradiation and no histological changes for minibeam irradiation.
More recent studies also investigated the dependence on minibeam size in order to mimic the way of the beam to the tumor and to elaborate the recommendations for the largest beneficial minibeam size [45]. A photon study on single beams spots showed that for beam sizes ≥ 3 mm, severe side effects occur and only beam sizes of up to 1 mm produced no side effects when applied alone. Guided by this study, the group investigated the effects of different proton minibeam sizes and irradiated mouse ears with 4 × 4 proton minibeams with a ctc of 1.8 mm and Gaussian shaped beams with sizes of σ = 95, 199, 306, 411 and 561 μm (standard deviation) [46]. They could show that each minibeam geometry induced less acute side effects compared to homogeneous irradiation (σ = 883 μm), again monitored by ear thickness and inflammation scoring.
The group of Prezado et al. at the Orsay proton therapy center [47] showed the positive effect of proton minibeams in rat brain. The whole (tumor-free) rat brain was irradiated with a mean dose of 25 Gy either homogeneously or in a minibeam geometry with planar beam sizes of 1.1 mm × 2 cm and a beam distance of 3.2 mm, generated via a multislit collimator. The peak dose for the minibeam geometry was 57 Gy and the valley dose 8.8 Gy. The rats were followed up for 6 months. Homogeneously irradiated rats showed a delayed weight gain with 15% less maximum weight compared to non-irradiated or minibeam treated mice. Additionally, homogeneously irradiated rats showed clinical symptoms such as apathy and reduced appetite (3 out of 8 had to be sacrificed). Furthermore, these rats showed permanent epilation and moist desquamation of the irradiated skin comparable to the effects seen in the studies of Girst et al. 2016 [44] and Sammer et al. 2019 [46]. In contrast, minibeam irradiated mice only showed reversible epilation in the path of the minibeam and no clinical symptoms or weight loss were observed. Further investigations performed on the homogeneously irradiated rat brains using MRI revealed severe damage in the hippocampal formation, hypothalamus, periaqueductal gray areas, basal forebrain and brainstem. Furthermore, the blood brain barrier was broken in various locations. By contrast, the minibeam irradiated rat brains showed no abnormalities in the MRI scans. Additional histopathological analysis revealed various lesions including neuro-inflammatory processes and necrosis in the homogeneously irradiated brains, whereas only one rat irradiated with minibeams showed lesions, which however were much less pronounced.
In a further study, Prezado and co-workers investigated the potential of pMBRT on tumor treatment using high-grade gliomas in rats. Here, they could show that irradiation of gliomas using minibeam geometry achieved tumor control in 22% of the mice. Again, no side effects were visible for 6 months after irradiation after applying a mean dose of 30 Gy with the same geometry than in the previous study leading to a peak dose of 70 Gy [48]. Furthermore, in their most recent study, they compared proton minibeam and conventional proton treatment in rat gliomas with a single shot mean dose of 25 Gy. Despite the absence of any detectable tumor after 170 days followup, the homogeneously irradiated group showed only 22% survival with substantial brain damage. In contrast, minibeam treatment led to tumor control with increased survival of up to 67% and less severe brain toxicity [49].
Fractionation, high dose and FLASH effect in minibeam therapy
All preclinical pMBRT studies performed so far have used single doses much higher than in clinical radiotherapy which resulted in no or only low side effects. In the BALB/c ear model, homogeneous irradiation with single doses higher than 10 Gy [44] and also fractionation using 4 daily fractions of up to 30 Gy [50] have shown dose-dependent adverse effects in mouse skin. This argues strongly for the performance of minibeam radiotherapy with high dose fractions and thus a lower total number of fractions, called hypofractionation. Nevertheless, the question remains, if fractionated minibeam therapy can lower the side effects and how the location of the minibeams between different fractions should be chosen to give the most positive effect. Several possibilities are explored in an ongoing study at SNAKE [51]. Here it is tested, if it is necessary to always hit the very same position of the minibeams, or never hit an irradiated position again, or if the beam location for each fraction is not of interest. The outcome of this study will clearly influence the technical feasibility of pMBRT, as patient positioning in the range of 100 μm would be necessary to ensure that the very same position is hit in every fraction. This would bring enormous challenges to patient positioning.
