Abstract
Luminescent metal-organic frameworks (LMOFs) are considered special candidates for the sensation and detection of particular analytes. Thiazolo[5,4-d]thiazoles (TTZs) are an ideal type of heterocycles for fluorophores with π-bridge moieties demonstrating structure-function characteristics. LMOFs with excellent sensing properties can be constructed by incorporating heterocyclic aromatic thiazolo[5,4-d]thiazole into the framework. This study has elaborated on LMOFs containing thiazolo[5,4-d]thiazole unit for the detection of environmental contaminants, such as toxic anions, aromatic compounds, and heavy metal ions.
Keywords
LMOF, Thiazole, Cd-MOFs, Zn-MOFs, Fluorescence sensor, Photoluminescence
Introduction
Porous coordination polymers (PCPs) or metal-organic frameworks (MOFs) have the potential for applications such as precursor for nanomaterial [1], gas storage [2], removal of heavy metal ions [3], luminescent sensor [4], catalyst [5], and so on. MOFs with appropriate luminescent behavior are called luminescent MOFs (LMOFs). The design and characterization of LMOFs have captured immense attention in the last decade, especially for developing various luminescent sensors, due to the merit of their high sensitivity and excellent selectivity. The photoluminescence of LMOFs can originate from organic ligands, lanthanide ions, and diverse luminescent guest species [6]. The selection of organic ligands containing π-conjugated or aromatic structures is an important section in the process of LMOFs design. LMOFs containing transition metals in a d10 electronic configuration (such as Cd2+ and Zn2+ ions) and highly conjugated organic linkers can emit strong linker-based luminescence [7].
Thiazole, a five-membered heterocycle containing sulfur and nitrogen atoms, is intrinsically fluorescent. Some thiazole derivatives have been used for the preparation of luminescence CPs and MOFs due to their fluorescent properties. Four 1D (one-dimensional) coordination polymers comprising the organic linker m-(2-thiazolyl) benzoic acid were synthesized and the luminescent properties in solutions were investigated. Upon excitation at =262 nm, m-(2-thiazolyl) benzoic acid and coordination polymers in water display an emission band centered at 348 nm assigned to the thiazole-containing ligand [8]. Violent luminescent with a strong sensitizer effect of the ligands for [Ln2Cl6(pym)2(thiazole)4] ? and [Ln2Cl6(pyz)(thiazole)6] ? (Ln = Tb, Er; pym= pyrimidine; pyz= pyrazine) can be observed by dominant ligand excitation and exclusive metal emission for VIS and NIR [9]. Among thiazole derivatives, thiazolo[5,4-d]thiazoles (TTZs) represent a rigid and planar backbone, strong π–π stacking, high oxidative stability, and luminescence [10]. The good luminescence feature and rigid π-conjugated system are the characteristics of 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole (DPTTZ). Meanwhile, it possesses properties of pyridine and thiazolo[5,4-d]thiazole, as well as multiple coordination sites. Hence, investigating the variety of structures and luminescence of MOFs, which are prepared through the mixed ligand strategy of DPTTZ and different dicarboxylate ligands is very interesting. The effective π???π stacking for electronic transmission can be obtained by the introduction of rigid planar ligand, DPTTZ, with π-conjugated and electron-poor heteroaromatic systems, resulting in outstanding photophysical properties that is an effective strategy for obtaining luminescent MOFs. This paper undertakes a useful study on the development and application of LMOFs containing thiazolo[5,4-d]thiazole unit for sensing environmental contaminants in aqueous solutions.
