Abstract
Production of high value recombinant pharmaceuticals using plant-based systems, otherwise called molecular pharming has greatly advanced over the past few years. In particular, recombinant prophylactic and therapeutic vaccines as well as therapeutic antibodies against infectious human viruses have been generated by stable expression using transgenic plant systems while transient expression has been accomplished using plant virus-based expression mechanisms. A majority of them have been shown to be effective in animal models while many have proved to be successful in human clinical trials. This mini-review focuses on recent developments in vaccine biopharming towards combating human viral infections addressed by the recent review of Venkataraman et al., 2021 in addition to taking a critical look of the perspectives presented in this review. Broadly, the current manuscript brings to light recent progresses made in molecular biopharming against viruses such as the HBV, HCV, HPV, Influenza, SARS-CoV-2 and the Zika virus through various plant-based modalities of expression. Importantly, this mini-review highlights the incidence of HIV infection, molecular determinants of HIV relevant to the development of plant-based anti-HIV biopharmaceuticals in addition to recent advancements in the expression of plant-based vaccines against HIV. Formulations such as plant viral VLPs and VNPs for display of viral antigens have been largely addressed in addition to the expression of therapeutic anti-viral antibodies in plant systems. The application of biopharmed vaccines against human viruses in medical practices and as emergency response prophylactics and therapeutics seems highly promising for the forthcoming future.
Introduction
Incidence of human viral diseases worldwide
Infectious human diseases are caused by a host of medically important viruses such as the HBV, HCV, HIV, HPV, influenza, SARS-CoV-2, dengue, WNV, chikungunya and zika viruses. They result in severe morbidity and mortality in affected individuals and result in millions of deaths every year. Amongst all of them, the recently emerging SARS-CoV-2 is responsible for over 228 million cases worldwide with nearly 5 million cases of death reported (WHO 2021). Each of these viruses cause typical symptomatic disease: for instance, HBV and HCV affect the liver causing liver cirrhosis and hepatocellular carcinoma while the influenza virus and the SARS-CoV-2 mainly affect the respiratory tract. For the influenza virus, there occurs a high degree of variability of the viral hemagglutinin (HA) antigen which results in the high antigenic variability of the virus. The HPV mostly spreads through sexual activity and in severe cases, results in cervical cancer. The HIV also spreads predominantly through sexual activity and causes impairment of the body’s immune system leaving it susceptible to a host of bacterial and viral diseases. The Zika virus causes Guillain-Barre syndrome in adults [1-3] and microcephaly in fetus. For many of these viruses such as HIV, HCV and Zika viruses, there is no vaccine available even as these viruses mutate and evolve mechanisms to evade the body’s immune mechanisms. Importantly, there is a dearth of low-cost, easily administrable prophylactic and therapeutic vaccines for these diseases in developing countries where incidence of high rates of infectious viral diseases is reported and herein there lies a compelling need to generate plant-based vaccines against these deadly diseases [4,5].
Advantages of Plant-Made Vaccines
By and large plants are being increasingly employed as biofactories for generation of viral vaccine antigens and anti-viral antibodies using genetic engineering mechanisms. Plants are intrinsically favorable for vaccine production as they incur low-cost and can be grown at large scales in industrial bioreactors or greenhouses. While parenteral vaccines require purification of the viral antigens, plant-derived oral vaccines do away with the time and cost incurred in the purification of these antigens due to their availability in the plant material consumed in order to provide immunity. Complex glycosylated antigens are efficiently expressed in plants which possess the inherent advantage of dispensing with risks such as contamination with human pathogens and endotoxins involved in equivalent insect, bacterial and mammalian cell systems [6]. Oral plant-made vaccines can be conveniently freezedried and formulated into tablets at low costs [7-9]. These oral vaccines are shielded by the plant cell wall in the ambient conditions of the stomach while undergoing slow release in the gut. This dispenses with the need for cold chain facilities to store and transport the plant material unlike the high-cost fermentation-based and conventional mammalian cell technologies.
Transgenic Plants Expressing Proteins from Infectious Human Viruses
The edible vaccine against HBV expressing HBsAg in maize [10] when administered orally increased IgA levels in the serum and IgA and IgG in the feces along with the elicitation of long-term memory both systemically and at the mucosal level. The preS2-S antigen was expressed in carrots [11] by incorporating signals for transport and retention within the endoplasmic reticulum. The 143 amino acids of the HCV core protein N-terminus were expressed in tobacco chloroplasts [12]. In another study [13], canola plants transgenic for the HCV core protein demonstrated immunogenicity in mice administered with the oilseeds and oil bodies of these plants. Elevated levels of IgG, Th1 responses and IL-4 cytokine levels were observed along with enhanced elicitation of IFN-gamma from CD4+ and CD8+ cells. The ethanol-inducible promoter was used to drive the chloroplast expression of the HPV16 L1 protein in Nicotiana tabacum plants which resulted in L1 protein accumulation at levels as high as 3 ug/mg plant material fresh weight.
The S1 protein and its smaller receptor binding domain (RBD) have been expressed against SARS-CoV-2 in tobacco plants by British American Tobacco (BAT) and its US subsidiary, Kentucky Bio-Processing (KBP). This has been successful in eliciting robust immune responses during pre-clinical studies [14] and is currently advancing into Phase 1/2 clinical trials [15]. This is a very promising vaccine candidate as it induces potent immune reactions in just a single dose and can be stored at room temperature (BAT 2020). The S1 protein and its RBD contain complex, mannose rich glycans, therefore necessitating their expression along with N-terminal signal peptides in order to secrete these proteins into the endomembrane compartment [16]. Similar plant-derived strategies have been employed by BAT to generate nearly 10 million doses of vaccines against Influenza and Ebola viruses within a months’ time and the prospects of manufacturing 1-3 million doses of the SARS-CoV-2 vaccine appear very promising [17]. The University of Western Ontario and the biotechnology company, Suncor in Canada have used algae for expressing the SARS-CoV-2 spike protein, based on which they have designed a SARS-CoV-2 diagnostic kit [18].
Transient Expression of Protein Antigens from Human Viruses
Lactuca sativa plants have been used to express the HBV S/preS121-47 antigen transiently which upon oral administration elicited immune reactions in mice even in the absence of any adjuvant. The resultant antibodies neutralized HBV infections in the HepG2-NTCP cell lines [19]. This shows great promise as a strong anti-HBV edible vaccine candidate. Transient agro-infiltration was used to express the HCV E1E2 heterodimer in Lactuca sativa plants [20]. Also, an E1E2 variant devoid of the E2 protein N-glycosylation site was separately produced. Both of these antigens generated weak IgM expression against HCV in mice although the latter showed higher IgA levels. Also, mucosal and systemic immunity was observed in mice wherein a priming injection with the HCV dimer produced in HEK293T cells followed by two subsequent oral booster shots with the lettuce expressed E1E2 dimer [20].
An influenza virus-derived fusion protein consisting of the conserved region of the HA2 subunit (76-130 amino acids) of Influenza A virus and the M2e peptide fused in the form of four tandem copies along with the Salmonella typhimurium flagellin protein was expressed transiently in Nicotiana benthamiana using a self-replicating vector based on PVX [21]. Upon intranasal administration of this fusion protein in its purified form, anti-M2e serum antibodies were found to be enhanced and this provided potent protection against influenza A virus strain A/ Aichi/2/68 (H3N2) challenge at lethal doses.
Transient expression in N. benthamiana of HPV 16 L1:L2 chimaeras consisting of conserved peptides 17-36, 56-81, 65-81, and 108-120 of the HPV L2 protein each of which were substituted into the DE loop of the HPV16 L1 protein at position 131 or into the C-terminus of the L1 protein at position 431, generated cross-neutralizing antibodies against other HPV types including HPV-11, 18 and 58 as well as against HPV-16 in mice [22]. In another study, transient expression of a trivalent HPV vaccine candidate against HPV types 35, 52 and 58 in N. benthamiana showed robust anti-HPV L1-specific humoral immunity to amounts similar to that induced by the classic HPV Gardasil vaccine [23].
Plant Virus VLP-Based Vaccines Expressed in Plants
Bivalent chimaeric VLPs composed of HBcAg presenting on their surface the immunological epitope of the ORF2 capsid protein of the Hepatitis E virus was expressed in infiltrated leaves of N. benthamiana using the vector, pEAQ-HT. The resultant plant extracts reacted robustly with the anti-HBcAg mAb and the swine serum positive for the anti-HEV IgG [24].
