Perspective
My team of chemists have formulated molecular iodine (I2) so it can be studied independent of other iodine species. The initial impetus for this effort was to allow for a higher concentration of biocidal I2 that would not evaporate into the atmosphere when applied to skin. The COVID pandemic increased the use of diluted PVP-I for nasal and oral prophylaxis which induced us to perform basic toxicological evaluations of pure I2. We did not believe that elevated concentrations of I2 would stain and irritate skin as literature suggests. Since dilution of Povidone Iodine (PVP-I) for nasal prophylaxis results in higher concentrations of I2 than neat 10% PVP-I, we wanted to demonstrate that this practice was likely to be safe. It was further reasoned that these formulations would allow an evaluation of I2 outgassing from skin as originally demonstrated by the leading expert of iodine disinfection in the 20th century, Waldermar Gottardi [1]. These questions were addressed and a novel approach for topical iodine disinfection is presented using high concentrations of I2 that can destroy pathogens for extended periods of time without staining or damaging skin [2].
The data we generated challenges two long-held beliefs that have inhibited the use of pure I2 in clinical and OTC settings. The scientific and medical communities believe that I2 is responsible for staining and cytotoxicity associated with aqueous iodine products such as PVP-I and Lugol’s Solution [3]. There is no controlled data in the literature that supports this assumption, and our data also calls this assumption into question. We demonstrated that I2 does not stain or irritate skin; this observation indicates that ingredients other than I2 in topical iodine disinfectants are responsible for these negative characteristics. Additionally, it is reasonable to ask, why wouldn’t pure I2 compositions be a preferred topical anti-microbial as compared to PVP-I and Lugol’s Solution?
Lack of staining – we demonstrated, in a straight-forward manner, how an emollient formulation containing pure I2 does not stain skin. The pure I2 emollient, with a concentration of 7500 ppm (>100 fold higher than I2 ppm found in PVP-I), was compared against 4 other topical formulations [2]. The pure I2 emollient was the only test area without any staining on the author’s left forearm (Figure 1). This experiment negates the argument that I2 is responsible for staining from iodophors such as PVP-I or from Lugol’s Solution.
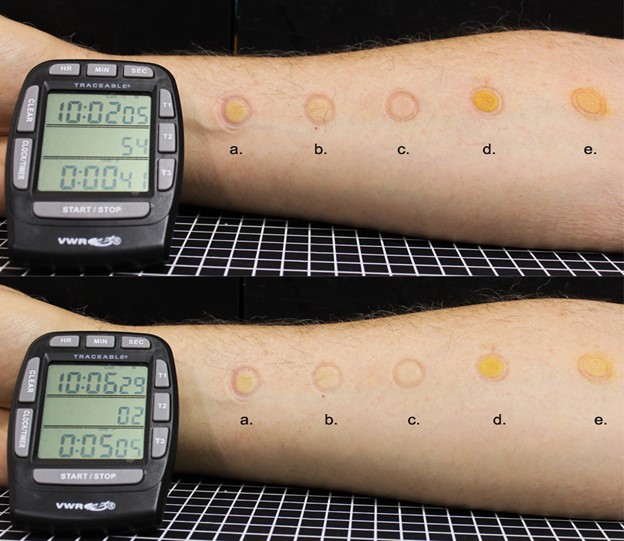
Figure 1. One milliliter of (a) 300 ppm I2 in water; (b) 10% povidone-iodine; (c) 7800 ppm I2 -glycerin, (d) Lugol’s solution and (e) iodine tincture were simultaneously contacted to the forearm of a volunteer for 3 min and residual was removed with a Dacron alcohol wipe. Images were taken immediately after application (top) and after 5 min (bottom). The glycerin composition with 7800 ppm of I2 did not stain the skin on the forearm of this 70-year-old white male and author of this manuscript, Jack Kessler Ph.D. in contrast to the four other topical
iodine formulations that contained much lower I2 concentrations.