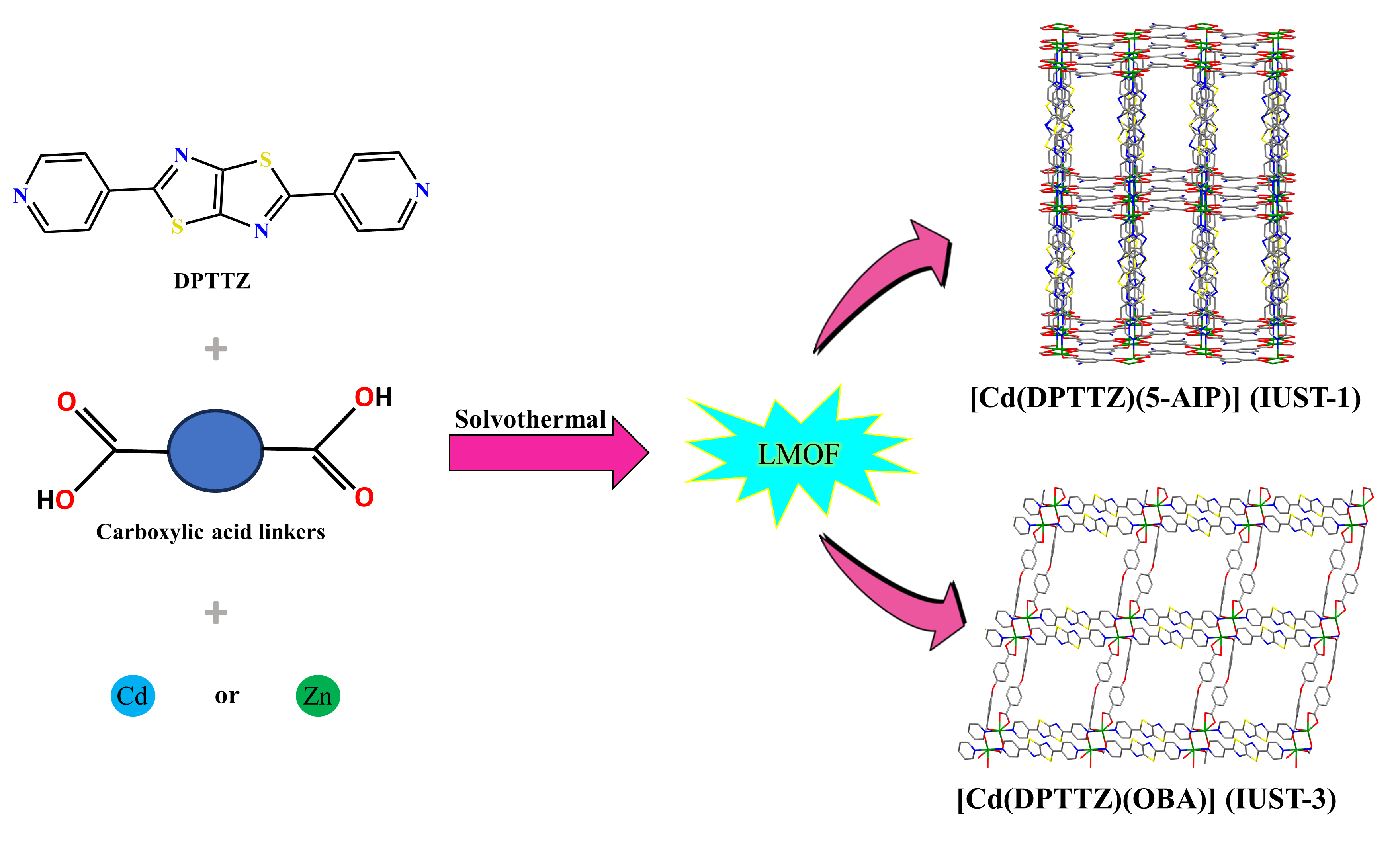
Design and Synthesis of LMOF Based on DPTTZ
Several different types of luminescent MOFs have been effectively explored as fluorescent probes for sensing applications. However, most of them are very poorly stable in water, which is undesirable for practical application. Therefore, the development of a LMOF for sensing materials in aqueous solutions is very important. The construction of MOFs based on d10 electron configurational metal ions and luminescent ligands is the desired strategy for exploring new LMOFs [11]. Incomparable LMOFs have been designed and fabricated by the dual-ligand strategy. LMOFs containing DPTTZ ligand were successfully fabricated using DPTTZ ligand in a mixture with dicarboxylate ligands and d10 metal cations (Cd2+ and Zn2+) via the solvothermal method (Figure 1). Synthesis conditions for LMOFs containing DPTTZ ligand can be affected by the change of metal ion and carboxylate ligands. Parameters e.g. type of crystallization temperature, solvent, and reaction time have important affection on the target LMOFs. The suitable temperature range for the synthesis of these compounds is between 80 to 120ºC. Also, most of these LMOFs have a reaction time of 24 to 72 hours. Solvents and a mixture of solvents such as pure DMF (N, N-dimethylformamide), pure water, DMF/ethanol, and DMF/water can be used for the preparation of LMOFs containing DPTTZ ligand. The PXRD (powder x-ray diffraction) analysis confirms the phase purity of all synthesized compounds and the yield of most products based on the DPTTZ ligand is more than 60%.
Figure 1. Design and synthesis of LOMFs based on DPTTZ ligand.