Production of influenza VLPs in plants has been pioneered worldwide by Medicago. Initially, vaccine candidates involving monovalent VLPs representing antigens from pandemic influenza strains including the H5N1 [25,26] and the H7N9 [27] were produced following which quadrivalent VLPs based on the HA antigen to combat seasonal flu were manufactured [28]. The latter met with great success in the completed phase 1 [29], phase 2 [30] and phase 3 human clinical trials [28]. Also, a couple of phase 3 trials were performed which revealed that plant-derived HA VLPs were more protective against influenza in adults at levels equivalent to the conventional seasonal flu vaccines generated in eggs [28]. This vaccine has progressed to come under active consideration as a universal influenza vaccine by public health agencies worldwide. An influenza HA vaccine based on TMV [31] was produced which successfully assembled into HA VLPs containing a novel glycosylation site of the H3N2 influenza 2017 strain and this was shown to be highly immunogenic. Significantly, this glycosylation site was not present in the egg-adapted vaccine strain [32]. These HA VLPs elicited expeditious pro-inflammatory cytokines from dendritic cells of human and mouse origin in vitro. Additionally, they stimulated T cells to exhibit antigenspecific reactions. Immunization of mice led to accretion of T- and B-cells along with dendritic cells in the draining lymph nodes. HA-only VLPs were shown to assemble and bud out of plant plasma membranes resulting in enveloped particles which protected against influenza in Phase I/II human clinical trials [33]. Rapid production of the H7N9 HA antigen protected ferrets and mice against influenza virus infection [25] in addition to proving successful in human clinical trials with high levels of cellular and humoral immunity [29]. H1 and H5 HA VLPs were expressed in plants [34] which showed interactions with human macrophages and monocytes which appeared more virus-like when compared to the egg-produced split vaccines.
Papaya mosaic virus (PMV) was used to display peptides derived from the M2 ion channel protein of influenza which stimulated antibody responses and protected mice against challenge with the H1N1 strain [35,36]. The above composition when combined with nanoparticles of nucleoprotein multimers protected mice against the H1N1 and H3N2 strains [37]. Moreover, these PMV VLPs possessed inherent adjuvant activity that significantly augmented immunogenicity and afforded 100% immunity against challenge with the WSN/33 influenza virus [38]. Display of peptides derived from the influenza nucleocapsid protein and the M1 matrix protein on the PMV surface showed strong in vitro reactions from B- and T-cells, while showing stimulation of antigen-specific CD8+ T cells in in vivo mouse models [39-41]. Zahmanova et al. [42] report the expression of a fusion protein composed of the M2e peptide of the influenza virus and the P2 loop of the ORF2 protein of HEV in N. benthamiana which obtained a high yield of the HEV capsid protein up to levels as high as 10% of the total soluble protein content. Notwithstanding, only the HEV 100-160 amino acids as well as the chimaeric M2e HEV 110-160 polypeptide were able to assemble into VLPs and the latter was capable of being recognized by anti-M2e antisera.
The trimeric H7 hemagglutinin protein of influenza was conjugated to the nanodiamond particle surface and expressed in N. benthamiana [43,44] which showed robust H7-specific IgG immune reaction in mice. Medicago’s candidate vaccine composed of the H5N1A/Indonesia/5/05 HA VLP was the first described case of administration of a plant-derived VLP vaccine into humans [27] which elicited 96% immunity in clinical trials. Resistance against influenza A H6N2 virus was observed in chickens administered with transiently expressed H6-derived influenza A VLP vaccine in N. benthamiana [45]. The HA protein of the influenza virus is the principal factor of virus neutralization and the only mandatory vaccine component. This protein is wellexpressed and folds correctly in plants due to which it has met with great success. The main benefit of plant-derived vaccines is their immense scalability for manufacture of vaccines for pandemic infections such as influenza. Additionally, they can be accommodated easily as ‘rapid response’ vaccines. Biotechnology organizations such as Fraunhofer USA Center for Molecular Biotechnology in Delaware, Kentucky Bioprocessing in Owensboro, the Project GreenVax consortium with partners from Texas A&M University system and G-Con from Texas as well as Medicago USA in North Carolina have been involved in the production of plant-based influenza vaccines [46]. Up to 10 million doses of the H1N1 influenza VLP vaccine was produced by Medicago in just 1 month as per cGMP Phase I regulations [47].
The HPV L2 protein was displayed on the surface of grapevine fanleaf virus VLPs considering that the L2 minor capsid protein is a preferred candidate to generate HPV vaccines that are widely protective [48]. The HBcAg VLPs were used to display the HPV L2 protein and additionally the L2 protein was fused to an immunoglobulin molecule that formed recombinant immune complexes (RIC) [49], both of which elicited good immune response and were even more robust when administered together as vaccine formulations.
Medicago has led the way ahead of other companies to develop a plant-based VLP vaccine against SARS-CoV-2 using their prior knowledge on plant-produced influenza VLPs. Specifically, they transformed the SARS-CoV-2 spike protein into N. benthamiana through Agrobacteriummediated transformation [50] which generated VLPs containing the spike protein as well as the plant lipid membrane. These VLPs contained a mutated version of the S protein possessing stabilizing point mutations namely, R667G, R668S and R670S 217 substitutions at the region of the S1/S2 cleavage site [51]. This vaccine has successfully passed through phase I clinical trials wherein it was shown to be safe, potently immunogenic and welltolerated. Presently, this VLP vaccine formulation is in phase 2-3 clinical trials and it is predicted that Medicago would produce as high as 10 million doses every month [52,53].
The SARS-CoV-2 S protein B- and T-cell epitopes were displayed on CPMV surface and this vaccine was administered parenterally [54]. A similar CPMV-derived strategy has been used to develop SARS-CoV-2 diagnostic kits that are highly stable, inexpensive to produce and can be stored at ambient temperatures for long periods of time that would prove valuable in resource-poor settings. A ubiquitin variant (UbV) analog capable of recognizing the SARS-CoV-2 deubiquitinase has been developed to control SARS-CoV-2 infection [55]. PMV-based viral nanoparticles carrying UbVs of these viruses can enter human cells and efficiently preclude virus infection. In this regard, PMV VNPs have already been shown to enter lung epithelial cells when used as a nasal spray. Hence, these PMV VLPs harboring the UbVs could be loaded into an inhaler so as to introduce it into the nasal cavity and lungs of SARS-CoV-2 infected patients. Our laboratory has been involved in engineering a Geminivirus, the Bean yellow dwarf virus to produce large amounts of the UbV protein against the SARS-CoV-2 and the Middle east respiratory syndrome virus (MERS) [56].
Properly assembled RICs and VLPs that express the ZE3 antigen of the Zika virus elicited strong anti-Zika neutralizing immune reactions in mice when both were administered separately while demonstrating a synergistic increase in neutralization levels upon codelivery of both RICs and VLPs. Plant-produced HBcAg VLPs displaying on their surface the ZE3 peptide also showed notable increases in neutralization reactions as well as increased antibody titers [1]. This was accompanied by significant enhancement of IFN-gamma levels implying potent Th1 or Th1/Th2 cell-mediated immune responses essential for virus neutralization. In another study [57], the EDIII envelope protein of the Zika virus was displayed on CMV particles which stimulated enhanced antibody levels and Zika virus neutralization while precluding the enhancement of infection by the dengue virus.
Dengvaxia, the sole licensed anti-dengue vaccine is not safe enough in young, seronegative patients and this results in a dire need for safe, effective alternatives. N. benthamiana plants were used to transiently express VLPs of the dengue virus containing structural proteins (SP) as well as a truncated version of the dengue non-structural protein lacking the RdRp and this formulation elicited strong immune responses [58]. This investigation shows how VLPs can be used optimally to generate viable vaccine candidates for combating enveloped infectious viruses.
Vaccines Generated Using Plant Virus- Derived Vectors
The small S HBsAg antigen was expressed at high yields (300 mg/kg wet weight) in N. benthamiana using the deconstructed TMV-derived cDNA MagniCON vector (Icon Genetics, Halle, Germany). This strategy met with great success as the recombinant antigen displayed the HBV ‘a’ antigenic determinant in its conformationally correct form and consisted of the full-length S protein with dimers linked through disulfides that assembled into functional VLPs [59]. HBV vaccine biopharming was first established by the Arizona Biodesign Institute [60] wherein they expressed the middle M polypeptide of the HBsAg in plants. Administration of this antigen in murine models elicited better B-cell immune response compared to that of the S protein [61]. Also, the MagniCON vector was used to express the HBcAg core antigen which yielded highly immunogenic VLPs that were expressed to levels as high as 2 g/kg wet weight [62].
