Abstract
Background: We recently published our multi-institutional experience performing primary robot-assisted retroperitoneal lymph node dissection (RA-RPLND) for men with non-seminomatous germ cell tumor (NSGCT). We concluded that primary RA-RPLND for NSGCT can be performed safely with low complication rates, acceptable early oncologic outcomes, and lower overall theoretical chemotherapy burden. In this commentary, we explore outcomes in clinical stage I patients stratified by clinical risk factors (RF) and estimate reductions in chemotherapy burden.
Methods: In our original study, we included clinical stage I and highly select clinical stage II patients. Clinical risk factors were defined as lymphovascular invasion (LVI) and/or predominance of embryonal carcinoma (EC) (>40%) in the orchiectomy specimen.
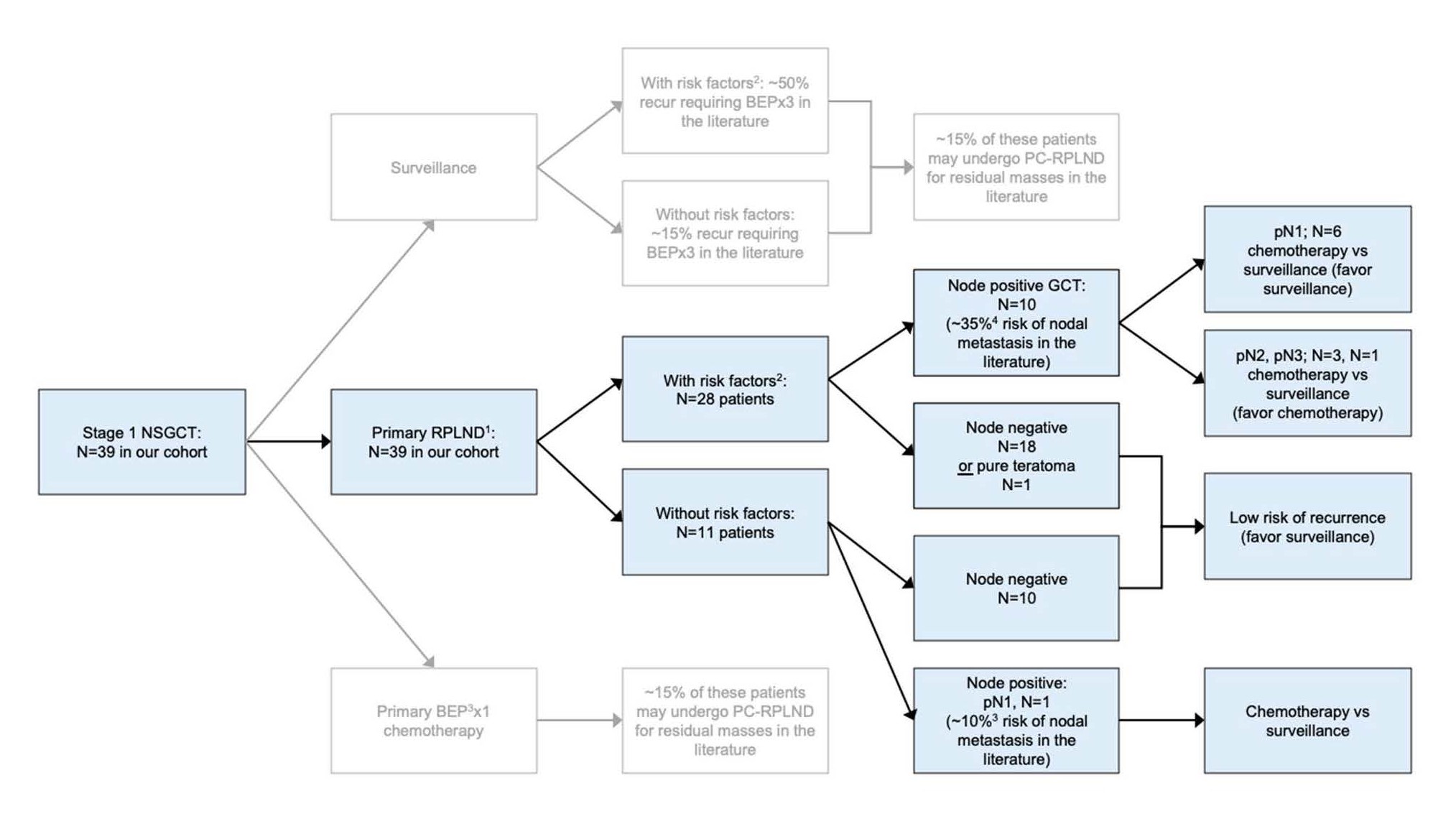
Results: 72% (28/39) of stage I patients that underwent RA-RPLND could be classified as belonging to the RF+ group (Figure 1). Among the RF+ group, 36% (10/28) had both LVI and EC (LVI+EC+). Of the LVI+EC+ patients, 70% had positive nodes (N+), whereas the rate was much lower in the LVI only (LVI+EC-) and EC only (LVI-EC+) groups (17% for both). Primary RA-RPLND allowed for accurate pathologic staging and avoidance of chemotherapy in the 90% and 64% of pN0 patients in the RF- and RF+ groups, respectively. Overall node positive rates were 36% and 9% for men with and without clinical risk factors, respectively. The majority of these node positive patients had pN1 disease and were thus candidates for post RPLND surveillance, thus reducing therapeutic burden and exposure to long-term toxicity.