Luminescent Properties
The luminescence properties of LMOFs containing DPTTZ ligand and central metal ions with d10 electron configuration such as zinc(II) and cadmium(II) have been investigated. In Table 1, excitation wavelengths and maximum emission are presented. The π → π* transition is the source of luminescence emission of the DPTTZ ligand. The luminescent characteristics of the LMOFs are different compared to the DPTTZ ligand and show a redshift. The redshift of emission for LMOFs to the free ligand can be mainly ascribed to the coordination of metal ions with the organic ligands, which may reduce the π-π* energy gaps of the ligands. Ligand-to-ligand charge transition (LLCT), ligand-to-metal charge transfer (LMCT), and intra-ligand charge transfer (ILCT) can lead to small, big, and very big redshifts. The small redshifts are commonly attributed to the metal perturbed intra-ligand transitions (MPILT) of ligands. The big redshifts can be due to the ligand-to-metal charge transfer in nature. The very big redshifts are assigned to ligand-to-ligand charge transfer. An enhancement in the luminescence intensity of LMOFs compared to the free ligands can be attributed to the increase in the ligand conformational rigidity due to their coordination with metal ions resulting in a decrease in the nonradiative decay of intra-ligand excited states [12]. However, some compounds display a slightly weaker luminescence intensity than those of their ligands due to the coordination of ligand to metal which reduces the rigidity of the ligand and increases the nonradiative energy loss. The discrepancy in the different coordination environments around metal ions and dicarboxylate linkers as well as the coordination and conformations of ligands can be the reasons for the complicated luminescent properties of these LMOFs.
|
LMOFs |
λex (nm) |
λem (nm) |
Ref. |
|
DPTTZ |
409 |
439-452 |
[12] |
|
{[Zn2(Py2TTz)(2-CH3-BDC)2]·2(DMF)·3(H2O)}n |
413 |
485 |
[12] |
|
{[Cd2(Py2TTz)2(2-CH3-BDC)2]·2(DMF)·2(C2H5OH)}n |
422 |
506 |
[12] |
|
{[Zn0.5(Py2TTz)0.5(2,5-di-CH3- BDC)0.5]·(DMF)}n |
423 |
464 |
[12] |
|
{[Zn(Py2TTz)(1,4-NDC)]·(DMF)·(H2O)}n |
434 |
508 |
[12] |
|
{[Zn(Py2TTz)0.5(2,6-NDC)]·(DMF)·(C2H5OH)}n |
424 |
463 |
[12] |
|
{[Cd2(Py2TTz)2(2,6-NDC)2]·3(DMF)·4(H2O)}n |
424 |
519 |
[12] |
|
{[Zn2(Py2TTz)2(5-CH3-BDC)2]·3(DMF)·3(H2O)}n |
404 |
497 |
[12] |
|
{[Cd2(Py2TTz)2(5-CH3-BDC)2]·3(DMF)·3(H2O)}n |
424 |
515 |
[12] |
|
[Cd(DPTTZ)(5-AIP)] (IUST-1) |
320 |
405 |
[14] |
|
[Cd(DPTTZ)(OBA)] (IUST-3) |
380 |
525 |
[15] |
|
{[Zn2(Py2TTz)2(BDC)2]·2(DMF)0.5(H2O)}n |
414 |
453 |
[16] |
|
{[Cd2(Py2TTz)2(BDC)2]·2(DMF)}n |
417 |
518 |
[16] |
|
[Zn(Py2TTz)(5-OH-IPA)]n |
275 |
460 |
[17] |
|
Note: Py2TTz: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; 2-CH3-BDC: 2-methyl-1,4-benzenedicarboxylic acid; DMF: N, N-dimethylformamide; 2,5-di-CH3-BDC: 2,5-dimethyl-1,4-benzenedicarboxylic acid; 1,4-NDC: 1,4-naphthalenedicarboxylic acid; 2,6-NDC: 2,6-naphthalenedicarboxylic acid; 5-CH3-BDC: 5-methyl-1,3-benzenedicarboxylic acid; DPTTZ: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; 5-AIP: 5-aminoisophthalic acid; IUST: Iran university science and technology; OBA: 4,4′-oxybis(benzoic acid); BDC: 1,4-benzenedicarboxylic acid; 5-OH-IPA: 5-hydroxyisophthalic acid dianion. |
|||
The photoluminescence behavior of pillared-paddle wheel framework [Zn2(NDC)2(DPTTZ)] with fluorophores (DPTTZ) and great chromophores (NDC) with complementary absorption and emission characteristics suitable for FRET (Fo?rster Resonance Energy Transfer) were investigated. The lowest-energy absorption peak of DPTTZ overlaps quite well with the emission peak of NDC, which enables ligand-to-ligand FRET and allows the MOF to display exclusively DPTTZ-centric blue emission (∼410 nm) irrespective of the excitation wavelength [13].