Biocompatibility with Ex vivo Human Skin
Biocompatibility of I2 (500, 2500, and 10000 ppm) with human skin was evaluated by performing an MTT assay. Adipose tissue was removed from freshly acquired human skin tissue. Intact human skin tissue explants (12 mm) were produced via biopsy punch and trimmed to an even thickness. Punched explants were transferred to the insert of a 6-well transwell cell culture plate with two explants per well. One mL of Roswell Park Memorial Institute 1640 medium (ATCC MTT Reagent containing 2% penicillin/streptomycin) was placed in each well below the transwell insert. Test agent (10 µL) were placed on top of explants and spread across the surface of the explant with sterile inoculation loops to ensure even coverage without allowing any formulation to go over the sides of the explant. Phosphate buffered saline (PBS), Tween 20, Triton X 100, SDS, and glycerin were run as controls. Cell viability was calculated by dividing the mean absorbance measured for test articles by the mean value for PBS. Reported. Triplicate (n=3 for MTT) samples were prepared per test item and timepoint and shown below in Table 1. At 3 and 7 hours there was no difference in skin cell viability between glycerin (carrier control) and any of the I2 treatments. At 7 hours the 10,000 ppm I2 control exhibited a higher mean cell viability than the glycerin control while Tween-20 (mild irritant), Triton-X100 (moderate irritant) and SDS (strong irritant) all reduced cell viability.
|
Test Agent |
Cell Viability Compared to PBS (mean ± SEM) |
|
|---|---|---|
| 3h | 7h | |
|
Glycerin |
140.3 ± 13.4 |
59.1 ± 2.84 |
|
10% Tween 20 |
110.3 ± 6.0 |
46.7 ± 5.5 |
|
1% Triton X 100 |
83.7 ± 11.1 |
22.3 ± 0.4 |
|
5% SDS |
59.9 ± 12.7 |
21.24 ± 3.8 |
|
500 ppm |
114.5 ± 8.9 |
58.0 ± 4.5 |
|
2500 ppm |
158.0 ± 5.15 |
54.0 ± 5.4 |
|
10,000 ppm |
127.4 ± 18.2 |
67.3 ± 11.3 |
Antiproliferative activity of I2 – Iodinated lipids were identified as mediators of the Wolff-Chaikoff effect over 25 years ago [4,5] and speculation on their effect on cancer cells followed [5-7]. Interaction of molecular iodine with lipid membranes leads to the formation of 5-hydroxy-6iodo-8,11,14-eicosatrienoicdeltalactone, an iodinated arachidonic acid which can be abbreviated as 6-IL. Experimental demonstration of cancer cell apoptosis [8-11] was demonstrated and Carmen Aceves has systematically explored the role of non-thyroidal iodine in tumor cell lines [12-20] and she is currently conducting studies to determine the effect of molecular iodine as adjuvant for conventional chemotherapy patients [21]. Thomasz and co-workers have recently demonstrated that 6-IL induces apoptosis in a xenograft colon cancer (HT29 cells) in a dose dependent manner [22,23]. IL-6 reduced tumor mass and microvessel density and downregulated VEGF and VEGF-R2. Aceves, Thomasz and others have proposed several possible pathways to explain the effect of IL-6 on angiogenesis including inhibition of Cox2 [24] and PPARγ agonism [6,8-10,20,25,26]. It is clear that more research is needed to elucidate a potential antiproliferative role for 6-IL/I2. However, it is also reasonable to speculate that IL-δ could be used as a chemotherapeutic agent for the treatment of cancer tissues, alone or combined with other therapies.
I2 Cytotoxicity
The metabolic activity of human skin treated with I2 concentrations up to 15,000 ppm was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Ex vivo human skin tissue (HST) procured postsurgically under an IRB exempt protocol was used to evaluate tissue compatibility with I2. Adipose tissue was removed from freshly acquired HST and tissue explants were produced via biopsy punch. Explants were transferred to 6-well transwell cell cultures plates with two explants per well. RPMI 1640 (1 mL) containing 2% penicillin/streptomycin was placed in each well below the transwell insert. Ten microliters of test agent (carrier control and I2-carrier at concentrations between 500 and 10,000 ppm) were placed on top of explants in quadruplicate (n=3) per test item. The percent viability of the human skin cells was quantified 3 & 7 hours post application and there was no difference in cell viability between the glycerin control and any of the I2 treated tissues. We demonstrated the absence of cytotoxicity with a test using an elevated concentration of I2 (1500 ppm) in a 24-hour direct contact test with mouse fibroblasts [2]. If pure I2 is not responsible for cytotoxicity in topical iodine disinfectants, which ingredients are? Leaving the observations in this study aside, the argument that I2 is responsible for cytotoxicity in PVP-I can be seen as questionable when we consider that that a 1/100 dilution of PVP-I, contains higher amounts of I2 but exhibits less toxicity [27-29].
