Abstract
Introduction: Recurrent joint bleeds in hemophilia lead to (irreversible) joint damage, so-called hemophilic arthropathy causing major morbidity amongst hemophilia patients. Progression of arthropathy is monitored by clinical examination and imaging, but sensitive joint outcome measurements detecting early and subclinical joint damage are lacking. Biochemical markers reflecting joint tissue turnover can potentially provide this significant information about the joint status.
Aim: To provide an update on our systematic review about blood and urinary biochemical markers in patients with HA and give an overview of the challenges in biomarker research.
Methods: We updated our systematic search in PubMed/EMBASE and selected all publications between September 9, 2019 and June 23, 2021. All articles were screened and eligible publications were allocated to one or several BIPED-categories.
Results: We found 220 new articles/abstracts of which twelve were eligible for inclusion. The results were in line with our previous review.Again, many studies focused on the differences between patients with hemophilia and (healthy) control groups. Cartilage markers (e.g. COL- 18N, PRO-C4, PRO-C8, C4M) were most promising for detecting joint damage progression or acute hemarthrosis.
Conclusion: Biomarkers may reflect pathophysiological processes and are theoretically useful in clinical practice and trials to accurately monitor joint damage progression. However, biomarker research comes with multiple pitfalls and challenges. Translation into daily practice is not yet achieved.
Keywords
Biochemical marker research, BIPED, Hemophilic arthropathy, Joint damage, Joint tissue turnover
Introduction
Hemophilia is a rare congenital bleeding disorder caused by a lack of or diminished activity of clotting factor VIII (hemophilia A) or IX (hemophilia B). This deficiency leads to an increase in spontaneous and traumatic bleeding especially in the large hinged joints. Joint bleeding may result in synovial inflammation and cartilage/bone damage, ultimately leading to irreversible hemophilic arthropathy (HA) [1]. Preventing hemarthroses and accurately monitoring joint status once arthropathy has developed, is of utmost importance to prevent invalidating arthropathy and subsequent major orthopedic interventions.
At present, the development and progression of arthropathy is monitored by clinical follow-up and imaging. Many different clinical tools and imaging modalities are used for the assessment of joint health, each with its own advantages and limitations. The value of clinical assessment tools is determined by its sensitivity, inter-observer variation, floor/ ceiling effects and the appropriateness in different patient populations. Imaging modalities also have issues with sensitivity, availability, costs, examination time and the need for sedation in young children [2-4].
Another approach for early and adequate detection of joint bleeds and subsequent processes leading to joint damage might be the use of biochemical markers. Markers reflecting dynamical changes in joint health upon bleeding or during joint damage progression may provide information about real-time joint status.
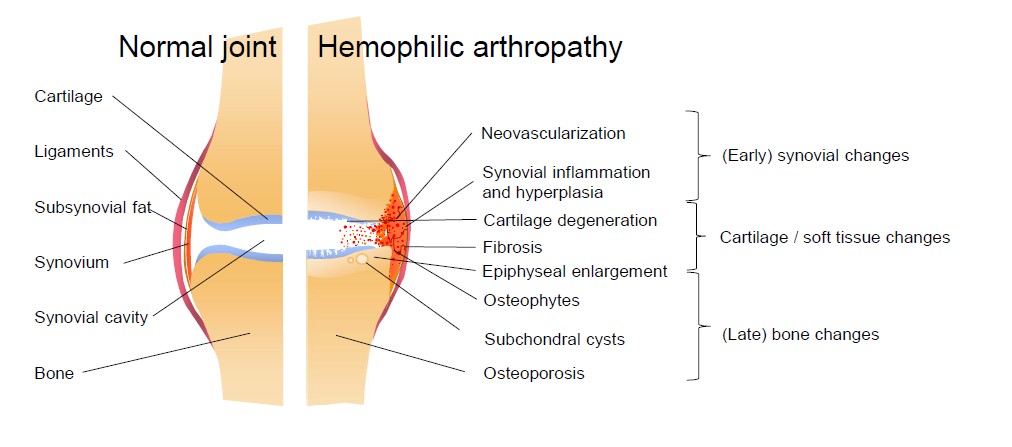
Upon joint bleeding, the synovial tissue is triggered by the presence of blood in the joint cavity. Synovial tissue is highly vascularized as it is responsible for removal of blood in the joint space and also for the production of synovial fluid for nutrition and lubrication of the joint. Blood entering the joint leads to an influx of inflammatory cells like macrophages, that remove blood from the joint cavity by phagocytosis. However, recurrent or ongoing joint bleeds overload the synovial capacity resulting in iron accumulation. The presence of iron triggers an inflammatory response and stimulates the proliferation of synoviocytes, which start producing cytokines and proteinases. The normally thin synovium becomes hypertrophic and needs more oxygen resulting in an increase of vascular endothelial growth factor (VEGF) and the formation of new fragile blood vessels. Besides the formation of new vessels accompanied by an increased risk of hemarthrosis, pro-inflammatory cytokines and proteases also upregulate cartilage-degrading enzymes. Together with the direct effects of blood on cartilage, chondrocyte apoptosis is induced. In a more advanced stage, blood in the joint cavity leads to bone changes like subchondral cysts and edema [1,5].