L1 capsid epitopes of HPV types 16, 18, 31 and 45 were expressed in vectors wherein the CMV replicase gene was inserted. This led to increased expression of these highly antigenic proteins to levels as much as 25-27 ug/mg total soluble protein [63] which was much better than that of the quadrivalent Gardasil vaccine. This implied that the CMV viral RdRp structural elements and their associated regulatory genes function as RNA silencing suppressors leading to multi-fold enhancements in the HPV antigen expression.
The HBcAg-zDIII fusion protein was expressed transiently in N. benthamiana using a MagnICON vector [1] via Agrobacterium infiltration. This showed anti- Zika humoral and cell-mediated immune responses in mouse models while triggering antibodies that did not enhance infection by the dengue virus. This proved to be an inexpensive and safe option for enabling Zika virus immunity.
Expression of Human Viral Polypeptides as Fusion Proteins
Reportedly, a PVX-based vector was used to express the HCV core protein which was fused to the HBsAg at its C-terminus. This construct was codon-optimized for efficacious expression in tobacco [64]. An enhancement in the core protein yield was observed upon co-expression of the Tomato bushy stunt virus p19 viral suppressor. Similar protein fusions involving the HCV peptide antigen and the PMV capsid protein have also been reported. The PMV-HCV E2 glycoprotein fusion was used as a vaccine to immunize mice which generated extended humoral immune response [65]. Oral HCV vaccines expressed in edible crops have been developed using the HCV E2 surface glycoprotein immunogenic R9 mimotope fused to carrier molecules such as the HBsAg [66], the CTB [67], the Alfalfa mosaic virus surface [68] and the CMV [69-71].
The influenza virus M2e peptide was fused to Salmonella typhimurium flagellin protein by Mardanova et al. [72] which elicited anti-M2e antibodies in mice and provided protection against challenges from various HCV strains even when administered at lethal doses. A wider range of protection could be achieved if both the M2e and HA2 influenza domains were administered together to formulate a single vaccine candidate [73-76]. Moreover, if the above strategy can be combined with a carrier VLP or an adjuvant, it could enhance their immunogenicity even further [75-77]. Tomato plant hairy roots were used to express the HPV16 E7 protein fused with SAPKQ, a nontoxic form of the Saponaria officinalis saporin protein [78]. This demonstrated anticancer activity against tumors in mice thereby serving as low-cost biofactories for developing therapeutic HPV vaccines.
In a study by the iBio American company, the SARSCoV- 2 major surface glycoprotein S (spike protein) was fused to a carrier protein lichenase from Clostridium thermocellum b-1,3-1,4-glucanase followed by expression of this fusion protein in plants [https://www.biospace. com/article/ibio-s-fastpharming-platform-producesdecoy- therapeutic-to-bind-tosars-cov-2/ (accessed on 16 September 2020)]. SARS-CoV-2 diagnostic reagents were expressed in plants by the South African company, Cape Bio Pharms (CBP) [79]. Several other biotechnology companies such as Nomad, Protalix, Ventria and Greenovation Biopharmaceuticals have joined the race for the development of plant-derived SARS-CoV-2 vacciens [80]. Additionally, academic institutions such as the Infectious Disease Research Center at the Laval Univeristy, Quebec, Canada in collaboration with Medicago is involved in developing therapeutic antibodies against SARSCoV- 2. Several countries including the USA, Mexico, UK, Germany, South Korea, South Africa and Thailand are also involved in molecular biopharming against SARS-CoV-2 [80].
The current precedency of other plant-derived vaccines against viruses such as influenza is an encouraging factor towards developing similar vaccines against SARS-CoV-2. While the SARS-CoV-2 spreads worldwide at pandemic proportions, manufacture of vaccines in plants affords low-cost, easy to administer, safe, stable and efficacious options to combat deadly viral diseases. An ideal vaccination regimen involving plant-based vaccines would combine initial parenteral administration of transiently expressed purified injectable vaccines developed in plants subsequently followed by oral boosters with plant material expressing the vaccine antigen [81].
Therapeutic Antibodies
Genetic engineering of plants has primed the development of homogenous, human-like ‘tailored’ glycans which have proved to be better than that of mammalian systems [82]. This becomes important because correct glycosylation is essential for stability and proper functioning of antibodies [83]. Elimination of endogenous b1,2-linked xylose and a1,3-linked fucose sugars that are plant-specific [84,85] and engineering of the plants to encode the entire human sialylation pathway has enabled the generation of plantderived antibodies having greater potency and increased capability to recognize immune receptors [86,87]. Safe and efficacious plant-based therapeutic antibodies have been expressed at high levels against viruses such as the dengue virus [88], west nile virus [89] and the chikungunya virus [90]. Ebola virus infections in rhesus macaques and humans have been successfully treated using antibodies expressed in glycoengineered plants [91-93].
Given the fact that rabies virus antibodies generated in equines are of varied quality and with limitations in their yield, there is a compelling need to synthesize plant-made anti-rabies antibodies. Powerful, broadlyneutralizing, correctly assembled anti-rabies antibodies in their humanized IgG form have been synthesized in transgenic N. tabacum plants [97] wherein they expressed anti-rabies activity equivalent to that of Mabs generated in hybridomas and were more efficacious than the currently available commercial rabies Ig vaccine (HRIG; Rabigam).
Recombinant plant-based antibodies against the SARSCoV- 2 can decelerate the progress of viral infection while giving the body adequate time to produce its own antibodies even before the infected patient develops the Covid-19 disease. The success of convalescent patient sera in diminishing the severity of Covid-19 disease symptoms provides proof-of-concept regarding the promise of therapeutic antibodies in precluding this disease [98,99]. Therefore, plants form ideal biofactories for the expression of antibodies for virus diagnosis and for passive immunotherapy.
TMV-based MagnICON vectors have been used to express three anti-Ebola, human-mouse chimaeric Mabs in N. benthamiana via agroinfiltration [91]. A cocktail of the above three Mabs provided 100% immunity against lethal challenge with Ebola virus in macaque models and were more potent than antibodies expressed in CHO cells. Another medley of anti-Ebola Mabs [93] designated the ZMapp was developed by biotechnology companies namely the Dreyfus of Toronto in collaboration with San Diego Mapp Biopharmaceuticals. This was subsequently manufactured by Kentucky Bio-Processing. ZMapp afforded 100% immunity from Ebola virus challenge in macaque models. Also, it was capable of reversing severe, advanced stages of Ebola disease in animals and led to full recovery. ZMapp was also capable of recognizing the greatly prevalent Ebola Guinea variant. Upon testing in humans, ZMapp proved to be efficacious and was seen to outperform the efficacy of other therapeutics against Ebola [100].
Besides, the cytokine storm elicited following SARSCoV- 2 infection can be suppressed by plant-made therapeutic antibodies in many severe cases of Covid-19 disease. Antibodies such as the tocilizumab/Actemra and sarilumab/Kevzara cocktail capable of recognizing the IL-6R (interleukin-6 receptor) and touted for rheumatoid arthritis therapy could be repurposed for Covid-19 immunotherapy and are presently under clinical trials [Long Island Press (2020), Swiss Broadcasting Corporation (2020)].
Plant-Based HIV Vaccines
Out of the 38 million people suffering with HIV infection, nearly 24 million are under antiretroviral therapy (ART) [101]. Every year about 700,000 new cases of HIV are reported and several of these patients die from full-blown chronic AIDS, a majority of which occur in Africa. There is a compelling need for the generation of a successful prophylactic anti-HIV vaccine considering the reality that several people where the HIV is endemic, cannot undergo ART due to poor socioeconomic status. Also, the application of ART necessitates life-long treatment accompanied by many side effects. A probable candidate anti-HIV vaccine was generated in 1987 in the USA which entered phase I clinical trials followed by more than 30 candidate vaccines reportedly in phase I/II human trials mainly in the USA and Europe [101].