As already discussed in a recent review by Eling et al. from ESRF [52], the effects seen in MRT or MBRT experiments at these ultra-high doses might already include the FLASH effect. While the dose rate used in the rat brain studies by Prezado was 2 Gy/min, the dose rate used for the minibeam irradiations at SNAKE [44] was approximately 75 Gy/s, so already in the FLASH region. Beside the large spatial fraction of the ear tissue between the minibeams spots that were spared completely from irradiation, this ultra-high dose rate within the minibeams might have contributed as well to the high tolerance of the ear skin to high dose minibeam irradiations. Using pMB FLASH would thus further reduce problems with intra-fractional movements such as breathing.
Technical parameters and limitations
Several theoretical studies on the size and arrangement of proton beams for pMBRT have been conducted. The studies in the group of Prezado rely on planar beams, i.e. beams that are small in one direction and large in the other. Identifying the best possible beam arrangement is necessary to get the best possible sparing of healthy tissue, while preserving tumor control.
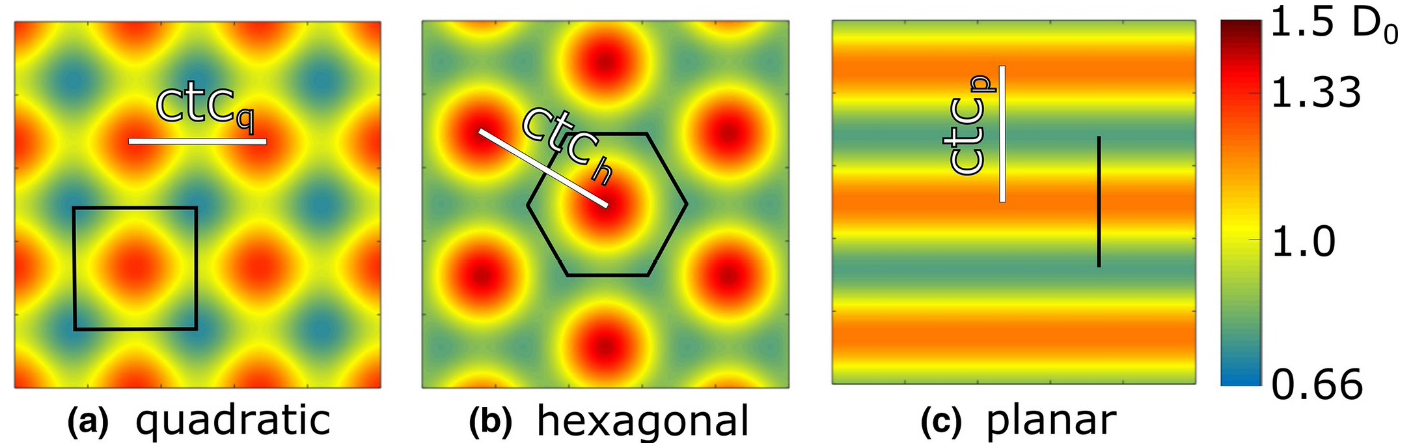
Sammer et al. [53] conducted a simulation study on this topic, where they simulated the cell survival from the skin entrance to the tumor for different beam geometries - planar, pencil with quadratic arrangement and pencil with hexagonal arrangement (Figure 2).
They chose the ctc distances in the different geometries such that the dose in the tumor was between 97.5% and 103.5% of the desired tumor dose. Dose distributions were calculated using MATLAB and LAP-CERR [54]. Physical doses were translated into theoretical cell survival using the linear quadratic model and mean α and β values for human cells from the database PIDE at 2 Gy and 10 Gy. It turned out that a lower cell survival on the way to the tumor can be expected using a planar beam arrangement compared to both pencil beam geometries, which are quite similar. This result clearly suggests the use of pencil beams instead of planar beams for future research and clinical implementation. In the hexagonal arrangement, the homogeneity criterion is met earlier compared to quadratic arrangement. For implementation, this means that for the same initial beam size and divergence the ctchexagonal can be chosen 1.14 times larger than the ctcquadratic, resulting in an additional slight enhancement of tissue sparing for the hexagonal arrangement.
The remaining question is now on how to prepare the minibeams for clinics, which have to be in the range of one millimeter or better below. There are two main methods of preparing a minibeam - collimation or focusing - which both have advantages and disadvantages. The beam size for collimated beams is limited by the scattering of the beams within the multislit collimator and at the edges – also leading to higher valley doses - and is in the range of several hundreds of micrometers [41]. In the first clinical implementation of pMBRT at the Institut Curie-Orsay proton therapy center, minibeams of 400 and 700 μm width were generated using 230 MeV protons [55]. A further limit to the usage of collimators is the production of secondary radiation, especially neutrons, due the stopping of the majority of particles in the collimator [56]. For minibeam therapy, the collimator has to be very close to the patient which further enhances the parasitic dose the patient is exposed to [57].