Sensing of Environmental Contaminants and Mechanism
According to the mentioned above, LMOFs based on DPTTZ ligand with strong fluorescence in the solid state and high stability to water were used to detect pollutants in water. The quenching constants (KSV) and detection limits (LOD) of the reported luminescent MOFs containing thiazolo[5,4-d]thiazole unit for sensing anions, cations, and aromatic compounds in water were reported in Table 2. The constant KSV and detection limits provide a direct measure of the quenching sensitivity. The high quenching constant and low detection limit represent high sensitivity. All these results indicate that LMOFs based on DPTTZ ligand can be used as an effective fluorescent “turn-off” probe for the detection of environmental contaminants in the water.
|
LMOFs |
detection limits (LOD)/ μM |
quenching constants (KSV)/ M-1 |
Ref. |
|
[Cd(DPTTZ)(5-AIP)] (IUST-1) |
2.6 (NB)/ 0.60 (Cr2O72−) |
5.84 × 104 (NB)/ 3.314 × 104 (Cr2O72−) |
[14] |
|
[Cd(DPTTZ)(OBA)] (IUST-3) |
0.52 (4-NA)/ 1.37 (CrO42−) |
1.03 × 105 (4-NA)/ 2.93 × 104 (CrO42−) |
[15] |
|
{[Zn2(Py2TTz)2(BDC)2]·2(DMF)0.5(H2O)}n |
0.93 (TNP)/ 0.91 (NZF) |
3.257× 104 (TNP)/ 1.726 × 104 (NZF) |
[16] |
|
{[Cd2(Py2TTz)2(BDC)2]·2(DMF)}n |
0.90 (TNP)/ 0.85 (NZF) |
4.063 × 104 (TNP)/ 4.538 × 104 (NZF) |
[16] |
|
[Zn(Py2TTz)(5-OH-IPA)]n |
0.125 (Hg) |
8.43× 104 (Hg) |
[17] |
|
[Zn2 (5-AIA)2(DPTTZ)]·DMF |
2.174± 0.06 × 10-6 (Hg) |
4.2 ×104 (40 ºC) and 5.5 ×104 (50 ºC) |
[18] |
|
[Zn2(TzTz)2(BDC)2]·2DMF |
4 (TNP)/ 4 (NZF) |
9 × 107 (Cr2O72−)/ 4.8 × 103 (MnO4−) |
[19] |
|
Note: 5-AIA: 5-aminoisophthalic acid; DPTTZ: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; DMF: N, N-dimethylformamide; 5-AIP: 5-aminoisophthalic acid; IUST: Iran university science and technology; OBA: 4,4′-oxybis(benzoic acid); Py2TTz: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; BDC: 1,4-benzenedicarboxylic acid; TzTz: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; 5-OH-IPA: 5-hydroxyisophthalic acid dianion; NB: Nitrobenzene; 4-NA: 4-Nitroaniline; TNP: 2,4,6-Trinitrophenol; NZF: Nitrofurazone. |
|||
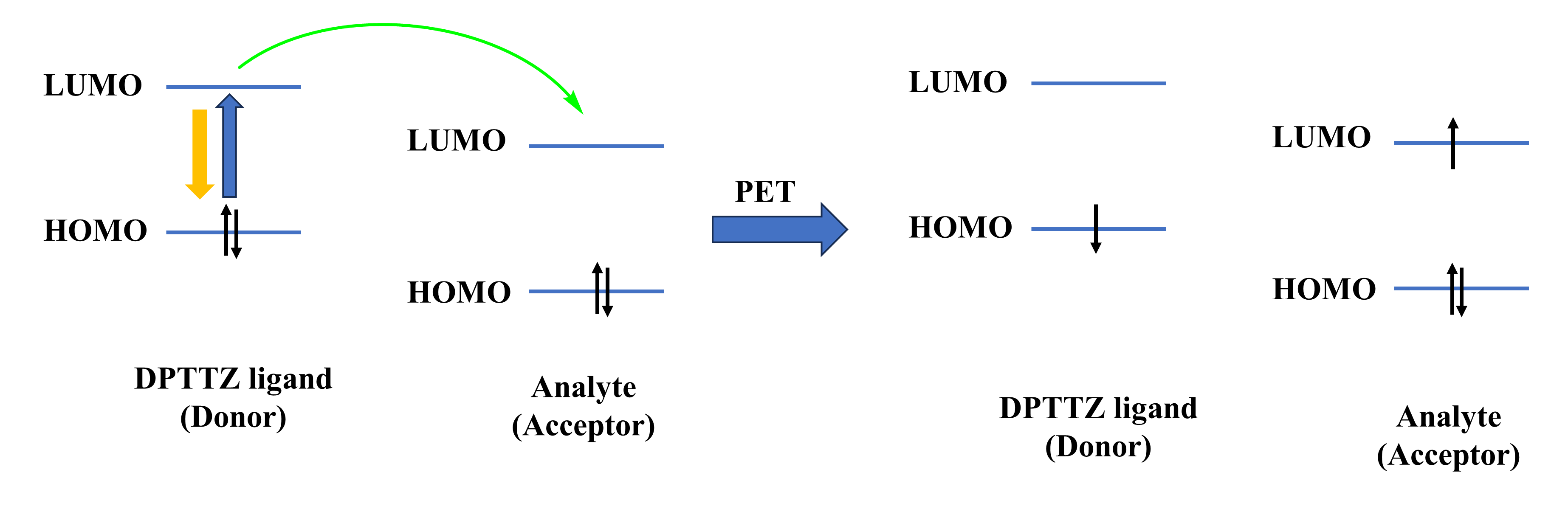
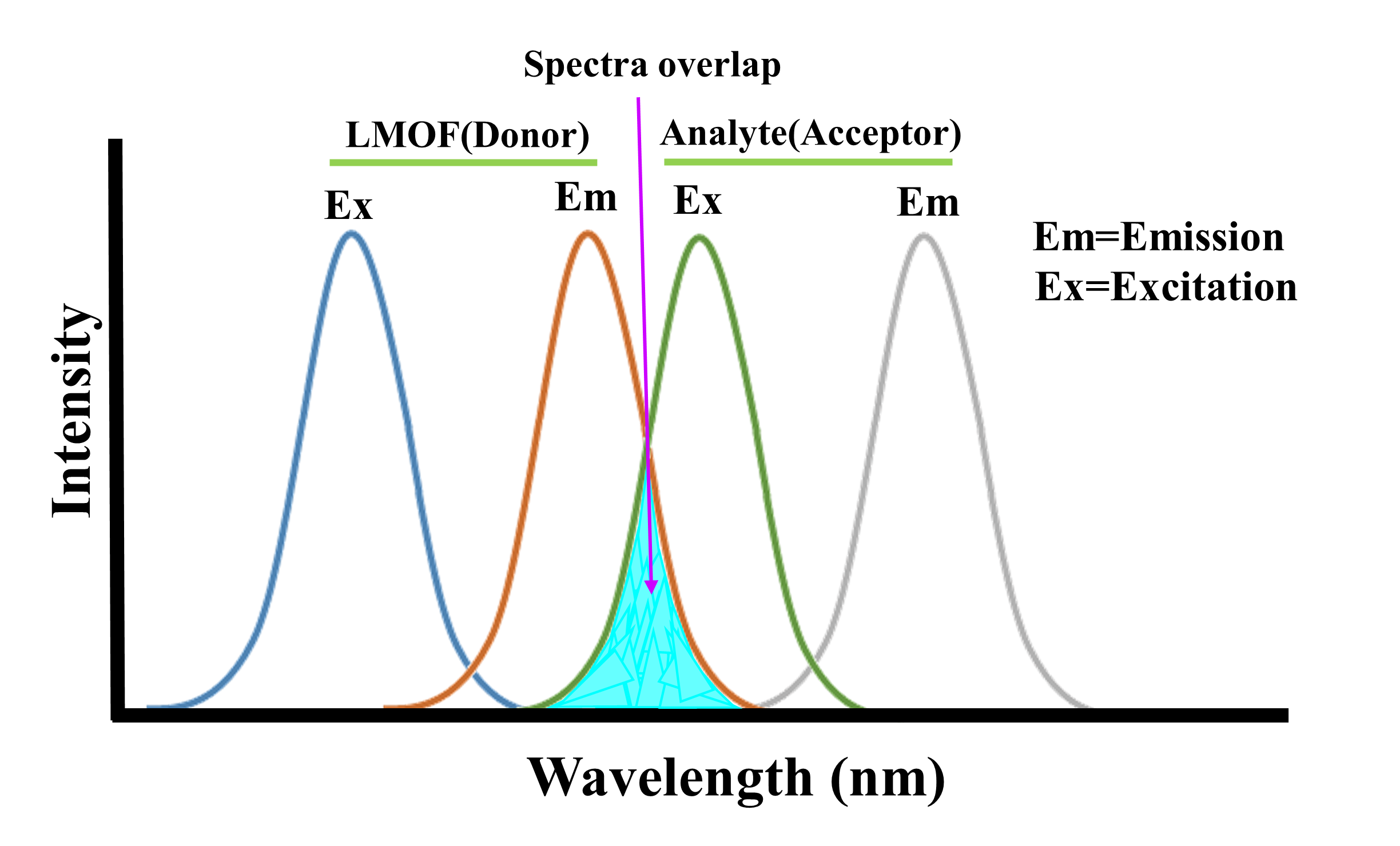