Although biochemical markers in theory could reflect the pathophysiologic processes induced by (recurrent) joint bleeding, translation as useful tool in clinical practice is not yet achieved. We published a systematic review with a clear overview of the existing literature on blood and urinary biochemical markers investigated in patients with HA [6]. Although promising in theory, biochemical marker research in HA is very heterogeneous, includes small and different patient populations and has a lack of a gold standard and therefore needs improvement. In the current article, we summarize the results of an updated search and provide an overview of the challenges and pitfalls in biomarker research in order to increase the quality and efficacy of biomarker research in HA.
Methods
Our initial systematic search with search terms ‘biomarkers’ AND ‘hemophilia’ OR ‘hemophilic arthropathy’ performed on September 9, 2019 was updated on June 23, 2021. Supplementary file 1 shows the detailed search strategy for PubMed and EMBASE. All new articles and abstracts were assessed for eligibility. Studies reporting on biochemical markers in blood or urine in patients with HA were included. Studies about biochemical markers in synovial tissue were excluded as these were considered not relevant for implementation in daily practice. Articles about bone turnover markers were only included when they correlated the markers with HA. Studies only evaluating bone turnover markers in correlation with bone mineral density fell outside the scope of this review. Included publications were analyzed and allocated to one or more BIPED-categories. This classification is based on the utility of biochemical markers (Table 1). The data are presented likewise.
| Category | Description |
|---|---|
| Burden of disease (B) | Biomarkers associated with the severity of hemophilia or severity of arthropathy |
| Investigative (I) | Biomarker research in animals or studying the changes upon a joint bleed |
| Prognostic (P) | Biomarkers predicting future outcomes (development or progression of arthropathy) in individual patients |
| Efficacy of intervention (E) | Biomarkers predicting whether an intervention will be efficacious and used to monitor the effects of an intervention or to determine which patients are eligible |
| Diagnostic (D) | Biomarkers with the capacity to identify hemophilic arthropathy in the general population or biomarker with the capacity to diagnose a joint bleed |
Results
Updated search results
220 new articles and abstracts were identified by searching PubMed (64) and Embase (156). Duplicates were systematically removed and 155 articles/abstracts were screened by title and abstract. After full text screening, seven articles and five abstracts were included. Publications were allocated to one or several BIPED-categories as summarized in Table 2.
|
Rf |
Biochemical marker | Investigation groups | Conclusions |
|---|---|---|---|
| Burden of disease | |||
|
1 |
S: 25-OH-vitD |
Children with hemophilia (n=38). Joint status assessed by questionnaire Physical Activity Score. Arthropathy defined as synovial swelling with restriction of movement. |
NSS: Correlation between PAS, presence of arthropathy and annual bleeding rate with 25-OH-vitD (values were not given). |
|
2 |
S:COL-18N |
PWH A, severe (n=50). Joint status: FISH and HJHS scores and X-rays and US. |
SS: Pt with =2 target joints higher COL-18N levels than pt with one or without target joints. Positive correlations between COL-18N level andtotalHJHS, US score and ABR. |
| 3 |
Collagen synthesis (PRO-C3/ C4/C5/C23)anddegradation(C2M/C3M/C4M2) |
PWHA/B(n=24),onprophylaxis(91%), with inhibitors (46%). Joint status: HJHS and HEAD US scores of knees, ankles, elbows. Group I (n=5): <2 hemarthroses; groupII (n=6): 2-20 hemarthroses; group III (n=13): >20 hemarthroses. Total: 30 joint sets assessed in 2 separate visits. |
SS: Correlation HEAD US score and PRO-C23 in joints with and without hemarthroses (60 joints). In joints with more than 2 hemarthroses clinical HJHS (20 joints) correlated with C4M2. HEAD US score for group I: correlation with PRO-C3as compared to groups II/III. |
|
4 |
S: TIMP-1, VEGF |