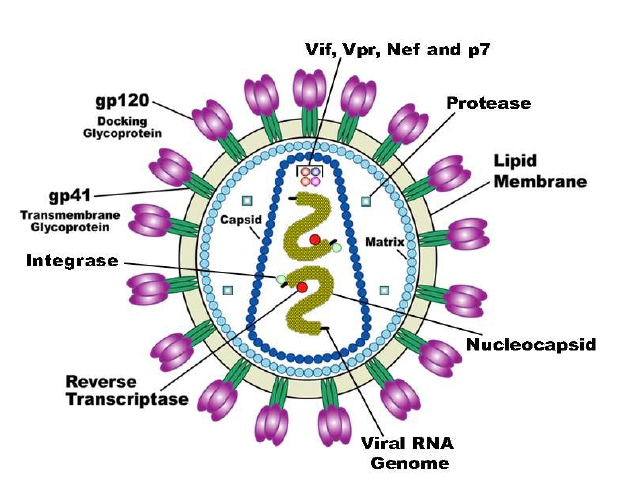
HIV belongs to the family of retroviruses called the lentiviruses with genome composed of two single-stranded RNAs and several viral polypeptides that are encapsidated into the enveloped viral capsid (Figure 1).
Figure 1: Schematic structu r e of the HIV virus. (Adapted from Medical Research Council, UK. Discovery of Key Component of HIV Virus Yields Drug Target. Drug discovery and development. August 11, 2016. [102]).
Out of the 2 HIV types reported namely the HIV-1 and HIV-2, HIV-1 and its subtypes are responsible for a majority of the AIDS pandemic. HIV-1 and HIV-2 share several similarities such as their key gene arrangement, methods of transmission, intracellular replication mechanisms and clinical outcomes, although both result in AIDS [102]. Nevertheless, HIV-2 shows lower transmissibility and diminished likelihood of advancement to AIDS. The basic mechanistic differences between infections caused by these two viruses demonstrate broader concerns of retroviral pathogenesis that are yet to be completely understood. Epidemiologically, HIV-2 occurs mostly in West Africa, while the HIV-1 has spread worldwide. In clinical terms, majority of individuals infected with HIV-2 remain nonprogressors in the long-term while most individuals infected with HIV-1 progress [103].
The envelope of the virus contains trimers of the HIV protein gp120 and gp41 heterodimers that interact with each other non-covalently. More than 12 subtypes of HIV- 1 and several of its recombinant forms are so far reported to be in circulation [104]. The pathogenicity of HIV is complicated due to the high mutation frequency of the major envelope protein gp120 and this has tremendous impact on anti-HIV immunity [105]. Therefore, this makes the HIV envelope highly variable in addition to the high rate of mutations in the viral genome (~1-10 mutations for every replication cycle of the virus). Besides the above, there occurs high conformational resilience along with glycan coverage. Additionally, HIV is capable of undergoing immune evasion through several mechanisms [106]. Polyvalent anti-HIV vaccines that are able to recognize conserved domains of the viral envelope can help in overcoming the high frequency of changes generated in the viral envelope [107].
Zhang et al. [108] generated transgenic tobacco expressing the HIV p24 capsid protein. Karasev et al. [109] expressed the Tat protein in spinach which elicited immune priming when administered following a primary injection with a DNA vaccine specific to the Tat protein. In another investigation [110], the HIV p24 antigen was expressed in the green alga, Chlamydomonas to levels as great as 0.25% of total cellular protein. An anti-HIV lectin called cyanovirin was expressed in tobacco [111]. The dense mannose glycans of the glycosylated HIV gp120 was targeted by the banana lectin [112] possessing activity against HIV replication. Recently, rice endosperm was engineered to express proteins such as cyanovirin-N, griffithsin (a lectin from algae) and HIV Mab 2G12 capable of neutralizing HIV by recognizing gp120 [113]. Upon expression of these proteins in lettuce and tobacco, this resulted in better virus neutralization in comparison with an equivalent cocktail generated conventionally. An anti- HIV polyvalent and multi-epitopic vaccine C4(V3)6 based on gp120 was produced in tobacco [8] and lettuce [114] both of which showed potent immune reactions against HIV [115]. A chimaeric protein containing epitopes of gp120 and gp41 was expressed in Physcomitrella patens (a moss plant) yielding a vaccine, pol-HIV which was found to elicit immune reactions in mice [48,116]. The DsRed fluorescent protein was designed to carry the HIV epitope ELDKWA that has the ability to capture the HIV neutralizing antibody 2F5, followed by its expression in transgenic tobacco [117]. This paved the way for generating and purifying high yields of idiotype-specific HIV mAbs. In another study, an immunogenic multi-epitopic HIV protein was expressed in tobacco which elicited g-IFN and T-helper cell reactions in mice upon oral administration [118].
Medicago Inc. expressed the HIV Env protein in fusion with the transmembrane and cytoplasmic tail domains of the influenza HA protein to generate HIV VLPs that budded successfully in plant cells [119,120]. This formulation afforded superior immunogenicity and shows great promise as a booster vaccine that could be employed in heterogenous prime-boost vaccination regimens. Different studies reported transgenic N. benthamiana [121] as well as transplastomic N. tabacum [122] expressing the HIV Gag-derived VLP-like molecule. Of these, the transgenic N. benthamiana demonstrated stabilized expression of the Gag protein and this was shown to interact with the co-expressed gp41 leading to the successful generation of enveloped VLPs. The above VLPs stimulated strong immunity to the Gag protein in murine models. CPMV was used to display a HIV-1 gp41 epitope on its surface which upon parenteral administration elicited neutralizing responses in mice against 3 strains of HIV [123-125].
Griffithsin, an effective inhibitor of HIV entry was produced in tobacco using a TMV-based vector which obtained yields of more than 1 g/kg fresh leaf weight. This possessed strong capability to recognize gp120 [126]. This resulted in the manufacture of 20 kg griffithsin per year at much lesser costs [127] compared that of similar production systems based on mammalian cells [128]. Large scale production of 2G12 and 2F5 anti-HIV neutralizing antibodies has been reported in transgenic rice [129], tobacco [130] and maize [131, 132]. Fraunhofer IME has acquired license for expressing HIV 2G12 antibodies in tobacco plants towards examination in human Phase I clinical trials. The 2G12 antibody has been expressed in rice [113] along with a couple of antiviral lectins enabling inexpensive synthesis of preformulated anti-HIV cocktails. In another reported study [133], MagnICON vectors were used to express CAP256-VRC26 anti-HIV bNAbs (broadly neutralizing antibodies) containing post-translational modifications in N. benthamiana. (Table 1) enlists some examples of plant-derived vaccines generated against HIV and subsequently tested in animal models [101].
|
HIV antigen expressed |
Plant expressed in |
Animal in which tested |
Immunogenicity detected |
Reference |
|
C4(V3)6 |
Lettuce |
Mice |
Elicitation of humoral and cell-mediated immune reactions |
[114]
|
|
C4V3 |
Tobacco |
Mice |
Generation of systemic mucosal antibody reactions and proliferation of T cells |
[136]
|
|
Multi-HIV |
Tobacco |
Mice |
Stimulation of humoral immune response |
[8]
|
|
Gp41 |
Tobacco |
Mice |
Strong CD4 and CD8 T cell as well as serum antibody immune responses |
[137]
|
|
p17/p24 |
Tobacco |
Mice |
Elicitation of humoral and T cell immunity |
[138]
|
|
p24 |
Arabidopsis |
Mice |
Stimulation of serum IgG |
[139]
|
|
p24 |
Tobacco |
Rabbit |
HIV-specific humoral immune reactions |
[140] |
|
C4(V3)6 and C4V3 are chimeric, multiepitopic proteins modeled based on the HIV gp120 and were engineered to test their immunization capability against HIV; multi-HIV represents the multi-epitopic chimaeric HIV protein derived from epitopes of gp41 and gp120 HIV proteins. (Adapted from Tremouillaux-Guiller et al. [101]). |
||||
When tyrosyl protein sulfotransferase was co-expressed in the above plants, this led to the incorporation of O-sulfated tyrosine in the complementarity determining portion of the heavy chain H3 loop of these bNAbs. Additionally, these antibodies folded and functioned in a manner similar to those expressed in mammalian cells.
They were also highly potent against some HIV subtype C strains. Thus, plants have proved to be optimal systems for multiple post-translational modifications towards production of antibodies for passive immunization against HIV as well as for serving as alternate therapies for the current HIV antiretroviral treatment schemes. Lately, transient expression of a fusion protein containing an anti-HIV bispecific broadly neutralizing antibody and lectin was demonstrated in N. benthamiana [134]. Also, N. benthamiana-based transient expression has been reported for the generation of the HIV Env gp140 antigen [135].