A particular method of fluorescence quenching (signal-off) has been used for designing this kind of LMOFs. The possible reasons for the quenching of the luminescence caused by environmental contaminants have been discussed as follows: structural collapse, RET (Resonance Energy Transfer), PET (Photoinduced Electron Transfer), and binding mechanism. The unaltered PXRD patterns of the frameworks after detecting experiments confirmed that the reduction in fluorescence intensity was not due to the decomposition of the framework’s structure. The PET mechanism was proposed due to the nature of the electron deficiency of analytes and the strong π-conjugated effect of ligand DPTTZ. Generally, analytes with low-lying LUMO (lowest unoccupied molecular orbital) are more capable of accepting electrons than the fluorescent ligand with higher energy, resulting in fluorescence quenching. Therefore, the excited state electrons can transfer from LMOFs (electron donors) to analytes (electron acceptors) (Figure 2a). The Stern-Volmer plot of the analytes exhibited a non-linear behavior, which indicates that RET can be another important reason for the luminescence quenching of LMOFs. As known, when the emission band of LMOF is effectively overlapped by the absorption band of the analyte, the resonance energy transfer from the LMOF to the analyte takes place and leads to luminescence quenching (Figure 2b).
(a)
(b)
Figure 2. (a) Schematic of photoinduced electron transfer process; (b) Schematic of resonance energy transfer process.
The luminescence quenching occurs through either static or dynamic interaction mechanisms, which can require different quantitative treatments. A non-fluorescent complex is formed between the ground state fluorophore and the quencher in static quenching, which is important in interdisciplinary applications [20]. The lifetimes of the [Zn2(5-AIA)2(DPTTZ)]·DMF and the MOF added with Hg(II) ions are the same (4.40 and 4.36 ns, respectively) that confirmed the static interaction of [Zn2(5-AIA)2(DPTTZ)]·DMF to Hg(II) ions. Also, The KSV for [Zn2(5-AIA)2 (DPTTZ)]·DMF at 40 and 50ºC were 4.2 ×104 and 5.5 ×104, respectively, indicating the static quenching mechanism [18]. The lifetime measurements of compounds {[Zn2(Py2TTz)2(BDC)2]·2(DMF)0.5(H2O)}n and {[Cd2(Py2TTz)2(BDC)2]·2(DMF)}n were performed at different