Children with severe hemophilia (n=50) on prophylaxis and on-demand therapy. Joint status: HJHS and MRI ankles/knees (Denver score). |
SS: Correlation (Rho) TIMP-1 and HJHS (-0.291), TIMP-1 and total MRI score (0.588), VEGF and HJHS (0.416), VEGF and total MRI score (0.517). |
|
5 |
P:NETs |
PWH (n=23). Chronic hemophilic synovitis assessed by HJHS. |
SS: Strong correlation of synovial and plasma levels of NETs (r=0.7). DNA and DNA-elastase in synovial fluid positively correlated with HJHS (r>0.5). |
|
6 |
S: IL-6, us-CRP |
PWH A, severe (n=20), prophylactic treatment. G1: no joint damage (n=6), G2 hemarthroses (n=14). |
SS: Median IL-6 lower in G1 vs G2 (1.0 vs 69.7 ug/ml). NSS: us-CRP (0.96 vs 1.98 mg/L). |
| 7 |
P: PRO-C5, PRO-C3, C2M, C3M |
PWH A/B, mild, moderate or severe (n=25). Joint status: HJHS or Pettersson. |
C2M correlated positively with Pettersson score (rs=0.46) and HJHS (rs=0.40) at base- line. HJHS and Pettersson score increased during the study, but C2M levels remained unchanged. No significant changes in other markers over time. |
| 8 |
Osteocalcin, P1NP, CTX-I, osteoprotegerin, sRANKL, COMP, IL-1ß, IL-6, TNF |
PWHA (n=117) receiving emicizumab. Joint status: HJHSat baseline and week 49. |
No significant differences in the measured biomarkers between patients with and without target joints. Mean baseline values were within normal ranges or similar to published levels in healthy individuals. Potential association of COMP with HJHS score at baseline (pearson = 0.46) |
|
9 |
S: 25-OH-vitD, ferritin, CTX, PINP, COMP |
PWH A/B, severe, on prophylactic treatment (16-49 yr). Joint status: MRI (IPSG score). |
No correlation with the presence or degree of hemophilic arthropathy (values were not given). |
| Investigative | |||
| Prognostic | |||
| 7 |
P: PRO-C4, C4M, PRO-C8, PRO-C23 |
PWH A/B, mild, moderate or severe (n=25). Joint status: HJHS or Pettersson. |
There were no differences in biomarkers in patients with or without joint progression as determined by any change in HJHS or Pettersson. No evidence that baseline biomarker concentrations predicted changes in HJHS or Pettersson. |
| Efficacy of intervention | |||
| 4 |
S: TIMP-1, VEGF |
Children with severe hemophilia (n=50) on prophylaxis vs on-demand therapy. | SS: Pt on prophylaxis therapy lower median VEGF (450 vs 1300 pg/mL) and TIMP-1 (220 vs 350 ng/mL). |
| 8 |
Osteocalcin, P1NP, CTX-I, osteoprotegerin, sRANKL, COMP, IL-1ß, IL-6, TNF |
PWH A (n=117) receiving emicizumab. |
None of the measured biomarkers (baseline and after 3, 6, 12 and 18 months of treatment) changed significantly during emicizumab prophylaxis. No significant differences in the measured biomarkers between PWH A previously on FVIII prophylaxis or on-demand treatment were seen at baseline. |
| Diagnostic | |||
| 1 |
S: 25-OH-vitD, calcium, phosphorus, alkaline phosphatase, parathormone, osteocalcin U: pyrilinks-D |
Children with hemophilia (n=38) vs age- and sex- matched healthy controls (n=38). |
SS: Children with hemophilia lower median 25-OH-vitD (7.20 vs 15.54 ng/mL); lower mean phosphorus (4.31 vs 4.68 mg/dL); higher mean alkaline phosphatase (511.76 vs 327.97 IU/L). NSS: Mean calcium (8.95 vs 8.94 mg/dL); median parathormone (18.35 vs 20.35 pg/ mL); median osteocalcin 9.67 vs 3.49 ng/ mL); median pyrilinks-D (23.11 vs 22.32 nmol DPD/mmol creatinine). |
| 2 |
S:COL-18N |
PWH A, severe (n=50) vs control group (not specified). |
SS: Higher median COL-18N levels in PWH. No difference in levels in PWH with and without inhibitors or NSAID use. |
| 4 |
S: TIMP-1, VEGF |
Children with severe hemophilia (n=50) vs healthy age-matched boys (n=50). |
SS: Children with hemophilia higher mean TIMP-1 (240 vs 150 ng/mL) and VEGF (600 vs 275 pg/mL). |
| 5 |
P:NETs |
PWH (n=23) vs healthy donors. |
DNA or DNA-elastase were detected in plasma of chronic hemophilic synovitis patients (assessed by HJHS) and were not detected in healthy donors. |
| 7 |
P: PRO-C4, C4M, PRO-C8, PRO-C23, C2M, PRO-C5, C3M, PRO-C3 |
PWH A/B, mild, moderate or severe (n=25). Assessment of bleed episode vs non bleed pain by MSKUS/PD. |