Therefore, a more favorable way of producing minibeams is by focusing a proton beam. Due to magnet stiffness and lens production variability resulting in chromatic aberrations, the possible achievable size for protons used for patient treatment is in the 100 μm range. This is still sufficient to meet the requirements of pMBRT, which requires beams (58]. The extraction mechanism for synchrotrons drastically changes the horizontal phase space [59,60] and in cyclotrons the degrader which is necessary to slow down the beam for patient treatment also destroys phase space [61], which makes proper focusing as chromatic aberrations occur. A promising accelerator technology, which was already introduced in the 1920s is the LINAC principle [62,63]. These linear radiofrequency accelerators provide pulsed beams of high brightness and ultra-high dose rates. This technology was supplanted by the - at that time - smaller and more cost efficient circular accelerators but is currently being rediscovered. The development in precise metal component production and radiofrequency technology possibly makes them smaller and more cost efficient. Currently, a prototype LINAC accelerator for patient treatment is under construction by the company AVO-ADAM [64] and will be located in London.
Conclusion
In this short review, we showed that proton minibeam radiotherapy alone and in combination with FLASH radiotherapy is a promising method for future tumor treatment. The unique depth dose distribution of particle radiation already spares the tissue behind the tumor. Superimposing this with spatial fractionation leads to additional sparing of the healthy tissue in front of the tumor. The beneficial effects for normal tissue have been shown in several preclinical animal studies using either mice or rats at the accelerator lab SNAKE in Munich and at the Orsay proton therapy center in France. First studies on tumor control using pMBRT have also been conducted in Orsay. Further proton minibeams with small animal platforms have been installed at the University of Maryland/ MD Anderson Cancer Center [40,41] and at the University of Washington in Seattle [65,66] in the last years.
A lot of technical questions arise for such a new technology, which are summarized in this article. One major question is related to the PVDR, i.e. peak and valley doses, in the normal tissue and in the tumor. Obviously, valley doses should be close to zero at the skin, as only this ensures least damage and thus least side effects. The question on the PVDR in the tumor cannot easily be answered. Following the ICRU constraints of 95% and 107% of desired tumor dose, clearly defines the PVDR as 107%/95% = 1.126. Nevertheless, this new method also gives the opportunity to really think in new paths. Giving up the restriction on maximum dose of 107% of tumor dose, further called pseudo-homogeneous, would open up many possibilities to fully exploit the potential of pMBRT and further decrease side effects. One of these possibilities would be the interlacing of beams from two or more entrance directions, i.e. adjusting position and spacing of the beams such that the “gaps” in the irradiation fields are filled from another direction. Simulations indicate that pseudo-homogeneous irradiation of the tumor is then feasible even with larger ctc distances. This again would reduce side effects in each channel due to the splitting of the dose in more then one channel as already exploited in conventional IMRT, with the additional benefit of pMBRT. To investigate the full potential of interlacing, further preclinical studies have to be conducted. However, interlacing – together with fractionation - would require an even higher delivery and positioning accuracy.
With pMBRT, higher radiation tolerance of tissue can be achieved resulting in the possibility of using higher doses per fraction. This opens the possibility of hypofractionation, reducing costs as well as physical and mental stress for the patient, but these treatment protocols have to be investigated in detail using preclinical studies. This opens the possibility of hypofractionation, reducing costs and physical and mental stress for the patient, but has to be investigated in detail using preclinical studies. The reproducibility of the spot positions between different fractions is already addressed in an ongoing study at SNAKE but one major remaining question is how much dose per fraction can be tolerated for certain beam sizes in the normal tissue by also considering tumor control.
Additionally, pMB FLASH radiotherapy seems to be an even more promising therapeutic approach. Some of the studies shown, already used FLASH dose rates and the results are all positive. Beside the biological FLASH effect, tumor irradiation using high dose rates has one clear and easy advantage: As irradiation time per minibeam, beam direction and also per fraction is shortened to the subsecond regime, motion management is made very simple. Breathing motion and even heartbeat are slow compared to irradiation times of one single minibeam in pMBRT. This opens the possibility to adapt the timing of beam irradiation to the patient movement and thus enhance treatment accuracy.