concentrations of analytes to show which quenching mechanism (dynamic or static) is dominant. KD and KS of compounds {[Zn2(Py2TTz)2(BDC)2]·2(DMF)0.5(H2O)}n and {[Cd2(Py2TTz)2(BDC)2]·2(DMF)}n based on the fluorescence decay data were calculated. The obtained results (Larger KS) display that the sensing process occurs through a static quenching pathway [16]. The XPS and crystal packing study represented the interaction of hard-based oxygen and soft acid Hg(II) for [Zn2(5-AIA)2(DPTTZ)]·DMF. The presence of a new peak at ~530.8 in the O 1s XPS spectra confirmed the Hg(II)-oxygen interaction, demonstrating the active site of the Hg(II) ion interaction. At the ground state, a complex formed with the analyte, showing a static quenching mechanism. Further, the turn-off quenching of the absorption spectra with a slight red shift in the UV-Vis spectroscopy shows the LMOF-mercury(II) interaction is a ground-state phenomenon. Also, the DFT (density functional theory) calculation underpinned the turn-off fluorescence quenching mechanism of the LMOF. According to the theoretical calculation, the LUMO of the mercury(II) ion sits between the HOMO and LUMO of 5-AIA, which conducted a bypass for the excited electron of 5-AIA to relax from its LUMO to the LUMO of the mercury(II) ion [18]. Also, XPS spectra supported the theory of interactions between Hg(II) and [Zn(Py2TTz)(5-OH-IPA)]n. The binding sites between sulfur and nitrogen atoms of the thiazole ring and Hg(II) were responsible for the luminescence quenching of [Zn(Py2TTz)(5-OH-IPA)]n. The XPS spectra after Hg(II) addition displayed the N 1s peak of the nitrogen atom of thiazole at 400.59 eV shifted to 401.62 eV and the S 2p core-level spectrum peak at 164.16 eV shifted to 164.62 eV. These results showed that the S and N atoms of the Py2TTz ligands were the binding sites between [Zn(Py2TTz)(5-OH-IPA)]n and Hg(II). These interactions were responsible for the change in the fluorescence intensity of [Zn(Py2TTz)(5-OH-IPA)]n. According to the DFT calculation, the decreased energy gap π → π* of 4.73 eV (Py2TTz) to 3.58 eV (Py2TTz-Hg(II)) caused easier electron transition and increased the probability of nonradiative transition, which resulted in red-shifted and quenching of fluorescence emission [17].