SS: PRO-C4, C4M, PRO-C8 increased during acute hemarthrosis (and not during painful episodes without hemarthrosis). A C4M level higher than 28.5 ng/ml was associated with a high risk for hemarthrosis (OR 7.7; 95% CI 1.2-51.2). NSS: PRO-C23, C2M, PRO-C5, C3M, PRO-C3. |
| 10 |
S: COMP, C1,2C, CS846, IL- 1ß, IL-6, TNFR1, TNFR2, vit D, CRP, MMP2, MMP8 U: CTX-II |
PWH A/B (n=18), moderate/severe on prophylaxis without joint bleeding within last 30 days vs age- matched healthy male controls (n=24). |
SS: IL-6 higher in PWH (median 1.79 vs 0.00 pg/mL), CRP higher in PWH (median 1.161 vs 0.49 mg/L), MMP2 lower in PWH (median 25.3 vs 29.4 ng/mL). NSS: vit D, MMP8, IL-1ß, TNFR1/2, COMP, C1,2C, CS846, uCTX-II (uCTX-II was 72% higher in PWH, not statistically significant). |
| 11 |
S/P: Cytokines, angiogenesis markers, acute phase proteins (SS differences will be mentioned in this table) |
Group 1: PWH A/B, severe, moderate, mild (n=63). Arthropathy in n=23. Without arthropathy in n=40. Group 2: RA pt (n=23). Group 3: healthy male controls (n=43). Arthropathy defined as painful swelling, functional impairment, typical radiology images and/or orthopaedic interventions. |
All levels are expressed as means. SS: Group 1 (pt without arthropathy) vs group 3: ferritin (213 vs 40 ng/mL) and a2-macroglobulin (237 vs 177 mg/dl), IL-7 (44.1 vs 18.5 pg/ mL), leptin (3309 vs 2127 pg/mL), PECAM-1 (5391 vs 4546 pg/mL), IL-10 (79 vs 22.8 pg/ mL), IL-12 (42.1 vs 23.5 pg/mL), IP-10 (786 vs 457 pg/mL) were increased. VEGFR-1 (278 vs 518 pg/mL), VEGFR-2 (3447 vs 4084 pg/ mL), HGF (945 vs 1362 pg/mL), follistatin MIP-1b (74.2 vs 124 pg/mL) were decreased. SS: Group 1 (pt without arthropathy) vs group 2: IL-7 (44.1 vs 20.2 pg/mL) increased, VEGFR-1 (278 vs 518 pg/mL) decreased. SS: Group 1 (pt with arthropathy) vs group 3: CRP (0.60 vs 0.12 mg/dL), ferritin (136 vs 40 ng/mL), a2-macroglobulin (234 vs 177 mg/ dL), leukocytes (6.89 vs 5.66 /nl), IP-10 (1041 vs 457 pg/mL), PECAM-1 (5089 vs 4546 pg/ mL), IL-10 (28.3 vs 22.8 pg/mL), IL-12 (33.1 vs 23.5 pg/mL), VEGF (20.7 vs 16.5 pg/mL) increased. MIP-1b (85.6 vs 124 pg/mL), HGF (894 vs 1362 pg/mL), VEGFR-2 (3394 vs 4084 pg/mL), TIE-2 (8030 vs 11153 pg/mL), IL-7 (13.3 vs 18.5 pg/mL), FGF basic (64.7 vs 85.7 pg/mL) decreased. SS: Group 1 (pt with arthropathy) vs group 2: ferritin (136 vs 40 ng/ml) decreased. |
|
12 |
S: CX3CL1 |
PWH A, severe with end-stage HA(n=20). Arthropathy defined as grade 4 on X-ray: Kellgren- Lawrence (26-53 yr). Control group (n=20): pt with end-stage OA (grade 3 Kellgren-Lawrence). |
SS: CX3CL1 elevated in PWH (7.16 vs 5.85 ng/ml). |
| References: 1. Ashrita; 2. Abdelhafez; 3. Acharya; 4. Andrawes; 5. Caviglia; 6. Detarsio; 7. Gopal; 8. Kiialainen; 9. Plut; 10. Putz; 11. Toenges; 12. Wojdasiewicz Abbreviations: COMP: Cartilage Oligomeric Matrix Protein; COL-18N: Endothelial type XVIII collagen; CS846: Chondroitin sulfate 846; CTX-I; CX3CL1: C-X3-C Motif Chemokine Ligand 1; C1,2C: Cartilage cleavage product; C2M/C3M/C4M2: MMP-degraded collagen type II/III/IV; FISH: Functional Independence Score in Hemophilia; HEAD-US: Hemophilia Early Arthropathy Detection with Ultrasound; HJHS: Hemophilia Joint Health Score; IL-6/1ß: Interleukin 6/1ß; IPSG: International Prophylaxis Study Group; MMP2/8: Matrix Metallopeptidase-2/8; MSKUS/ PD: High-resolution musculoskeletal ultrasound with power Doppler; MRI: Magnetic Resonance Imaging; NETs: Neutrophil Extracellular Traps; NNS: Non statistically significant; NSAID: Non-Steroidal Anti-inflammatory Drug; PRO-C3/C4/C5/C23: Pro-peptide of type III/IV/V/ XXIII collagen; pt: patient; PWH: Patients with Hemophilia; P1NP: N-terminal propeptide of type I procollagen; SS: Statistically Significant; sRANKL: Soluble Receptor Activator of Nuclear Factor Kappa-B Ligand; TIMP-1: Tissue Inhibitor of Metalloproteinase 1; TNF(R1/R2): Tumor Necrosis Factor (Receptor 1/2); us CRP: Ultra-sensitive C-reactive protein; VEGF: Vascular Endothelial Growth Factor; vs: versus; 25-OH-vitD: 25-hydroxy-vitamin D |
|||
Figure 1: Pathophysiological changes in hemophilic arthropathy
Burden of disease