Conclusion
In accordance with the intention of this mini-review, a majority of the details on plant-based vaccines against important human viruses as discussed above have been clearly presented in the paper by Venkataraman et al. [81]. This paper conveys useful up-to-date information on developments in this area in addition to plant based therapeutic antibodies for prevention and treatment of human viral diseases. Specifically, this review discusses vaccines against important human viruses such as HPV, HCV, HBV, Influenza virus, HIV, Zika virus and SARSCoV- 2. Highlighted are details regarding the worldwide incidence of these viral diseases, molecular characteristics of these viruses that are of relevance to vaccine design and recent advancements in the synthesis of plantderived vaccines against these important viruses. This article shows that by virtue of being highly effectual, low-cost and facile to administer, plant-based vaccines present great promise in confronting the global incidence of viral diseases in the near future. Also, in light of the urgent necessity to control the HIV/AIDS epidemic worldwide, plant-based vaccine expression systems can be of tremendous value in prophylaxis and therapy of HIV disease. Once an efficacious plant derived HIV vaccine is developed, plant molecular pharming could be a good solution for combating HIV infections as they present several advantages over other vaccine/therapeutic production platforms [101]. A gamut of plant-based products such as antiretroviral microbicides, vaccine bioformulations and broadly neutralizing monoclonal antibodies are currently under development that could have several positive implications on the epidemiology, prevalence and incidence of HIV. At the least, plantbased vaccines can be administered as booster shots in heterologous prime/boost regimens considering that large quantities of vaccine material are needed for repeated booster vaccinations [137].
Taking into consideration all of the latest literature presented in this mini-review as above, the work by Venkataraman et al. [81] presents a comprehensive picture of recent plant-based vaccines against the most important infectious human viruses. Nevertheless, the details of plant-derived vaccines against other human viruses such as the rift valley fever virus, the respiratory syncytial virus, the chikungunya virus, west nile virus and the SARS-CoV-1 need to be addressed and this could the subject for another review.
Conflict of Interest
The author declares no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
References
2. Attar N. ZIKA virus circulates in new regions. Nat Rev Microbiol. 2016; 14: 62.
3. Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case control study. Lancet. 2016; 387: 1531-1539.
4. Murad S, Fuller S, Menary J, Moore C, Pinneh E, Szeto T, et al. Molecular Pharming for low and middle income countries. Current Opinion in Biotechnology. 2020 Feb 1;61:53-9.
5. Tsekoa TL, Singh AA, Buthelezi SG. Molecular farming for therapies and vaccines in Africa. Curr Opin Biotechnol. 2019; 61: 89-95.
6. Aggarwal, S. What’s fueling the biotech engine-2008. Nat Biotechnol. 2009; 27: 987-993.
7. Su J, Zhu L, Sherman A, Wang X, Lin S, Kamesh A, et al. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015; 70: 84-93.
8. Rosales-Mendoza S, Rubio-Infante N, Monreal- Escalante E, Govea-Alonso DO, García-Hernández AL, Salazar-González JA, et al. Chloroplast expression of an HIV envelop-derived multiepitope protein: towards a multivalent plant-based vaccine. Plant Cell Tissue Organ Cult. 2014;116(1):111-23.
9. Rosales-Mendoza S, Salazar-González JA. Immunological aspects of using plant cells as delivery vehicles for oral vaccines. Expert Rev Vaccines. 2014;13(6):737-49.
10. Hayden CA, Fischer ME, Andrews BL, Chilton HC, Turner DD, Walker JH, et al. Oral delivery of wafers made from HBsAg-expressing maize germ induces long-term immunological systemic and mucosal responses. Vaccine. 2015;33(25):2881-6.
11. Rosales-Mendoza S, Tello-Olea MA. Carrot cells: a pioneering platform for biopharmaceuticals production. Mol Biotechnol. 2015;57(3):219-32.
12. Madesis P, Osathanunkul M, Georgopoulou U, Gisby MF, Mudd EA, Nianiou I, Tsitoura P, Mavromara P, Tsaftaris A, Day A. A hepatitis C virus core polypeptide expressed in chloroplasts detects anti-core antibodies in infected human sera. Journal of biotechnology. 2010 Feb 15;145(4):377-86.
13. Mohammadzadeh S, Roohvand F, Ehsani P, Salmanian AH, Ajdary S. Canola oilseed-and Escherichia coli-derived hepatitis C virus (HCV) core proteins adjuvanted with oil bodies, induced robust Th1-oriented immune responses in immunized mice. Apmis. 2020 Nov;128(11):593-602.
14. Gretler C. Tobacco-Based Coronavirus Vaccine Poised for Human Tests Bloomberg, May 15. 2020. Available online: https://www.bloomberg.com/news/ articles/2020-05-15/cigarette-maker-s-coronavirusvaccine- poised-for-human-tests (Accessed on 24 December 2020).
15. Palca J. Tobacco plants contribute key ingredient for COVID-19 Vaccine. 2020. Available online: https://www. npr.org/sections/health-shots/2020/10/15/923210562/ tobacco-plants-contribute-key-ingredient-for-covid-19- vaccine (Accessed on 20 December 2020).
16. Krokhin O, Li Y, Andonov A, Feldmann H, Flick R, Jones S, et al. Mass Spectrometric Characterization of Proteins from the SARS Virus: A Preliminary Report. Molecular & Cellular Proteomics. 2003 May 1;2(5):346- 56.
17. Mullan K. Tobacco Giant BAT Says It Could be Making 1 to 3 Million COVID-19 Vaccines a Week by June. 2020. Available online: https://www.derryjournal.com/news/ people/tobacco-giant-bat-says-it-could-be-making-1-3- million-covid-19-vaccines-week-june-2526933 (Accessed on 24 December 2020).
18. Makay, C. Algae Tasked with Making COVID-19 Kits. 2020. Available online: https://phys.org/news/2020-04- algae-taskedcovidkits.html (Accessed on 24 December 2020).
19. Dobrica MO, Lazar C, Paruch L, van Eerde A, Clarke JL, Tucureanu C, Caras I, Ciulean S, Onu A, Tofan V, Branzan A. Oral administration of a chimeric hepatitis B Virus S/preS1 antigen produced in lettuce triggers infection neutralizing antibodies in mice. Vaccine. 2018 Sep 11;36(38):5789-95.
20. Clarke JL, Paruch L, Dobrica MO, Caras I, Tucureanu C, Onu A, Ciulean S, Stavaru C, Eerde A, Wang Y, Steen H. Lettuce-produced hepatitis C virus E1E2 heterodimer triggers immune responses in mice and antibody production after oral vaccination. Plant biotechnology journal. 2017 Dec;15(12):1611-21.
21. Blokhina EA, Mardanova ES, Stepanova LA, Tsybalova LM, Ravin NV. Plant-produced recombinant Influenza A virus candidate vaccine based on flagellin linked to conservative fragments of M2 protein and hemagglutintin. Plants. 2020 Feb;9(2):162.
22. Chabeda A, van Zyl AR, Rybicki EP, Hitzeroth II. Substitution of human papillomavirus type 16 L2 neutralizing epitopes into L1 surface loops: the effect on virus-like particle assembly and immunogenicity. Frontiers in plant science. 2019 Jun 20;10:779.
23. Naupu PN, van Zyl AR, Rybicki EP, Hitzeroth II. Immunogenicity of plant-produced Human Papillomavirus (HPV) virus-like particles (VLPs). Vaccines (Basel). 2020;8(4):740.
24. Zahmanova G, Mazalovska M, Takova K, Toneva V, Minkov I, Peyret H, et al. Efficient production of chimeric hepatitis B virus-like particles bearing an Epitope of hepatitis E virus capsid by transient expression in Nicotiana benthamiana. Life (Basel). 2021;11(1):64.
25. Pillet S, Racine T, Nfon C, Di Lenardo TZ, Babiuk S, Ward BJ, et al. Plant-derived H7 VLP vaccine elicits protective immune response against H7N9 influenza virus in mice and ferrets. Vaccine. 2015;33(46):6282-9.
26. Pillet S, Aubin É, Trépanier S, Poulin J-F, Yassine- Diab B, Ter Meulen J, et al. Humoral and cell-mediated immune responses to H5N1 plant-made virus-like particle vaccine are differentially impacted by alum and GLASE adjuvants in a Phase 2 clinical trial. NPJ Vaccines. 2018;3:3.
27. Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS One. 2010;5(12):e15559.
28. Ward BJ, Makarkov A, Séguin A, Pillet S, Trépanier S, Dhaliwall J, et al. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18-64 years) and older adults (≥ 65 years): two multicentre, randomised phase 3 trials. The Lancet. 2020 Nov 7;396(10261):1491-503.
29. Pillet S, Aubin É, Trépanier S, Bussière D, Dargis M, Poulin J-F, et al. A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol. 2016;168:72-87.