The active biocide in topical iodine disinfectants is the I2 molecule [27,30-32]. Once absorbed into the skin, I2 immediately begins outgassing [1]. While outgassing from skin, the I2 molecule can inactivate pathogens [1,33]. Observations indicate that I2 partitions into the hydrophobic regions of the dermis/hypodermis. The I2 slowly diffuses from the hydrophobic tissue for 6 or more hours until I2 can no longer be measured [2].
A careful reading of the medical and chemical literature related to “iodine” illuminates several current dynamics: (a) the medical community understandably associates the word “iodine” with thyroid hormones; (b) does not understand aqueous “iodine” chemistry which determines the properties of the topical I odine products currently in use [33,34]; and (c) there is an abundance of independent experimental data based on distinct experimental approaches that demonstrate an anti-inflammatory [35-39] and antiproliferative activity for molecular iodine and IL-6.
The data presented here may suggest a new model for surgical lavage [40-44], skin antisepsis and provides a practical I2 dosage form to explore clinical utility as an anti-inflammatory agent [35-37,39,45-49]. This formulation approach provides the possibility of converting skin into an antimicrobial material for hours while providing emollience.
Is pure I2 a natural antimicrobial with broader clinical utility?
References
2. Freeman C, Duan E, Kessler J. Molecular iodine is not responsible for cytotoxicity in iodophors. J Hosp Infect 2022.
3. Capriotti K, Capriotti JA. Topical iodophor preparations: chemistry, microbiology, and clinical utility. Dermatol Online J. 2012;18(11):1.
4. Dugrillon A, Uedelhoven WM, Pisarev MA, Bechtner G, Gartner R. Identification of delta-iodolactone in iodide treated human goiter and its inhibitory effect on proliferation of human thyroid follicles. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1994;26(10):465-9.
5. Langer R, Burzler C, Bechtner G, Gartner R. Influence of iodide and iodolactones on thyroid apoptosis. Evidence that apoptosis induced by iodide is mediated by iodolactones in intact porcine thyroid follicles. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2003;111(6):325-9.
6. Gartner R, Rank P, Ander B. The role of iodine and delta-iodolactone in growth and apoptosis of malignant thyroid epithelial cells and breast cancer cells. Hormones (Athens, Greece). 2010;9(1):60-6.
7. Rosner H, Torremante P, Moller W, Gartner R. Antiproliferative/cytotoxic activity of molecular iodine and iodolactones in various human carcinoma cell lines. No interfering with EGF-signaling, but evidence for apoptosis. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2010;118(7):410-9.
8. Shrivastava A, Tiwari M, Sinha RA, Kumar A, Balapure AK, Bajpai VK, et al. Molecular iodine induces caspase-independent apoptosis in human breast carcinoma cells involving the mitochondria-mediated pathway. Journal of Biological Chemistry. 2006 Jul 14;281(28):19762-71.
9. Singh P, Godbole M, Rao G, Annarao S, Mitra K, Roy R, et al. Inhibition of autophagy stimulate molecular iodine-induced apoptosis in hormone independent breast tumors. Biochemical and Biophysical Research Communications. 2011;415(1):181-6.
10. Rösner H, Möller W, Groebner S, Torremante P. Antiproliferative/cytotoxic effects of molecular iodine, povidone-iodine and Lugol’s solution in different human carcinoma cell lines. Oncol Lett. 2016;12(3):2159-62.
11. Zhang L, Sharma S, Zhu LX, Kogai T, Hershman JM, Brent GA, et al. Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Research. 2003;63(16):5065-72.
12. Aceves C, Anguiano B, Delgado G. Is iodine a gatekeeper of the integrity of the mammary gland? Journal of Mammary Gland Biology and Neoplasia. 2005;10(2):189-96.
13. Garcia-Solis P, Alfaro Y, Anguiano B, Delgado G, Guzman RC, Nandi S, et al. Inhibition of N-methyl-N-nitrosourea-induced mammary carcinogenesis by molecular iodine (I2) but not by iodide (I-) treatment Evidence that I2 prevents cancer promotion. Molecular and Cellular Endocrinology. 2005;236(1-2):49-57.