All nine studies in this category investigated the correlation of biomarkers with the degree of HA. As in our initial systematic review, the assessment of the degree of HA was very heterogeneous using questionnaires, physical examination scores and radiological scores. However, the recently published articles included here show a shift towards the use of the Hemophilia Joint Health Score (HJHS), ultrasound (US) and magnetic resonance imaging (MRI), while X-rays were only used twice. Different cartilage markers, e.g. endothelial specific isoform of type XVIII collagen (COL-18N), tissue inhibitor of metalloproteinase (TIMP-1), degraded type II collagen (C2M) and inflammatory markers, e.g. VEGF, interleukin-6 (IL-6), neutrophil extracellular traps (NETs) showed statistically significant correlations with the degree of HA, while bone markers did not show this correlation [7–15]. Kjeld et al. previously showed that the cartilage marker COL- 18N was significantly associated with the annual bleeding rate (ABR), a parameter directly associated with the degree of HA on X-rays [16]. An abstract by Abdelhafez also showed that this marker might be promising as it showed a statistically significant positive correlation with total HJHS, US score and ABR. Also, patients with two or more target joints had significantly higher levels of COL-18N than patients with one or without target joints [8].
Investigative
None of the included publications studied biochemical markers in animals or the change of biochemical markers upon a joint bleeding.
Prognostic
Biochemical markers predicting future outcomes such as the risk for joint damage progression in a particular patient are most useful for daily practice. However, publications with such study design are very limited. In addition to the previously investigated serum type II collagen degradation (Coll2-1), cartilage oligomeric matrix protein (COMP), chondroitin sulfate 846 (CS846) and urinary C-terminal telopeptide of type II collagen (CTX-II) [17,18], we now found a study investigating the prognostic value of other cartilage markers: pro-peptide of type IV, VIII, XXIII collagen and degraded collagen type IV (PRO-C4/C8/C23, C4M). No differences were found in biomarkers in patients with or without joint damage progression as determined by any change in HJHS or Pettersson score on X-ray. Also, there was no evidence that baseline biomarker concentrations predicted changes in HJHS or Pettersson score [13].
Efficacy of intervention
Two studies investigated the efficacy of treatment on biochemical markers. Children with severe hemophilia on prophylactic clotting factor replacement therapy showed lower median VEGF and TIMP-1 levels than children with on-demand therapy [10]. In a large cohort of patients with hemophilia A (n=117) receiving emicizumab, none of the measured cartilage (COMP), bone (osteocalcin, N-terminal propeptide of type I procollagen (P1NP), CTX-I, osteoprotegerin, receptor activator of nuclear factor kappa-B ligand (RANKL)) and inflammatory markers (IL-1β, IL-6, tumor necrosis factor (TNF)) changed significantly after 3, 6, 12 and 18 months of emicizumab prophylaxis. Also, no statistically significant differences were seen in patients previously on FVIII prophylaxis or on-demand treatment [14]. This is in line with our previous finding of a conference abstract reporting no statistical significant differences in cartilage markers Coll2- 1 and COMP between patients treated on-demand and on prophylactic basis [18].
Diagnostic
This category contains studies comparing markers between patients with hemophilia and control populations. This gives insight in the pathophysiologic processes involved in HA and can also elucidate differences with other diseases with joint involvement. For implementation in daily practice, this approach is less relevant as HA is a long-term outcome of a disease already diagnosed. Studies reporting about the capacity of biomarkers to diagnose a joint bleeding or to differentiate between a bleeding episode or a flare of HA, were also allocated to the Diagnostic-category. Our current search identified only one article using this approach and only investigated cartilage markers. PRO-C4, C4M and PRO-C8 showed a statistically significant increase during acute hemarthrosis assessed by US and not during a painful episode without a joint bleeding [13].