|
LMOFs |
Mechanism |
Ref. |
|
[Cd(DPTTZ)(5-AIP)] (IUST-1) |
NB (RET)/ Cr2O72− (RET) |
[14] |
|
[Cd(DPTTZ)(OBA)] (IUST-3) |
4-NA (RET and PET)/ CrO42− (RET) |
[15] |
|
{[Zn2(Py2TTz)2(BDC)2]·2(DMF)0.5(H2O)}n |
TNP (RET and PET)/ NZF (RET and PET) |
[16] |
|
{[Cd2(Py2TTz)2(BDC)2]·2(DMF)}n |
TNP (RET and PET)/ NZF (RET and PET) |
[16] |
|
[Zn2(TzTz)2(BDC)2]·2DMF |
Cr2O72− (RET)/ MnO4− (RET) |
[19] |
|
[Zn(Py2TTz)(5-OH-IPA)]n |
Hg (PET and Coordination) |
[17] |
|
Note: 5-AIP: 5-aminoisophthalic acid; DPTTZ: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; IUST: Iran university science and technology; OBA: 4,4′-oxybis(benzoic acid); Py2TTz: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; BDC: 1,4-benzenedicarboxylic acid; DMF: N, N-dimethylformamide; TzTz: 2,5-bis(4-pyridyl)thiazolo[5,4-d]thiazole; 5-OH-IPA: 5-hydroxyisophthalic acid dianion; NB: Nitrobenzene; 4-NA: 4-Nitroaniline; TNP: 2,4,6-Trinitrophenol; NZF: Nitrofurazone. |
||
Conclusion
In summary, LMOFs with superior sensing properties can be fabricated by introducing heterocyclic aromatic thiazolo[5,4-d]thiazole into the framework. Hence, it is interesting to introduce DPTTZ and different dicarboxylate ligands into MOFs by the mixed ligand strategy. LMOFs based on thiazolo[5,4-d]thiazole were successfully fabricated using DPTTZ ligand in a mixture with dicarboxylate ligands and d10 metal cations (Cd2+ and Zn2+) via the solvothermal method. Exclusively, one advantage of these fluorescent compounds is their great selectivity and sensitivity for the detection of cations, anions, and aromatic compounds in water through a fluorescence quenching effect with a low detection limit and high quenching constant. However, the limited hydrolytic stability of some DPTTZ-based LMOFs such as Zn2(NDC)2(DPTTZ) could prevent practical usage of this LMOF as a sensor in aqueous solution. This study can be used for the design and assessment of the feasibility of a wide range of LMOFs as sensors for other analytes. Meanwhile, MOFs based on DPTTZ ligand have the potential to be used as fluorescence-based biosensors.
Acknowledgment
We gratefully acknowledge the Iran University of Science and Technology (IUST) for providing materials and some facilities.
Author Contributions
Akram Karbalaee Hosseini: Conceptualization, Writing – Original draft preparation. http://orcid.org/0009-0009-9900-3458; Azadeh Tadjarodi: Project administration Supervision, Review & Editing. http://orcid.org/0000-0003-0496-2322.
Competing Interests
The authors declare no competing interests.
Additional Information
Correspondence and requests for materials should be addressed to A.T.
References
2. Gonçalves DV, Snurr RQ, Lucena SM. Impact of H2O and CO2 on methane storage in metal–organic frameworks. Adsorption. 2019;25:1633-42.
3. Hosseini AK. Tadjarodi A. Novel Zn metal-organic framework with the thiazole sites for fast and efficient removal of heavy metal ions from water. Scientific Reports. 2023;13(1):11430.
4. Fan YK, Zhang WY, Zhang SS, Yan YT, Zhong K, Zhang Y, et al. Four new water-stable metal-organic frameworks based on diverse metal clusters: Syntheses, structures, and luminescent sensing properties. Journal of Solid State Chemistry. 2019;269:386-95.