Finally, the technology for producing such beams is, although not common, already existing, but will have to be adapted to the special requirements of minibeam fractionation, interlacing and FLASH pMBRT. New technological developments which aim to execute pMBRT and combine it with FLASH have to face and solve the challenges and chances discussed in this review. Already new technologies are emerging such as the PHASER system [67] for FLASH therapy and the LINAC principle [62,64] for pMBRT.
We suggest that for these emerging technologies, currently established systems such as fixed beam or usage of gantries should be critically evaluated. And the most promising technology should be used to achieve best possible results regarding accuracy, positioning and timing. Finally, we encourage everybody to critically but also open-mindedly evaluate the possibility of hypofractionation using these technologies to further enhance the positive effects for the patient during treatment. Nevertheless, new developments should not only include technological developments but also should have a closer look to the involvement of the immune system and evaluate the possibilities of combining immunotherapy with the new radiotherapy methods. Common immunotherapy drugs e.g. antagonizing CTLA4 [68] or blocking PD-1/PD-L1 pathway [69] are promising to enhance tumor radiosensitivity and might further enhance the prospects of pMBRT and FLASH therapy.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors are funded by BMBF? project 02NUK031A (LET-Verbund) and DFG Cluster of Excellence: Munich- Centre for Advanced Photonics.
Acknowledgement
We kindly thank Matthias Sammer for providing Figure 1, Günther Dollinger for the inspiring discussions and Gerd Datzmann for his support and proof-reading.
References
2. Podgorsak EB, editor. Radiation oncology physics: A handbook for teachers and students. Vienna; 2005.
3. Köhler AA. Method of Deep Roentgen Irradiation without Injury to the Skin. Archives of The Roentgen Ray 1909; 14(5):141-142.
4. Slatkin DN, Dilmanian FA, Spanne PO, inventors. Method for microbeam radiation therapy: U.S. Patent.
5. Laissue JA, Geiser G, Spanne PO, Dilmanian FA, Gebbers JO, Geiser M, Wu XY, Makar MS, Micca PL, Nawrocky MM, Joel DD. Neuropathology of ablation of rat gliosarcomas and contiguous brain tissues using a microplanar beam of synchrotron-wiggler-generated x rays. International journal of cancer. 1998 Nov 23;78(5):654-60.
6. Anschel DJ, Bravin A, Romanelli P. Microbeam radiosurgery using synchrotron-generated submillimetric beams: a new tool for the treatment of brain disorders. Neurosurg Rev 2010; 34(2):133-42.
7. Grotzer MA, Schültke E, Bräuer-Krisch E, Laissue JA. Microbeam radiation therapy: Clinical perspectives. Phys Med 2015; 31(6):564-7.
8. Prezado Y, Deman P, Varlet P, Jouvion G, Gil S, Le Clec’H C, Bernard H, Le Duc G, Sarun S. Tolerance to dose escalation in minibeam radiation therapy applied to normal rat brain: long-term clinical, radiological and histopathological analysis. Radiation research. 2015 Aug 18;184(3):314-21.
9. Schültke E, Bräuer-Krisch E, Blattmann H, Requardt H, Laissue JA, Hildebrandt G. Survival of rats bearing advanced intracerebral F 98 tumors after glutathione depletion and microbeam radiation therapy: conclusions from a pilot project. Radiat Oncol 2018; 13(1): 89.
10. Prezado Y, Sarun S, Gil S, Deman P, Bouchet A, Le Duc G. Increase of lifespan for glioma-bearing rats by using minibeam radiation therapy. J Synchrotron Radiat 2012; 19(Pt 1): 60–5.
11. Favaudon V, Caplier L, Monceau V, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014; 6(245): 245ra93.
12. HORNSEY S, ALPER T. Unexpected Dose-rate Effect in the Killing of Mice by Radiation. Nature 1966; 210(5032):212–3.
13. Field SB, Bewley DK. Effects of Dose-rate on the Radiation Response of Rat Skin. International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine 1974; 26(3):259–67.
14. Hendry JH, Moore JV, Hodgson BW, Keene JP. The Constant Low Oxygen Concentration in All the Target Cells for Mouse Tail Radionecrosis. Radiat Res 1982; 92(1):172.
15. Montay-Gruel P, Petersson K, Jaccard M, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiotherapy and Oncology 2017; 124(3):365-9.
16. Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, Bouchaab H. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clinical Cancer Research. 2019 Jan 1;25(1):35-42.
17. Loo BW, Schuler E, Lartey FM, Rafat M, King GJ, Trovati S, Koong AC, Maxim PG. (P003) delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. International Journal of Radiation Oncology• Biology• Physics. 2017 Jun 1;98(2):E16.
18. Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond JF, Moeckli R. Treatment of a first patient with FLASH- radiotherapy. Radiotherapy and oncology. 2019 Jul 11.
19. Schmid TE, Dollinger G, Hauptner A, et al. No evidence for a different RBE between pulsed and continuous 20 MeV protons. Radiat Res 2009; 172(5):567-74.
20. Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, Molls M, Röper B. Relative biological effectiveness of pulsed and continuous 20 MeV protons for micronucleus induction in 3D human reconstructed skin tissue. Radiotherapy and Oncology. 2010 Apr 1;95(1):66-72.
21. Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, Auer S, Friedl AA, Schmid E, Molls M, Röper B. The effectiveness of 20 MeV protons at nanosecond pulse lengths in producing chromosome aberrations in human-hamster hybrid cells. Radiation research. 2011 Mar 25;175(6):719-27.
22. Zlobinskaya O, Schmid TE, Dollinger G, et al. Differences in Gamma-H2AX Foci Formation after Irradiation with Continuous and Pulsed Proton Beams. In: Dössel O, Schlegel WC, editors. World Congress on Medical Physics and Biomedical Engineering: 7 - 12 September, 2009, Munich, Germany; [WC 2009; 11th international congress of the IUPESM. Berlin: Springer 2009; 142-5.
23. Zlobinskaya O, Dollinger G, Michalski D, Hable V, Greubel C, Du G, Multhoff G, Röper B, Molls M, Schmid TE. Induction and repair of DNA double-strand breaks assessed by gamma-H2AX foci after irradiation with pulsed or continuous proton beams. Radiation and environmental biophysics. 2012 Mar 1;51(1):23-32.
24. Zlobinskaya O, Siebenwirth C, Greubel C, Hable V, Hertenberger R, Humble N, Reinhardt S, Michalski D, Röper B, Multhoff G, Dollinger G. The effects of ultra- high dose rate proton irradiation on growth delay in the treatment of human tumor xenografts in nude mice. Radiation research. 2014 Feb 13;181(2):177-83.
25. Auer S, Hable V, Greubel C, et al. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat Oncol 2011; 6(1):1-8.
26. Dollinger G, Datzmann G, Hauptner A, Hertenberger R, Körner HJ, Reichart P, Volckaerts B. The Munich ion microprobe: Characteristics and prospect. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms. 2003 Sep 1;210:6-13.
27. Hauptner A, Dietzel S, Drexler GA, Reichart P, Krücken R, Cremer T, Friedl AA, Dollinger G. Microirradiation of cells with energetic heavy ions. Radiation and environmental biophysics. 2004 Feb 1;42(4):237-45.
28. Bourhis J, Montay-Gruel P, Jorge PG, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M, Bochud F, Moeckli R, Germond JF. Clinical translation of FLASH radiotherapy: Why and how?. Radiotherapy and Oncology. 2019 Jun 25.
29. Weiss H, Epp ER, Heslin JM, Ling CC, Santomasso A. Oxygen Depletion in Cells Irradiated at Ultra-high Dose-rates and at Conventional Dose-rates. International Journal of Radiation Biology and Related Studies in Physics, Chemistry and Medicine 1974; 26(1):17-29.
30. Maxim PG, Keall P, Cai J. FLASH radiotherapy: Newsflash or flash in the pan? Med Phys 2019.
31. Zlobinskaya O, Girst S, Greubel C, Hable V, Siebenwirth C, Walsh DW, Multhoff G, Wilkens JJ, Schmid TE, Dollinger G. Reduced side effects by proton microchannel radiotherapy: study in a human skin model. Radiation and environmental biophysics. 2013 Mar 1;52(1):123-33.
32. Prezado Y, Fois GR. Proton-minibeam radiation therapy: a proof of concept. Med Phys 2013; 40(3): 31712.
33. Prescribing, Recording, and Reporting Proton-Beam Therapy (ICRU report No. 78) 2007; (78).
34. Girst S, Greubel C, Reindl J, Siebenwirth C, Zlobinskaya O, Dollinger G, Schmid TE. The influence of the channel size on the reduction of side effects in microchannel proton therapy. Radiation and environmental biophysics. 2015 Aug 1;54(3):335-42.