Studies comparing hemophilia patients and control patients showed both increased and decreased levels of cartilage, bone and synovial inflammation markers [7,8,10,11,19–21]. These discrepancies were also noticed in our initial systematic review.
Discussion
Biochemical markers reflecting dynamical changes in the joint might be valuable for early detection of joint damage and can help in monitoring joint deterioration. Moreover, markers reflecting joint tissue turnover can elucidate the pathophysiological processes in hemophilic joints which in the future hopefully results in targeted treatments to prevent progression. In clinical practice, biochemical markers might be used as a diagnostic tool to distinguish hemarthrosis from arthropathy flares. Gopal et al. showed that degradation and synthesis markers of collagen, found in the basement membranes, synovial blood vessels and synovial lining, were both elevated during a joint bleeding and not during a flare of HA. This suggests a transient nature of vascular remodeling processes directly associated with a joint bleeding [13].
It is doubtful whether these markers can really assist with diagnosing joint bleeds. By the time markers of acute hemarthrosis increase, decisions about the appropriate treatment should have been made already. Therefore, it is necessary to determine a precise time course of biomarker clearance and select markers with a rapid increase after a joint bleed in order to be clinically relevant. However, markers with a relatively slow increase can still be useful as they may assist in detecting non-symptomatic subclinical bleeds. These markers can also be used to accurately monitor joint status in clinical trials investigating new hemostatic agents. Since annual bleeding rates are no longer sensitive enough with the current effective treatments, sensitive joint measurements are necessary. Otherwise, a potential benefit of new therapies can only be monitored by a very long follow-up.
Biochemical marker research is challenging. It takes years before changes in the joint can be detected by imaging. In order to prevent irreversible joint damage and subsequent major orthopedic surgeries, it is of utmost importance to identify patients at high risk of developing arthropathy or patients who progress rapidly. Biochemical markers may have the capacity to reflect ongoing ‘real-time’ changes in the joint and detect deterioration a lot earlier than imaging to intervene timely. However, cartilage and bone turnover are, instead of synovial inflammation, relatively slow and longterm processes and might not be captured within the time frame of a typical study [13]. Longitudinal study designs with multiple biomarker measurements are essential to detect long-term complications. These multiple measurements can also diminish inter-individual differences. Combined indexes of biomarkers capturing several pathophysiological changes can also be considered to decrease heterogeneity [17].
Besides individual differences in biomarker levels between patients, comparison of research of biochemical markers is also hampered by heterogeneous joint status assessment tools used as reference. Different approaches are used to define joint health (e.g. physical examination scores, X-ray, US, MRI) and these methods all have their specific limitations and discrepancies. In order to compare biochemical marker performances, it is necessary to homogenize joint assessment and use appropriate scores. Moreover, the degree of joint damage in patients with a similar bleeding history varies and heterogeneity in clinical phenotype is observed. Some patients suffer from chronic synovitis, while other patients have evident cartilage and bone damage [22]. Hypothetically, these different phenotypes may require different treatment regimens. For example, patients with a synovial driven phenotype may benefit from anti-inflammatory drugs like celecoxib. Biochemical marker research can help in understanding the underlying pathophysiologic mechanisms and may predict which patients respond to an intervention. Developing such markers can be facilitated by correlating specific biochemical marker with subscores with a focus on synovial changes or osteochondral changes (e.g. subscores in the International Prophylaxis Study Group (IPSG) score).
Another point to consider is the influence of co-morbidity, like the presence of hepatitis C virus (HCV) and human immunodeficiency virus (HIV) or low bone mineral density. These comorbidities may affect biomarker levels. Also, different ages may partly explain differences in (bone) markers [23,24]. Finally, obesity and liver and kidney dysfunction may influence systemic biomarker metabolism. Local joint conditions are probably best reflected by local fluids and in this way may be helpful in discovering new potential biomarkers without the variability of systemic differences. However, practical objections and ethical issues complicate this, especially in patients with a bleeding disorder where synovial fluid punctures come with a risk. Although this approach might be helpful during discovery, translation into clinical practice will not occur as synovial fluid punctures are not desirable for joint damage screening during routine care.