Conclusion: Primary RA-RPLND can be safely performed with low complication rates and acceptable short term oncologic outcomes. Assessing clinical risk factors when deciding on treatment may further improve outcomes by helping to identify clinical stage I patients who are more likely to be stage II and thus benefit most from adjuvant treatment with RPLND.
Keywords
Neoplasms, Germ cell and embryonal neoplasms, Robotic surgical procedures, Lymph node excision, Testicular neoplasms
Introduction
Retroperitoneal lymph nodes are often the first landing site of metastatic disease in men with testicular cancer. Primary retroperitoneal lymph node dissection (RPLND) for clinical stage I NSGCT can accurately surgically stage patients who may have nodal micrometastases, and in some cases, can serve as the primary therapy when volume of metastasis is low. The risk of relapse from chemoresistant tumors and teratoma is lowered with surgical control of the retroperitoneum. Moreover, relapse after negative primary RPLND is uncommon (<1%) [1] and frequently is curable with chemotherapy, when it does occur. In addition, follow-up regimens to monitor for recurrence can often be simplified.
RPLND, historically performed with an open surgical approach, is increasingly being performed using a minimally invasive robotic approach [2,3] with significantly shorter length of stays (~1-2 days) than open surgery. In a recent paper [4], we presented complication rates and oncologic outcomes from a multi-institutional series of men undergoing primary robot-assisted retroperitoneal lymph node dissection (RA-RPLND) between 2014 and 2019 by eight urologists experienced in testis cancer and robotic surgery. We concluded that robot-assisted RPLND can be safely performed with similar oncologic outcomes as an open approach, while providing an option that may also reduce the need for chemotherapy.
In addition to RPLND, men with stage I NSGCTs are also candidates for active surveillance [5] or chemotherapy [6]. Selection of management strategy depends on several factors including patient preference and the relative importance of treatment toxicities, ability to adhere to surveillance, physician comfort with RPLND, risk factors and probability of relapse. Adhering to surveillance can often be challenging for many patients with large national studies [7] showing 30% of all surveillance patients received no surveillance imaging (abdominal/chest) or tumor marker tests within the first year of diagnosis.
Chemotherapy, specifically the standard regimen of bleomycin, etoposide, cisplatin (BEP), is another guideline management strategy for stage I NSGCT. Risk of relapse is reduced by approximately 90% [8] with single-cycle treatment in stage I patients. However, chemotherapy, even at lower dosages, can have pulmonary, neural, renal, and metabolic toxicities [9,10]. Moreover, in the case of teratoma, chemotherapy does not reduce the risk of recurrence and requires close surveillance imaging. Given the high likelihood of long-term cure for men with stage I NSGCT regardless of treatment option, there has been an increasing focus in experienced centers to minimize chemotherapy through upfront RPLND. In this commentary, we further investigate outcomes of patients with stage I NSGCT undergoing RA-RPLND stratified by clinical risk factor status, with a focus on the potential reduction in chemotherapy burden through primary surgical staging.
Figure 1. 1Primary retroperitoneal lymph node dissection (RPLND) performed robotically for all patients. 2Lymphovascular invasion and embryonal component predominance. 3Nicolai N, Miceli R, Artusi R, Piva L, Pizzocaro G, Salvioni R. “A simple model for predicting nodal metastasis in patients with clinical stage I nonseminomatous germ cell testicular tumors undergoing retroperitoneal lymph node dissection only." The Journal of Urology. 2004;171:172-176.