35. Girst S, Marx C, Bräuer-Krisch E, Bravin A, Bartzsch S, Oelfke U, Greubel C, Reindl J, Siebenwirth C, Zlobinskaya O, Multhoff G. Improved normal tissue protection by proton and X-ray microchannels compared to homogeneous field irradiation. Physica Medica. 2015 Sep 1;31(6):615-20.
36. Mohiuddin M, Curtis DL, Grizos WT, Komarnicky L. Palliative treatment of advanced cancer using multiple nonconfluent pencil beam radiation: a pilot study. Cancer. 1990 Jul 1;66(1):114-8.
37. Withers HR, Thames HD. Dose fractionation and volume effects in normal tissues and tumors. Am J Clin Oncol 1988; 11(3):313-29.
38. Straile WE, Chase HB. The Use of Elongate Microbeams of X-Rays for Simulating the Effects of Cosmic Rays on Tissues: A Study of Wound Healing and Hair Follicle Regeneration. Radiat Res 1963; 18(1):65.
39. Dilmanian FA, Morris GM, Hainfeld JF, inventors. Methods for implementing microbeam radiation therapy: U.S. Patent.
40. Dilmanian FA, Eley JG, Krishnan S. Minibeam therapy with protons and light ions: physical feasibility and potential to reduce radiation side effects and to facilitate hypofractionation. Int J Radiat Oncol Biol Phys 2015; 92(2):469-74.
41. Dilmanian FA, Eley JG, Rusek A, Krishnan S. Charged Particle Therapy with Mini-Segmented Beams. Front Oncol 2015; 5:269.
42. Dilmanian FA, Qu Y, Feinendegen LE, Peña LA, Bacarian T, Henn FA, Kalef-Ezra J, Liu S, Zhong Z, McDonald JW. Tissue-sparing effect of x-ray microplanar beams particularly in the CNS: is a bystander effect involved?. Experimental hematology. 2007 Apr 1;35(4):69-77.
43. Belyakov OV, Mitchell SA, Parikh D, Randers- Pehrson G, Marino SA, Amundson SA, Geard CR, Brenner DJ. Biological effects in unirradiated human tissue induced by radiation damage up to 1 mm away. Proceedings of the National Academy of Sciences. 2005 Oct 4;102(40):14203-8.
44. Girst S, Greubel C, Reindl J, Siebenwirth C, Zlobinskaya O, Walsh DW, Ilicic K, Aichler M, Walch A, Wilkens JJ, Multhoff G. Proton minibeam radiation therapy reduces side effects in an in vivo mouse ear model. International Journal of Radiation Oncology* Biology* Physics. 2016 May 1;95(1):234-41.
45. Sammer M, Teiluf K, Girst S, Greubel C, Reindl J, Ilicic K, Walsh DW, Aichler M, Walch A, Combs SE, Wilkens JJ. Beam size limit for pencil minibeam radiotherapy determined from side effects in an in-vivo mouse ear model. PloS one. 2019 Sep 4;14(9):e0221454.
46. Sammer M, Zahnbrecher E, Dobiasch S, et al. Proton pencil minibeam irradiation of an in-vivo mouse ear model spares healthy tissue dependent on beam size. Plos One 2019; (Under review).
47. Prezado Y, Jouvion G, Hardy D, Patriarca A, Nauraye C, Bergs J, González W, Guardiola C, Juchaux M, Labiod D, Dendale R. Proton minibeam radiation therapy spares normal rat brain: Long-Term Clinical, Radiological and Histopathological Analysis. Scientific reports. 2017 Oct 31;7(1):14403.
48. Prezado Y, Jouvion G, Patriarca A, Nauraye C, Guardiola C, Juchaux M, Lamirault C, Labiod D, Jourdain L, Sebrie C, Dendale R. Proton minibeam radiation therapy widens the therapeutic index for high-grade gliomas. Scientific reports. 2018 Nov 7;8(1):16479.
49. Prezado Y, Jouvion G, Guardiola C, Gonzalez W, Juchaux M, Bergs J, Nauraye C, Labiod D, De Marzi L, Pouzoulet F, Patriarca A. Tumor Control in RG2 Glioma- Bearing Rats: A Comparison Between Proton Minibeam Therapy and Standard Proton Therapy. International Journal of Radiation Oncology* Biology* Physics. 2019 Jun 1;104(2):266-71.