14. Garcia-Solis P, Guadalupe D, Anguiano B, Aceves C. Differential Uptake and Signaling of Molecular Iodine (I2) in Lactating, Virgin, or Neoplastic Mammary Glands. 13th International Thyroid Meeting, Buenos Aires, Arg. Thyroid. 2005;15(Supplement 1):S-128.
15. Aceves OA-HBAGDC. Uptake and antiproliferative effect of molecular iodine in the MCF-7 breast cancer cell line. Endocrine-related Cancer. 2006;13:11.
16. Nunez-Anita RE, Arroyo-Helguera O, Cajero-Juárez M, López-Bojorquez L, Aceves C. 6-Iodo-d-lactone identified as novel ligand of peroxisome proliferator-activated receptors (PPARs). 78th Annual Meeting of The American Thyroid Association, New York, USA,October. Abstract 2007 Oct (Vol. 42).
17. Aranda N, Arroyo-Helguera O, Delgado G, Aceves C, Anguiano B. Iodine reduces proliferation without affecting metabolism and functionality of human prostate cancer cells. 78th ATA Meeting. 2007; Abstract 114.
18. Arroyo-Helguera O, Rojas Edl Castillo E, Carmen A. Characterization of Cell Cycle Arrest and Apoptosis Pathways Involved in the Antineoplastic Effect of Molecular Iodine and 6-Iodo-D-Lactone (6-IL) in Mornal and Cancer Breast Cells.
19. Vega-Riveroll LR-A J; Rombero-Romo J, Delgado G, Mondragon P, Aceves C. Apoptotic and Antiproliferative Effects of Iodine Supplements on Human Breast Cancer Tumors.
20. Nunez-Anita RE, Arroyo-Helguera O, Cajero-Juarez M, Lopez-Bojorquez L, Aceves C. A complex between 6-iodolactone and the peroxisome proliferator-activated receptor type gamma may mediate the antineoplastic effect of iodine in mammary cancer. Prostaglandins & Other Lipid Mediators. 2009;89(1-2):34-42.
21. Moreno-Vega A, Vega-Riveroll L, Ayala T, Peralta G, Torres-Martel JM, Rojas J, et al. Adjuvant Effect of Molecular Iodine in Conventional Chemotherapy for Breast Cancer. Randomized Pilot Study. Nutrients. 2019;11(7).
22. Thomasz L, Oglio R, Rossich L, Villamar S, Perona M, Salvarredi L, et al. 6 Iodo-δ-lactone: a derivative of arachidonic acid with antitumor effects in HT-29 colon cancer cells. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2013;88(4):273-80.
23. Romina O, Federico B, Leonardo S, Jennifer M, Carla R, Marina P, et al. 6 Iodo-delta lactone inhibits angiogenesis in human HT29 colon adenocarcinoma xenograft. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2022;186:102507.
24. Aceves C, Mendieta I, Anguiano B, Delgado-González E. Molecular Iodine Has Extrathyroidal Effects as an Antioxidant, Differentiator, and Immunomodulator. International Journal of Molecular Sciences. 2021;22(3).
25. Aceves C, Garcia-Solis P, Arroyo-Helguera O, Vega-Riveroll L, Delgado G, Anguiano B. Antineoplastic effect of iodine in mammary cancer: participation of 6-iodolactone (6-IL) and peroxisome proliferator-activated receptors (PPAR). Molecular Cancer. 2009;8:33.
26. Cuenca-Micó O, Delgado-González E, Anguiano B, Vaca-Paniagua F, Medina-Rivera A, Rodríguez-Dorantes M, et al. Effects of Molecular Iodine/Chemotherapy in the Immune Component of Breast Cancer Tumoral Microenvironment. Biomolecules. 2021;11(10).
27. Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidone-iodine solutions. J Clin Microbiol. 1982;15(4):635-9.
28. Atemnkeng MA, Plaizier-Vercammen J, Schuermans A. Comparison of free and bound iodine and iodide species as a function of the dilution of three commercial povidone-iodine formulations and their microbicidal activity. Int J Pharm. 2006;317(2):161-6.
29. Atemnkeng MA, Plaizier-Vercammen JA. Comparison of free iodine as a function of the dilution of two commercial povidone-iodine formulations. J Pharm Belg. 2006;61(1):11-3.