Currently, blood and urinary biomarkers are not used in clinical practice. Many studies included in our systematic review and in this article focus on the differences of patients with hemophilia and (healthy) controls. However, we consider these comparisons as clinically non-relevant as hemophilia is an already diagnosed disease and joint damage is a longterm complication. Although this research can help in understanding normal ranges in the general population, a different approach is necessary before these markers can be implemented in daily practice and clinical trials. We recommend comparisons of biomarker levels in patients with hemophilia with and without arthropathy to find promising biomarkers. Results from these cross-sectional studies could be used in longitudinal follow-up studies, e.g. after a joint bleed or during (new) treatments. The focus should not only be on the performance of one individual biomarker, but combined indexes reflecting pathophysiological processes should be considered as well. Moreover, the homogeneity of study designs and sample sizes should be increased and assessments of the joint status be improved. Widespread introduction of biochemical markers in daily practice would require it to be easily collectable from patients during a quick and safe procedure. Finally, markers should be stable during transport and storage and analyzing these markers is preferably not labor intensive.
The development of clinically relevant biomarkers may require different approaches. In osteoarthritis, a degenerative joint disease with pathophysiological similarities, there has been an increased focus on regulatory mechanisms underlying the pathogenesis and as such on the role of microRNAs. MicroRNAs are non-coding RNAs regulating post-transcriptional gene expression, e.g. gene expression in chondrogenesis. These microRNAs are essential for the cellular function and up- or downregulation of microRNAs has been associated with osteoarthritis [25,26]. Although promising in osteoarthritis, only one study investigated the role of microRNAs in HA. They found that microRNAs regulating inflammatory mediators were significantly elevated in the acute hemarthrosis model. In the chronic hemarthrosis model, other pathologically relevant microRNAs (e.g. VEGF signaling) were discovered. This stage-specific microRNA expression may have potential in both monitoring and treating HA [27]. The ultimate goal of these different approaches is translation into daily practice. Ideally, biochemical markers are measured in blood and urine during regular clinical follow-up and these markers can help in detecting subclinical inflammation or ongoing joint damage not noticed by the patient. In this way, eventually together with easily accessible point-of-care ultrasound, we can closely monitor the joint status and adapt treatment regimens when small deteriorations occur, resulting in improved personalized medicine for hemophilia patients.
Conflicts of Interest
No conflicts of interest.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions Statement
E.D.P. van Bergen: Conceptualization, Writing – Original Draft, Visualization
S.C. Mastbergen: Writing – Review & Editing
F.P.J.G. Lafeber: Supervision
R.E.G. Schutgens: Writing – Review & Editing
L.F.D. van Vulpen: Conceptualization, Writing – Review & Editing
References
2. Gouw SC, Timmer MA, Srivastava A, de Kleijn P, Hilliard P, Peters M, et al. Measurement of joint health in persons with haemophilia: A systematic review of the measurement properties of haemophiliaspecific instruments. Haemophilia. 2019;25(1):e1–10.
3. Doria AS. State-of-the-art imaging techniques for the evaluation of haemophilic arthropathy: present and future. Haemophilia. 2010 Jul;16:107-14.
4. Seuser A, Khayat CD, Negrier C, Sabbour A, Heijnen L. Evaluation of early musculoskeletal disease in patients with haemophilia: Results from an expert consensus. Blood Coagul Fibrinolysis. 2018;29(6):509–20.
5. van Vulpen LFD, Thomas S, Keny SA, Mohanty SS. Synovitis and synovectomy in haemophilia. Haemophilia. 2021 Feb;27:96-102.
6. van Bergen EDP, van Vulpen LFD, Schutgens REG, Mastbergen SC, Lafeber FPJG. Biochemical marker research in hemophilic arthropathy: A systematic review. Blood Reviews. 2021 May 1;47:100781.
7. Ashritha A, Delhi Kumar CG, Sahoo J, Nalini P. Evaluation of Bone Mineral Density in Children With Hemophilia: An Observational Case- Control Study. Journal of pediatric Hematology. 2019 Oct 1;41(7):511-4.
8. Abdelhafez N, Abdel Gawad Tantawy A, Ahmad Safwat N, Anwar El Sayed El Seteha K. Endothelial specific isoform of type XVIII collagen (COL-18N): A marker of vascular integrity in hemophilic arthropathy. Research and Practice in Thrombosis and Haemostasis. 2020;4(0):423.
9. Acharya S, Choi-Rosen J, Stanco J, Adler R. Ultrasound and extracellular matrix (ECM) turnover markers for monitoring prophylaxis in hemophilia. Pediatric Blood & Cancer. 2021;68(0):S18–9.
10. Andrawes NG, Saker HM, Salah El-Din NY, Abd Elhakim Hussain M. Tissue-inhibitors of metalloproteinase-1 and vascularendothelial growth-factor in severe haemophilia A children on low dose prophylactic recombinant factor VIII: Relation to subclinical arthropathy. Haemophilia. 2020;26(4):607–14.