50. Dombrowsky AC, Schauer J, Sammer M, Blutke A, Walsh DW, Schwarz B, Bartzsch S, Feuchtinger A, Reindl J, Combs SE, Dollinger G. Acute Skin Damage and Late Radiation-Induced Fibrosis and Inflammation in Murine Ears after High-Dose Irradiation. Cancers. 2019 May;11(5):727.
51. Schmid TE, Hunger A, Sammer M, Zahnbrecher E, Reindl J, Ilicic K, Walsh D, Greubel C, Schwarz B, Wilkens JJ, Dollinger G. PV-0570: Proton minibeam radiation therapy (pMBRT): a novel approach to minimize normal tissue damage. Radiotherapy and Oncology. 2018 Apr 1;127:S300.
52. Eling L, Bouchet A, Nemoz C, Djonov V, Balosso J, Laissue J, Bräuer-Krisch E, Adam JF, Serduc R. Ultra high dose rate Synchrotron Microbeam Radiation Therapy. Preclinical evidence in view of a clinical transfer. Radiotherapy and Oncology. 2019 Jul 12.
53. Sammer M, Greubel C, Girst S, Dollinger G. Optimization of beam arrangements in proton minibeam radiotherapy by cell survival simulations. Med Phys 2017; 44(11):6096-104.
54. Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys 2003; 30(5):979-85.
55. Peucelle C, Nauraye C, Patriarca A, Hierso E, Fournier- Bidoz N, Martínez-Rovira I, Prezado Y. Proton minibeam radiation therapy: Experimental dosimetry evaluation. Medical physics. 2015 Dec;42(12):7108-13.
56. Guardiola C, Peucelle C, Prezado Y. Optimization of minibeam generation by mechanical collimation in proton minibeam radiation therapy. Physica Medica 2016; 32:291.
57. Schneider U, Agosteo S, Pedroni E, Besserer J. Secondary neutron dose during proton therapy using spot scanning. International Journal of Radiation Oncology*Biology*Physics 2002; 53(1):244-51.
58. Amaldi U, Bonomi R, Braccini S, Crescenti M, Degiovanni A, Garlasché M, Garonna A, Magrin G, Mellace C, Pearce P, Pittà G. Accelerators for hadrontherapy: from Lawrence cyclotrons to linacs. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2010 Aug 11;620(2-3):563-77.
59. Bryant PJ, Borri G, Crescenti M, Badano L, Maier AT, Weisser L, Reimoser S, Pavlovic M, Knaus P, Pullia M, Benedikt M. Proton-Ion Medical Machine Study (PIMMS), 2. 2000 Aug 1.
60. Badano L, Crescenti M, Holy P, Maier AT, Knaus P, Bryant PJ, Benedikt M, Pullia M, Rossi S. Proton-Ion Medical Machine Study (PIMMS), 1. 1999 Mar 2.
61. Jongen Y, Laycock S, Abs M, et al. The proton therapy system for the NPTC: equipment description and progress report. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms 1996; 113(1):522-5.
62. Ising G. Prinzip einer Methode zur Herstellung von Kanalstrahlen hoher Voltzahl. Ark. Mat. Astron. Fys. 1924; 18:1-4.
63. Wideröe R. Über ein neues Prinzip zur Herstellung hoher Spannungen. Archiv f. Elektrotechnik 1928; 21(4):387-406.
64. AVO-ADAM. First LIGHT System: In Central London. Available from: URL: https://www.avoplc.com/Our- Technology/Toward-the-First-Installation-of-LIGHT.
65. Lee E, Meyer J, Sandison G. Collimator design for spatially-fractionated proton beams for radiobiology research. Phys. Med. Biol. 2016; 61(14):5378.
66. Meyer J, Stewart RD, Smith D, Eagle J, Lee E, Cao N, Ford E, Hashemian R, Schuemann J, Saini J, Marsh S. Biological and dosimetric characterisation of spatially fractionated proton minibeams. Physics in Medicine & Biology. 2017 Nov 21;62(24):9260.
67. Maxim PG, Tantawi SG, Loo BW. PHASER: A platform for clinical translation of FLASH cancer radiotherapy. Radiotherapy and Oncology 2019.
68. Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 2011; 17(22):6958-62.
69. Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, Singh S. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non- human primates. Cancer immunology research. 2014 Sep 1;2(9):846