30. Favero MS. Iodine--champagne in a tin cup. Infect Control. 1982;3(1):30-2.
31. Horn D, Ditter W. Physico-chemical basis of the microbicidal effect of aqueous PVP-iodine solutions. In: Hierholzer G, Görtz G,editors. PVP-Iodine in der operativen Medizin, Berlin, Heidelberg:Springer; 1984.
32. Wada H, Nojima Y, Ogawa S, Hayashi N, Sugiyama N, Kajiura T, et al. Relationship between Virucidal Efficacy and Free Iodine Concentration of Povidone-Iodine in Buffer Solution. Biocontrol Science. 2016;21(1):21-7.
33. Gottardi W. Iodine and disinfection: theoretical study on mode of action, efficiency, stability, and analytical aspects in the aqueous system. Archiv der Pharmazie. 1999;332(5):151-7.
34. Nuckolls C. Chemical Considerations Related to The Dilution of Commercial 10% Povidone-Iodine For Use In The COVID-19 Pandemic. 2020.
35. Wormser U, Brodsky B, Green BS, Arad-Yellin R, Nyska A. Protective effect of povidone-iodine ointment against skin lesions induced by sulphur and nitrogen mustards and by non-mustard vesicants. Archives of Toxicology. 1997;71(3):165-70.
36. Wormser U. Early topical treatment with povidone-iodine ointment reduces, and sometimes prevents, skin damage following heat stimulus. Burns. 1998;24(4):383.
37. Wormser U, Sintov A, Brodsky B, Nyska A. Topical iodine preparation as therapy against sulfur mustard-induced skin lesions. Toxicology and Applied Pharmacology. 2000;169(1):33-9.
38. Wormser U, Brodsky B, Reich R. Topical treatment with povidone iodine reduces nitrogen mustard-induced skin collagenolytic activity. Archives of Toxicology. 2002;76(2):119-21.
39. Wormser U, Sintov A, Brodsky B, Amitai Y, Nyska A. Protective effect of topical iodine preparations upon heat-induced and hydrofluoric acid-induced skin lesions. Toxicol Pathol. 2002;30(5):552-8.
40. Lores ME, Ortiz JR, Rossello PJ. Peritoneal lavage with povidone-iodine solution in experimentally induced peritonitis. Surgery, Gynecology & Obstetrics. 1981;153(1):33-8,
41. Radulesco T, Lechien JR, Saussez S, Hopkins C, Michel J. Safety and Impact of Nasal Lavages During Viral Infections Such as SARS-CoV-2. Ear Nose Throat J. 2020:145561320950491.
42. Wu PH, Cheng PC, Chang CM, Lo WC, Cheng PW. Efficacy of Povidone-Iodine Nasal Irrigation Solution After Sinonasal Surgery: A Randomized Controlled Study. The Laryngoscope. 2021.
43. Kumar KS, Reddy GV, Naidu G, Pandiarajan R. Role of povidone iodine in periapical surgeries: Hemostyptic and anti-inflammatory? Ann Maxillofac Surg. 2011;1(2):107-11.
44. Shindo K, Funai S, Kuroda K, Wakano T, Nishimura K. Clinical study on the antiseptic effect of povidone-iodine solution for the surgical field of digestive tract operations. Dermatology. 2002;204 Suppl 1:47-51.
45. Sugimoto K, Ishikawa N, Terano T, Kitukawa Y, Kubosawa H, Ito S, et al. The importance of bacterial superantigens produced by Staphylococcus aureus in the treatment of atopic dermatitis using povidone-iodine. Dermatology. 2006;212 Suppl 1:26-34.
46. Moore K, Thomas A, Harding KG. Iodine released from the wound dressing Iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int J Biochem Cell Biol. 1997 Jan;29(1):163-71.
47. Beukelman CJ, van den Berg AJ, Hoekstra MJ, Uhl R, Reimer K, Mueller S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel) for wound healing in vitro. Burns. 2008;34(6):845-55.
48. Wang L, Qin W, Zhou Y, Chen B, Zhao X, Zhao H, et al. Transforming growth factor β plays an important role in enhancing wound healing by topical application of Povidone-iodine. Sci Rep. 2017;7(1):991.
49. Wormser U, Brodsky B, Green BS, Arad-Yellin R, Nyska A. Protective effect of povidone iodine ointment against skin lesions induced by chemical and thermal stimuli. J Appl Toxicol. 2000;20 Suppl 1:S183-5.