30. Pillet S, Couillard J, Trépanier S, Poulin J-F, Yassine- Diab B, Guy B, et al. Immunogenicity and safety of a quadrivalent plant-derived virus like particle influenza vaccine candidate-Two randomized Phase II clinical trials in 18 to 49 and ≥50 years old adults. PLoS One. 2019;14(6):e0216533.
31. Mallajosyula JK, Hiatt E, Hume S, Johnson A, Jeevan T, Chikwamba R, et al. Single-dose monomeric HA subunit vaccine generates full protection from influenza challenge. Hum Vaccin Immunother. 2014;10(3):586-95.
32. Zost SJ, Parkhouse K, Gumina ME, Kim K, Diaz Perez S, Wilson PC, et al. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc Natl Acad Sci U S A. 2017;114(47):12578-83.
33. Ward BJ, Landry N, Trépanier S, Mercier G, Dargis M, Couture M, et al. Human antibody response to N-glycans present on plant-made influenza virus-like particle (VLP) vaccines. Vaccine. 2014;32(46):6098-106.
34. Makarkov AI, Chierzi S, Pillet S, Murai KK, Landry N, Ward BJ. Plant-made virus-like particles bearing influenza hemagglutinin (HA) recapitulate early interactions of native influenza virions with human monocytes/ macrophages. Vaccine. 2017;35(35):4629-36.
35. Carignan D, Thérien A, Rioux G, Paquet G, Gagné M-ÈL, Bolduc M, et al. Engineering of the PapMV vaccine platform with a shortened M2e peptide leads to an effective one dose influenza vaccine. Vaccine. 2015;33(51):7245-53.
36. Thérien A, Bédard M, Carignan D, Rioux G, Gauthier- Landry L, Laliberté-Gagné M-È, et al. A versatile papaya mosaic virus (PapMV) vaccine platform based on sortasemediated antigen coupling. J Nanobiotechnology. 2017;15(1).
37. Bolduc M, Baz M, Laliberté-Gagné M-È, Carignan D, Garneau C, Russel A, et al. The quest for a nanoparticlebased vaccine inducing broad protection to influenza viruses. Nanomedicine. 2018;14(8):2563-74.
38. Denis J, Acosta-Ramirez E, Zhao Y, Hamelin M-E, Koukavica I, Baz M, et al. Development of a universal influenza A vaccine based on the M2e peptide fused to the papaya mosaic virus (PapMV) vaccine platform. Vaccine. 2008;26(27-28):3395-403.
39. Babin C, Majeau N, Leclerc D. Engineering of papaya mosaic virus (PapMV) nanoparticles with a CTL epitope derived from influenza NP. J Nanobiotechnology. 2013;11(1):10.
40. Laliberté-Gagné M-È, Bolduc M, Thérien A, Garneau C, Casault P, Savard P, et al. Increased immunogenicity of full-length protein antigens through sortase-mediated coupling on the PapMV vaccine platform. Vaccines (Basel). 2019;7(2):49.
41. Leclerc D, Beauseigle D, Denis J, Morin H, Paré C, Lamarre A, et al. Proteasome-independent major histocompatibility complex class I cross-presentation mediated by papaya mosaic virus-like particles leads to expansion of specific human T cells. J Virol. 2007;81(3):1319-26.
42. Zahmanova GG, Mazalovska M, Takova KH, Toneva VT, Minkov IN, Mardanova ES, et al. Rapid high-yield transient expression of swine hepatitis E ORF2 capsid proteins in Nicotiana benthamiana plants and production of chimeric hepatitis E virus-like particles bearing the M2e Influenza Epitope. Plants. 2019;9(1):29.
43. Pushko P, Tretyakova I. Influenza virus like particles (VLPs): Opportunities for H7N9 vaccine development. Viruses. 2020;12(5):518.
44. Pham NB, Ho TT, Nguyen GT, Le TT, Le NT, Chang H-C, et al. Nanodiamond enhances immune responses in mice against recombinant HA/H7N9 protein. J Nanobiotechnology. 2017;15(1):69.
45. Smith T, O’Kennedy MM, Wandrag DBR, Adeyemi M, Abolnik C. Efficacy of a plant-produced virus-like particle vaccine in chickens challenged with Influenza A H6N2 virus. Plant Biotechnol J. 2020;18(2):502-12.
46. Pellerin C. DARPA Effort Speeds Biothreat Response. In American Forces Press Service: U.S. Department of Defense; 2010.
47. DARPA Makes 10 Million Strides in the Race to Contain a Hypothetical Pandemic. 2012. Available online: http:// www.darpa.mil/NewsEvents/Releases/2012/07/25.aspx (Accessed on 25 July 2012).
48. Yazdani R, Shams-Bakhsh M, Hassani-Mehraban A, Arab SS, Thelen N, Thiry M, et al. Production and characterization of virus like particles of grapevine fanleaf virus presenting L2 epitope of human papillomavirus minor capsid protein. BMC Biotechnol. 2019;19:81.
49. Diamos AG, Larios D, Brown L, Kilbourne J, Kim HS, Saxena D, et al. Vaccine synergy with virus-like particle and immune complex platforms for delivery of human papillomavirus L2 antigen. Vaccine. 2019;37(1):137-44.
50. Krenek P, Samajova O, Luptovciak I, Doskocilova A, Komis G, Samaj J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol Adv. 2015;33(6 Pt 2):1024-42.
51. Ward BJ, Gobeil P, Séguin A, Atkins J, Boulay I, Charbonneau P-Y, et al. Phase 1 trial of a candidate recombinant virus-like particle vaccine for covid-19 disease produced in plants. bioRxiv. 2020.
52. Rosales-Mendoza S, Márquez-Escobar VA, González- Ortega O, Nieto-Gómez R, Arévalo-Villalobos JI. What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines (Basel). 2020;8(2):183.
53. Phillip Morris International. 2020. Available online: https://www.pmi.com/media-center/news/ pmi-announces-medicago-tosupply-up-to-76-milliondoses- of-its-plant-derived-covid-19-vaccine-candidate (Accessed on 24 October 2020).
54. Lopez-Ramirez MA, Soto F, Wang C, Rueda R, Shukla S, Silva-Lopez C, et al. Built-in active microneedle patch with enhanced autonomous drug delivery. Adv Mater. 2020;32(1):e1905740.
55. Clemente V, D’Arcy P, Bazzaro M. Deubiquitinating enzymes in coronaviruses and possible therapeutic opportunities for COVID-19. Int J Mol Sci. 2020;21(10):3492.
56. Hefferon KL. DNA virus vectors for vaccine production in plants: Spotlight on geminiviruses. Vaccines (Basel). 2014;2(3):642-53.
57. Cabral-Miranda G, Lim SM, Mohsen MO, Pobelov IV, Roesti ES, Heath MD, et al. Zika virus-derived E-DIII protein displayed on immunologically optimized VLPs induces neutralizing antibodies without causing enhancement of Dengue virus infection. Vaccines (Basel). 2019;7(3).
58. Ponndorf1 D, Meshcheriakova Y, Thuenemann EC, Alonso AD, Overman R, Holton N, et al. Plant-made dengue virus-like particles produced by coexpression of structural and non-structural proteins induce a humoral immune response in mice. Plant Biotech J. 2021;19:745- 756.
59. Huang Z, LePore K, Elkin G, Thanavala Y, Mason HS. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol J. 2008;6(2):202-9.
60. Rybicki EP. Plant-based vaccines against viruses. Virol J. 2014;11(1):205.
61. Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen CJ, Thanavala Y, et al. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine. 2005;23(15):1851-8.
62. Huang Z, Santi L, LePore K, Kilbourne J, Arntzen CJ, Mason HS. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice.Vaccine. 2006;24(14):2506-13.
63. Salyaev RK, Rekoslavskaya NI, Stolbikov AS. The new plant expression system for the development of vaccines against papillomaviruses. Dokl Akad Nauk. 2019;484(4):503-6.
64. Mohammadzadeh S, Khabiri A, Roohvand F, Memarnejadian A, Salmanian AH, Ajdary S, et al. Enhanced-transient expression of hepatitis C virus core protein in Nicotiana tabacum, a protein with potential clinical applications. Hepat Mon. 2014;14(11):e20524.
65. Denis J, Majeau N, Acosta-Ramirez E, Savard C, Bedard M-C, Simard S, et al. Immunogenicity of papaya mosaic virus-like particles fused to a hepatitis C virus epitope: evidence for the critical function of multimerization. Virology. 2007;363(1):59-68.