Methods
Charts of men undergoing primary robot-assisted RPLND between 2014 and 2019 by eight urologists experienced in testis cancer and robotic surgery at five separate institutions were retrospectively reviewed [4]. We further categorize stage I patients as high risk based on National Comprehensive Cancer Network (NCCN) guidelines which include the presence of lymphovascular invasion (LVI), discontinuous spermatic cord invasion, or invasion of the scrotum [6]. We also include percent embryonal in the primary tumor as another commonly used criteria to predict recurrence risk [1,11,12]. We compare the RARPLND lymph node counts and node positive (N+) rates, as well as chemotherapy burden between patients with risk factors (RF+) and without risk factors (RF-). Within the RF+ group, the presence of lymphovascular invasion (LVI+EC-), predominance of an embryonal carcinoma component (LVI-EC+), or presence of both is presented (LVI+EC+) [13].
Results
This exploratory analysis of our original cohort of 49 patients undergoing RA-RPLND for NSGCT showed that the majority of patients (39/49) had stage I NSGCT and that clinical risk factors (RF+) were predominant in 28 of 39 of these patients (Table 1). Within the RF+ group, 36% (10/28) had both LVI and EC (LVI+EC+). Of the LVI+EC+ patients, 70% had positive nodes (N+), whereas the rate was much lower in the LVI only (LVI+EC-) and EC only (LVI-EC+) groups (17% for both).
| Types of Bond | Average node count |
Number of patients with positive nodes |
Number of patients that received adjuvant chemo |
Number of patients that needed salvage** |
|
|---|---|---|---|---|---|
| Without risk factors | 11 | 35 | 1 | 0 | 0 |
| With risk factors* | 28 | 30 | 12 | 6 | 3 |
| (LVI+EC-) 6 | 6 | 32 | 1 | 0 | 0 |
| (LVI-EC+) | 12 | 25 | 2 | 2 | 0 |
| (LVI+EC+) | 10 | 32 | 7 | 4 | 3 |
Table 1: *LVI: Lymphovascular Invasion, EC: Embryonal Component predominance
**salvage patients did not receive adjuvant treatment
Primary RA-RPLND allowed for accurate pathologic staging with overall N+ rates of 9% and 36% for the RFand RF+, respectively. In our cohort, the decision was independently made at the discretion of the oncologist and urologic oncologist to administer adjuvant chemotherapy. The 1 RF- patient with positive nodes was pN1; he did not receive adjuvant chemotherapy and has had no evidence of recurrence close to 4 years after RPLND. Within the RF+ group, 6 patients were pN1, 3 patients were pN2 and 1 patient was pN3. Adjuvant chemotherapy was administered to 3, 1 and 1 men within each group, respectively. 3 patients recurred, of whom 2 were RF+ pN1 and 1 was RF+ pN2, requiring salvage chemotherapy.
Upfront RPLND allowed many patients to avoid chemotherapy. Specifically, within the RF- and RF+ groups, 10/11 (91%) and 18/28 (64%), respectively, had negative nodes or pure teratoma on RA-RPLND specimen. A theoretical analysis for reduced chemotherapy burden for stage I patients within our cohort shows that primary RA-RPLND reduced the chemotherapy burden by up to 83%. This was estimated by assuming the 12 patients with clinical stage I who were pathologic stage II would have likely received at least 3 cycles of BEP or 4 cycles of EP according to NCCN guidelines, if they were observed and eventually recurred. Instead, surgical staging revealed 3 patients with pN2-pN3 disease who were given 6 total cycles of adjuvant chemotherapy. The remainder of patients, including the one pN+ patient with pure teratoma, were not given chemotherapy and are being observed after RARPLND. Thus, 30 cycles, or 83% of potential chemotherapy burden was reduced through primary RA-RPLND.
Discussion
Primary RA-RPLND allowed for accurate pathologic staging with overall N+ rates of 9% and 36% for the RFand RF+, respectively. The majority of stage I NSGCT had clinical risk factors (72%). Within the RF+ group, 70% of patients with dual risk factors (LVI+EC+) were N+, whereas the rates were lower (17%) in those with a single risk factor alone. The LVI+EC+ were more likely to have node positive disease and were thus more likely receive adjuvant or salvage chemotherapy. Half of pN1- pN3 patients received adjuvant chemotherapy, with lower cumulative regimens than they would have received if they recurred without primary upfront surgery.
RA-RPLND pathology revealed pN0 in 90% and 64% of patients with RF- and RF+, respectively. These patients, in addition to the one patient with teratoma, were able to avoid chemotherapy altogether. Among the N+ patients, the majority (64%) had pN1 and were thus candidates for post RPLND surveillance [6], reducing the therapeutic burden and exposure to long-term toxicity.