5. Moghadaskhou F, Hosseini AK, Tadjarodi A, Abroudi M. Amino-induced cadmium metal-organic framework based on thiazole ligand as a heterogeneous catalyst for the epoxidation of alkenes. Scientific Reports. 2023;13(1):15391.
6. Kukkar P, Kukkar D, Sammi H, Singh K, Rawat M, Singh P, et al. A facile means for the improvement of sensing properties of metal-organic frameworks through control on the key synthesis variables. Sensors and Actuators B: Chemical. 2018;271:157-63.
7. Hidalgo‐Rosa Y, Treto‐Suárez MA, Schott E, Zarate X, Páez‐Hernández D. Sensing mechanism elucidation of a chemosensor based on a metal‐organic framework selective to explosive aromatic compounds. International Journal of Quantum Chemistry. 2020;120(23):e26404.
8. Staderini S, Tuci G, D'Angelantonio M, Manoli F, Manet I, Giambastiani G, et al. Zinc coordination polymers containing the m‐(2‐thiazolyl) benzoic acid spacer: synthesis, characterization and luminescent properties in aqueous solutions. Chemistry Select. 2016; 1(6): 1123-31.
9. Dannenbauer N, Zottnick SH, Müller-Buschbaum K. Thiazole and the diazines pyrazine and pyrimidine as sensitizers for lanthanide luminescence from VIS to NIR. Zeitschrift fur Anorganische und Allgemeine Chemie. 2017;643:1513-18.
10. Zani L, Calamante M, Mordini A, Reginato G. Thiazolo [5, 4-d] thiazole-based compounds: emerging targets in materials science, organic electronics and photovoltaics. Targets in Heterocyclic Systems. 2013;87.
11. Mercuri G, Giambastiani G, Rossin A. Thiazole-and Thiadiazole-based metal-organic frameworks and coordination polymers for luminescent applications. Inorganics. 2019;7(12):144.
12. Zhai ZW, Yang SH, Luo P, Li LK, Du CX, Zang SQ. Dicarboxylate‐induced structural diversity of luminescent Zn(II)/Cd(II) metal–organic frameworks based on the 2, 5‐Bis (4‐pyridyl) thiazolo [5, 4‐d] thiazole ligand. European Journal of Inorganic Chemistry. 2019;2019(22):2725-2734.
13. Khatun A, Panda DK, Sayresmith N, Walter MG, Saha S. Thiazolothiazole-based luminescent metal-organic frameworks with ligand-to-ligand energy transfer and Hg2+-sensing capabilities. Inorganic Chemistry. 2019;58(19):12707-15.
14. Hosseini AK, Pourshirzad Y, Tadjarodi A. A water-stable luminescent cadmium-thiazole metal-organic framework for detection of some anionic and aromatic pollutants. Journal of Solid State Chemistry. 2023;317:123676.
15. Hosseini AK, Tadjarodi A. Luminescent Cd coordination polymer based on thiazole as a dual-responsive chemosensor for 4-nitroaniline and CrO42- in water. Scientific Reports. 2023;13(1):269.
16. Zhai ZW, Yang SH, Cao M, Li LK, Du CX, Zang SQ. Rational design of three two-fold interpenetrated metal–organic frameworks: luminescent Zn/Cd-metal–organic frameworks for detection of 2, 4, 6-trinitrophenol and nitrofurazone in the aqueous phase. Crystal Growth & Design. 2018; 18(11):7173-82.
17. Li X, Xiu D, Shi J, Miao J, Yu Y, Song H, et al. Visual Hg(II) sensing in aqueous solution via a new 2, 5-Bis (4-pyridyl) thiazolo [5, 4-d] thiazole-based fluorescence coordination polymer. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2022;265:120367.
18. Nath A, Thomas GM, Hans S, Vennapusa SR, Mandal S. Crystal packing-driven selective Hg(II) ion sensing using thiazolothiazole-based water-stable zinc metal–organic framework. Inorganic Chemistry. 2022;61(4): 2227-33.
19. Safaei S, Wang J, Junk PC. Incorporation of thiazolothiazole fluorophores into a MOF structure: a highly luminescent Zn(II)-based MOF as a selective and reversible sensor for Cr2O72− and MnO4− anions. Journal of Solid State Chemistry. 2021;294:121762.
20. Genovese D, Cingolani M, Rampazzo E, Prodi L, Zaccheroni N. Static quenching upon adduct formation: a treatment without shortcuts and approximations. Chemical Society Reviews. 2021;50(15):8414-27.