66. Mohammadzadeh S, Roohvand F, Memarnejadian A, Jafari A, Ajdary S, Salmanian A-H, et al. Co-expression of hepatitis C virus polytope-HBsAg and p19-silencing suppressor protein in tobacco leaves. Pharm Biol. 2016;54(3):465-73.
67. Nemchinov LG, Liang TJ, Rifaat MM, Mazyad HM, Hadidi A, Keith JM. Development of a plant-derived subunit vaccine candidate against hepatitis C virus. Arch Virol. 2000;145(12):2557-73.
68. Attar AE, Shamloul A, Shalaby A, Riad B, Saad A, Mazyad H, et al. Expression of chimeric HCV peptide in transgenic tobacco plants infected with recombinant alfalfa mosaic virus for development of a plant-derived vaccine against HCV. African J Biotechnol. 2004;3:7.
69. Natilla A, Piazzolla G, Nuzzaci M, Saldarelli P, Tortorella C, Antonaci S, et al. Cucumber mosaic virus as carrier of a hepatitis C virus-derived epitope. Arch Virol. 2004;149(1):137-54.
70. Piazzolla G, Nuzzaci M, Tortorella C, Panella E, Natilla A, Boscia D, et al. Immunogenic properties of a chimeric plant virus expressing a hepatitis C virus (HCV)- derived epitope: new prospects for an HCV vaccine. J Clin Immunol. 2005;25(2):142-52.
71. Nuzzaci M, Piazzolla G, Vitti A, Lapelosa M, Tortorella C, Stella I, et al. Cucumber mosaic virus as a presentation system for a double hepatitis C virus-derived epitope. Arch Virol. 2007;152(5):915-28.
72. Mardanova ES, Kotlyarov RY, Kuprianov VV, Stepanova LA, Tsybalova LM, Lomonosoff GP, et al. Rapid high-yield expression of a candidate influenza vaccine based on the ectodomain of M2 protein linked to flagellin in plants using viral vectors. BMC Biotechnol. 2015;15(1):42.
73. Ameghi A, Pilehvar-Soltanahmadi Y, Baradaran B, Barzegar A, Taghizadeh M, Zarghami N, et al. Protective immunity against homologous and heterologous influenza virus lethal challenge by immunization with new recombinant chimeric HA2-M2e fusion protein in BALB/C mice. Viral Immunol. 2016;29(4):228-34.
74. Deng L, Kim JR, Chang TZ, Zhang H, Mohan T, Champion JA, et al. Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology. 2017;509:82-9.
75. Stepanova LA, Mardanova ES, Shuklina MA, Blokhina EA, Kotlyarov RY, Potapchuk MV, et al. Flagellin-fused protein targeting M2e and HA2 induces potent humoral and T-cell responses and protects mice against various influenza viruses a subtypes. J Biomed Sci. 2018;25(1).
76. Stepanova LA, Kotlyarov RY, Shuklina MA, Blochina EA, Sergeeva MV, Potapchuk MV, et al. Influence of the linking order of fragments of HA2 and M2e of the influenza A virus to flagellin on the properties of recombinant proteins. Acta Naturae. 2018;10(1):85-94.
77. Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5(10):1157-63.
78. Massa S, Paolini F, Marino C, Franconi R, Venuti A. Bioproduction of a therapeutic vaccine against human Papillomavirus in tomato hairy root cultures. Front Plant Sci. 2019;10:452.
79. Nogrady, B. How SARS-CoV-2 TestsWork and What’s Next in COVID-19 Diagnostics. The Scientist 2020.
80. Rosales-Mendoza S. Will plant-made biopharmaceuticals play a role in the fight against COVID-19? Expert Opin. Biol Ther. 2020;20:545-548.
81. Venkataraman, S, Hefferon, K, Makhzoum, A, Abouhaidar, MG.Combating Human Viral Diseases: Will Plant-Based Vaccines Be the Answer? Vaccines 2021, 9,761.
82. Montero-Morales L, Steinkellner H. Advanced Plant- Based Glycan Engineering. Front Bioeng Biotechnol. 2018;6.
83. Mastrangeli R, Palinsky W, Bierau H. Glycoengineered antibodies: towards the next-generation of immunotherapeutics. Glycobiology. 2019;29(3):199- 210.
84. Zeitlin L, Pettitt J, Scully C, Bohorova N, Kim D, Pauly M, et al. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A. 2011;108(51):20690-4.
85. Marusic C, Pioli C, Stelter S, Novelli F, Lonoce C, Morrocchi E, et al. N-glycan engineering of a plantproduced anti-CD20-hIL-2 immunocytokine significantly enhances its effector functions. Biotechnol Bioeng. 2018;115(3):565-76.
86. Castilho A, Strasser R, Stadlmann J, Grass J, Jez J, Gattinger P, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J Biol Chem. 2010;285(21):15923-30.
87. Castilho A, Neumann L, Daskalova S, Mason HS, Steinkellner H, Altmann F, et al. Engineering of sialylated mucin-type O-glycosylation in plants. J Biol Chem. 2012;287(43):36518-26.
88. Dent M, Hurtado J, Paul AM, Sun H, Lai H, Yang M, et al. Plant produced anti-dengue virus monoclonal antibodies exhibit reduced antibody dependent enhancement of infection activity. J Gen Virol. 2016;97:3280-3290.
89. Sun H, Chen Q, Lai H. Development of antibody therapeutics against flaviviruses. Int J Mol Sci. 2017;19(1): 54.
90. Hurtado J, Acharya D, Lai H, Sun H, Kallolimath S, Steinkellner H, et al. In vitro and in vivo efficacy of antichikungunya virus monoclonal antibodies produced in wild-type and glycoengineered Nicotiana benthamiana plants: Glycoengineered anti-Chikungunya monoclonal antibody. Plant Biotechnol J. 2020;18(1):266-73.
91. Olinger GG Jr, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc Natl Acad Sci U S A. 2012;109(44):18030-5.
92. Lyon GM, Mehta AK, Varkey JB, Brantly K, Plyler L, McElroy AK, et al. Clinical care of two patients with Ebola virus disease in the United States. N Engl J Med. 2014;371(25):2402-9.
93. Qiu X, Wong G, Audet J, Bello A, Fernando L, Alimonti JB, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514(7520):47-53.
94. Huang Z, Chen Q, Hjelm B, Arntzen C, Mason H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol Bioeng. 2009;103(4):706-14.
95. Huang Z, Phoolcharoen W, Lai H, Piensook K, Cardineau G, Zeitlin L, et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng. 2010;106(1):9- 17.
96. Diamos AG, Mason HS. Chimeric 30 flanking regions strongly enhance gene expression in plants. Plant Biotechnol J. 2018;16:1971-1982.
97. Dolleweerd CJ, Teh AY, Banyard AC, Both L, Lotter- Stark HC, Tsekoa T, et al. Engineering, expression in transgenic plants and characterisation of e559, a rabies virus-neutralising monoclonal antibody. J Infect Dis. 2014;210:200-208.
98. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582-9.
99. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490-6.
100. Wilson J, Dellorto D. 9 Questions about This New Ebola Drug. Atlanta, GA, USA: CNN; 2014.
101. Tremouillaux-Guiller J, Moustafa K, Hefferon K, Gaobotse G, Makhzoum A. Plant-made HIV vaccines and potential candidates. Curr Opin Biotechnol. 2020;61:209- 16.
102. Medical Research Council, UK. Discovery of Key Component of HIV Virus Yields Drug Target. Drug discovery and development. August 11, 2016.
103. Nyamveya S, Hegedust A, Jaye A, Rowland S, Flanagan KL, Macallan DC. Comparing HIV-1 and HIV- 2 infection: Lessons for viral immunopathogenesis. Rev Med Virol. 2013;23:221-240.
104. Stephenson KE, Neubauer GH, Reimer U, Pawlowski N, Knaute T, Zerweck J, et al. Quantification of the epitope diversity of HIV-1-specific binding antibodies by peptide microarrays for global HIV-1 vaccine development. J Immunol Methods. 2015;416:105-23.
105. Haddox HK, Dingens AS, Hilton SK, Overbaugh J, Bloom JD. Mapping mutational effects along the evolutionary landscape of HIV envelope. Elife. 2018;7.
106. Rathore U, Purwar M, Vignesh VS, Das R, Kumar AA, Bhattacharyya S, et al. Bacterially expressed HIV-1 gp120 outer-domain fragment immunogens with improved stability and affinity for CD4-binding site neutralizing antibodies. J Biol Chem. 2018;293(39):15002-20.