In our primary paper, primary RA-RPLND was shown to be safe with low rates of complications. Furthermore, oncologic outcomes were comparable to historical controls with 31% of clinical stage I patients being pathologic stage II after primary RPLND. There was a higher predominance of positive lymph nodes for patients with pre-operative clinical risk factors. This further highlights the critical need to consider the entire clinical picture and risk for recurrence when deciding on treatment strategy. Although upfront surgical staging may be more beneficial for men with stage I NSGCT who have risk factors, men without risk factors may also benefit if compliance to surveillance is challenging.
Limitations of this analysis include it’s exploratory nature and small sample size. Results should be confirmed in further studies. However, we maintain high confidence in the results of our primary findings that RA-RPLND can be safely performed with comparable complication rates given our data is multi-institutional and similar to many other RA-RPLND cohorts.
Conclusion
Primary RA-RPLND has mounting evidence that it can be a safe diagnostic procedure with therapeutic benefits in patients with pure teratoma or low volume stage II disease. This, combined with consideration of patient’s RF+ status, can simplify followup schedules and allow for a more informed decision making discussion pre-operatively.
Disclosures
Dr. William Huang serves as a consultant for Intuitive Surgical, Inc.
References
2. Pearce SM, Golan S, Gorin MA, Luckenbaugh AN, Williams SB, Ward JF, et al. Safety and Early Oncologic Effectiveness of Primary Robotic Retroperitoneal Lymph Node Dissection for Nonseminomatous Germ Cell Testicular Cancer. European Urology. 2017;71(3):476-82.
3. Rocco NR, Stroup SP, Abdul-Muhsin HM, Marshall MT, Santomauro MG, Christman MS, et al. Primary robotic RLPND for nonseminomatous germ cell testicular cancer: a two-center analysis of intermediate oncologic and safety outcomes. World Journal of Urology. 2019.
4. Taylor J, Becher E, Wysock JS, Lenis AT, Litwin MS, Jipp J, et al. Primary Robot-assisted Retroperitoneal Lymph Node Dissection for Men with Nonseminomatous Germ Cell Tumor: Experience from a Multi-institutional Cohort. European Urology Focus. 2020.
5. Kollmannsberger C, Moore C, Chi KN, Murray N, Daneshmand S, Gleave M, et al. Non-risk-adapted surveillance for patients with stage I nonseminomatous testicular germ-cell tumors: diminishing treatmentrelated morbidity while maintaining efficacy. Annals of Oncology. 2010;21(6):1296-301.
6. Gilligan T, Lin DW, Aggarwal R, Chism D, Cost N, Derweesh IH, et al. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2019;17(12):1529-54.
7. Yu HY, Madison RA, Setodji CM, Saigal CS. Quality of surveillance for stage I testis cancer in the community. Journal of Clinical Oncology. 2009;27(26):4327-32.
8. Tandstad T, Dahl O, Cohn-Cedermark G, Cavallin-Stahl E, Stierner U, Solberg A, et al. Risk-adapted treatment in clinical stage I nonseminomatous germ cell testicular cancer: the SWENOTECA management program. Journal of Clinical Oncology. 2009;27(13):2122-8.
9. Groot HJ, van Leeuwen FE, Lubberts S, Horenblas S, de Wit R, Witjes JA, et al. Platinum exposure and causespecific mortality among patients with testicular cancer. Cancer. 2020;126(3):628-39.
10. Abouassaly R, Fossa SD, Giwercman A, Kollmannsberger C, Motzer RJ, Schmoll HJ, et al. Sequelae of treatment in long-term survivors of testis cancer. European Urology. 2011;60(3):516-26.
11. Lago-Hernandez CA, Feldman H, O’Donnell E, Mahal BA, Perez V, Howard S, et al. A refined risk stratification scheme for clinical stage 1 NSGCT based on evaluation of both embryonal predominance and lymphovascular invasion. Annals of Oncology. 2015;26(7):1396-401.
12. Divrik RT, Akdogan B, Ozen H, Zorlu F. Outcomes of surveillance protocol of clinical stage I nonseminomatous germ cell tumors-is shift to risk adapted policy justified? The Journal of Urology. 2006;176(4 Pt 1):1424-29; discussion 9-30.
13. Nicolai N, Miceli R, Artusi R, Piva L, Pizzocaro G, Salvioni R. A simple model for predicting nodal metastasis in patients with clinical stage I nonseminomatous germ cell testicular tumors undergoing retroperitoneal lymph node dissection only. The Journal of Urology. 2004;171(1):172- 6.