11. Caviglia H, Oneto P, Landro ME, Daffunchio C, Douglas Price AL, Schattner M, et al. Dna and neutrophil extracellular traps release, novel potential biomarkers and therapeutic targets of joint damage in hemophilia. Haemophilia. 2020;26(0):42.
12. Detarsio G, Pérez S, Carbonell M, Castro J, Williams M, Davoli M, et al. Interleukin-6 (IL-6) and ultra sensitive c reactive protein (USCRP) as markers of joint damage in hemophilia. Biocell. 2020;44(0):9.
13. Gopal S, Barnes RFW, Cooke EJ, Zhou JY, Levin I, Emery P, et al. Systemic vascular basement membrane markers linked to synovial vascular remodeling are biomarkers of hemarthrosis in patients with hemophilia. Journal of Thrombosis and Haemostasis. 2021;19(5):1200–11.
14. Kiialainen A, Niggli M, Kempton CL, Castaman G, Chang TY, Paz- Priel I, et al. Bone and joint health markers in persons with hemophilia A (PwHA) treated with emicizumab in HAVEN 3. Blood. 2019;134(0).
15. Plut D, Faganel Kotnik B, Preložnik Zupan I, Kljucevšek D, Vidmar G, Snoj Ž, et al. Detection and evaluation of haemophilic arthropathy: Which tools may be considered more reliable. Haemophilia. 2020;(April):1–8.
16. Kjeld NG, Hua B, Karsdal MA, Sun S, Manon-Jensen T. The endothelial specific isoform of type XVIII collagen correlates to annual bleeding rate in haemophilia patients. PLoS One. 2018 Jan 1;13(1).
17. Pulles AE, Mastbergen SC, Foppen W, Schutgens REG, Lafeber FPJG, van Vulpen LFD. The combination of urinary CTX-II and serum CS-846: Promising biochemical markers to predict radiographic progression of haemophilic arthropathy—An exploratory study. Haemophilia. 2018 Jul;24(4):e278-80.
18. Sun X, Zhuang J, Zhou X, Liu Z, Sun J. Relationship between serum cartilage turnover biomarkers and hemophilic arthropathy severity in adult patients with severe hemophilia A in China. Research and Practice in Thrombosis and Haemostasis. 2019;3(0):273–4.
19. Putz P, Durstberger S, Kaufmann C, Klinger M, Plessl K, Rejtö J, et al. 3D gait analysis, haemophilia joint health score, leg muscle laterality and biomarkers of joint damage: A cross-sectional comparative assessment of haemophilic arthropathy. Haemophilia. 2020;26(6):e323–33.
20. Toenges R, Wittenbrink A, Miesbach W. Biomarkers and immunological parameters in haemophilia and rheumatoid arthritis patients: a comparative multiplexing laboratory study. Haemophilia. 2021;27(1):e119–26.
21. Wojdasiewicz P, Poniatowski LA, Kotela A, Skoda M, Pyzlak M, Stangret A, et al. Comparative Analysis of the Occurrence and Role of CX3CL1 (Fractalkine) and Its Receptor CX3CR1 in Hemophilic Arthropathy and Osteoarthritis. J Immunol Res. 2020;2020.
22. van Vulpen LFD, Mastbergen SC, Lafeber FPJG, Schutgens REG.Differential effects of bleeds on the development of arthropathy – basic and applied issues. Haemophilia. 2017;23(4):521–7.
23. Reingold JS, Wanke C, Kotler DP, Lewis CE, Tracy R, Heymsfield S, et al. Association of HIV Infection and HIV/HCV Coinfection With C-Reactive Protein Levels The Fat Redistribution and Metabolic Change in HIV Infection (FRAM) Study Design: Cross-sectional study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) cohort and controls from the Cor-onary Artery Risk Development in Young Adults (CARDIA) study. Journal of acquired immune deficiency syndromes (1999). 2008 Jun 1;48(2):142.
24. Iorio A, Fabbriciani G, Marcucci M, Brozzetti M, Filipponi P. Bone mineral density in haemophilia patients: A meta-analysis. Thromb Haemost. 2010 Mar;103(3):596–603.
25. Oliviero A, Della Porta G, Peretti GM, Maffulli N. MicroRNA in osteoarthritis: Physiopathology, diagnosis and therapeutic challenge. British Medical Bulletin. 2019;130(1):137–47.
26. Wu C, Tian B, Qu X, Liu F, Tang T, Qin A, et al. MicroRNAs play a role in chondrogenesis and osteoarthritis (review). International Journal of Molecular Medicine. 2014;34(1):13–23.
27. Sujanthi E, Sarangi P, Amit S, Ananthan AS, Jamora C, Vemula P, et al. Small RNAs influence the pathophysiology of hemophilic synovitis and arthropathy. Research and Practice in Thrombosis and Haemostasis. 2020;4(0):395.