107. Marusic C, Vitale A, Pedrazzini E, Donini M, Frigerio L, Bock R, et al. Plant-based strategies aimed at expressing HIV antigens and neutralizing antibodies at high levels. Nef as a case study. Transgenic Res. 2009;18(4):499-512.
108. Zhang GG, Rodrigues L, Rovinski B, White KA. Production of HIV-1 p24 protein in transgenic tobacco plants. Mol Biotechnol. 2002;20(2):131-6.
109. Karasev AV, Foulke S, Wellens C, Rich A, Shon KJ, Zwierzynski I, et al. Plant based HIV-1 vaccine candidate: Tat protein produced in spinach. Vaccine. 2005;23(15):1875-80.
110. Barahimipour R, Neupert J, Bock R. Efficient expression of nuclear transgenes in the green alga Chlamydomonas: synthesis of an HIV antigen and development of a new selectable marker. Plant Mol Biol. 2016;90(4-5):403-18.
111. Opdensteinen P, Clodt JI, Müschen CR, Filiz V, Buyel JF. A combined ultrafiltration/diafiltration step facilitates the purification of cyanovirin-N from transgenic tobacco extracts. Front Bioeng Biotechnol. 2018;6:206.
112. Hopper JTS, Ambrose S, Grant OC, Krumm SA, Allison TM, Degiacomi MT, et al. The tetrameric plant lectin BanLec neutralizes HIV through bidentate binding to specific viral glycans. Structure. 2017;25(5):773-782.e5.
113. Vamvaka E, Farré G, Molinos-Albert LM, Evans A, Canela-Xandri A, Twyman RM, et al. Unexpected synergistic HIV neutralization by a triple microbicide produced in rice endosperm. Proc Natl Acad Sci U S A. 2018;115(33):E7854-62.
114. Govea-Alonso DO, Gómez-Cardona EE, Rubio- Infante N, García-Hernández AL, Varona-Santos JT, Salgado-Bustamante M, et al. Production of an antigenic C4 (V3) 6 multiepitopic HIV protein in bacterial and plant systems. Plant Cell Tissue Organ Cult. 2013;113:73-79.
115. Loh H-S, Green BJ, Yusibov V. Using transgenic plants and modified plant viruses for the development of treatments for human diseases. Curr Opin Virol. 2017;26:81-9.
116. Orellana-Escobedo L, Rosales-Mendoza S, Romero- Maldonado A, Parsons J, Decker EL, Monreal-Escalante E, et al. An Env-derived multi-epitope HIV chimeric protein produced in the moss Physcomitrella patens is immunogenic in mice. Plant Cell Rep. 2015;34(3):425-33.
117. Rühl C, Knödler M, Opdensteinen P, Buyel JF. A linear epitope coupled to DsRed provides an affinity ligand for the capture of monoclonal antibodies. J Chromatogr A. 2018;1571:55-64.
118. Rubio-Infante N, Govea-Alonso DO, Romero- Maldonado A, Garcia-Hernandez AL, Ilhuicatzi-Alvarado D, Salazar-Gonzalez JA, et al. A plant-derived derived multi-HIV antigen induces broad immune responses in orally immunized mice. Mol Biotechnol. 2015;57:662-674.
119. D’Aoust MA, Couture MM, Lavoie PO, Vezina LP. Virus Like Particle Production in Plants. Patent. 2012;number WO2012083445(A1).
120. Wang B-Z, Liu W, Kang S-M, Alam M, Huang C, Ye L, et al. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into viruslike particles. J Virol. 2007;81(20):10869-78.
121. Kessans SA, Linhart MD, Matoba N, Mor T. Biological and biochemical characterization of HIV-1 Gag/dgp41 virus-likeparticles expressed in Nicotiana benthamiana. Plant Biotechnol J. 2013;11:681-690.
122. Scotti N, Alagna F, Ferraiolo E, Formisano G, Sannino L, Buonaguro L, et al. High-level expression of the HIV-1 Pr55gag polyprotein in transgenic tobacco chloroplasts. Planta. 2009;229(5):1109-22.
123. Porta C, Spall VE, Loveland J, Johnson JE, Barker PJ, Lomonossoff GP. Development of cowpea mosaic virus as a high-yielding system for the presentation of foreign peptides. Virology. 1994;202(2):949-55
124. McLain L, Porta C, Lomonossoff GP, Durrani Z, Dimmock NJ. Human immunodeficiency virus type 1-neutralizing anti-bodies raised to a glycoprotein 41 peptide expressed on the surface of a plant virus. AIDS Res Hum Retrovir. 1995;11:327-334.
125. McLain L, Durrani Z, Wisniewski LA, Porta C, Lomonossoff GP, Dimmock NJ. Stimulation of neutralizing antibodies to human immunodeficiency virus type 1 in three strains of mice immunized with a 22 amino acid peptide of gp41 expressed on the surface of a plant virus. Vaccine. 1996;14(8):799-810.
126. Fuqua JL, Wanga V, Palmer KE. Improving the large scale purification of the HIV microbicide, griffithsin. BMC Biotechnol. 2015;15(1):12.
127. Alam A, Jiang L, Kittleson GA, Steadman KD, Nandi S, Fuqua JL, et al. Technoeconomic modeling of plantbased griffithsin manufacturing. Front Bioeng Biotechnol. 2018;6.
128. Nandi S, Kwong AT, Holtz BR, Erwin RL, Marcel S, McDonald KA. Techno-economic analysis of a transient plant-based platform for monoclonal antibody production. MAbs. 2016;8(8):1456-66.
129. Vamvaka E, Twyman RM, Murad AM, Melnik S, Teh AY-H, Arcalis E, et al. Rice endosperm produces an underglycosylated and potent form of the HIV neutralizing monoclonal antibody 2G12. Plant Biotechnol J. 2016;14(1):97-108.
130. Ma JK-C, Drossard J, Lewis D, Altmann F, Boyle J, Christou P, et al. Regulatory approval and a first-inhuman phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J. 2015;13(8):1106-20.
131. Rademacher T, Sack M, Arcalis E, Stadlmann J, Balzer S, Altmann F, et al. Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV- 1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol J. 2008;6(2):189-201.
132. Ramessar K, Rademacher T, Sack M, Stadlmann J, Platis D, Stiegler G, et al. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci U S A. 2008;105(10):3727-32.
133. Singh AA, Pooe O, Kwezi L, Lotter-Stark T, Stoychev SH, Alexandra K, et al. Plant-based production of highly potent anti-HIV antibodies with engineered posttranslational modifications. Sci Rep. 2020;10(1):6201.
134. Seber Kasinger LE, Dent MW, Mahajan G, Hamorsky KT, Matoba N. A novel anti-HIV-1 bispecific bNAb-lectin fusion protein engineered in a plant-based transient expression system. Plant Biotechnol J. 2019;17(8):1646- 56.
135. Margolin E, Chapman R, Meyers AE, van Diepen MT, Ximba P, Hermanus T, et al. Production and immunogenicity of soluble plant-produced HIV-1 subtype C envelope gp140 immunogens. Front Plant Sci. 2019;10:1378.
136. Rubio-Infante N, Govea-Alonso DO, Alpuche- Solís ÁG, García-Hernández AL, Soria-Guerra RE, Paz- Maldonado LMT, et al. A chloroplast-derived C4V3 polypeptide from the human immunodeficiency virus (HIV) is orally immunogenic in mice. Plant Mol Biol. 2012;78(4-5):337-49.
137. Kessans SA, Linhart MD, Meador LR, Kilbourne J, Hogue BG, Fromme P, et al. Immunological characterization of plant-based HIV-1 Gag/Dgp41 viruslike particles. PLoS One. 2016;11(3):e0151842.
138. Meyers A, Chakauya E, Shephard E, Tanzer FL, Maclean J, Lynch A, et al. Expression of HIV-1 antigens in plants as potential subunit vaccines. BMC Biotechnol. 2008;8(1):53.
139. Lindh I, Bråve A, Hallengärd D, Hadad R, Kalbina I, Strid Å, et al. Oral delivery of plant-derived HIV-1 p24 antigen in low doses shows a superior priming effect in mice compared to high doses. Vaccine. 2014;32(20):2288- 93.
140. Pérez-Filgueira DM, Brayfield BP, Phiri S, Borca MV, Wood C, Morris TJ. Preserved antigenicity of HIV- 1 p24 produced and purified in high yields from plants inoculated with a tobacco mosaic virus (TMV)-derived vector. J Virol Methods. 2004;121(2):201-8.