Abstract
The clinical spectrum of COVID-19 ranges from asymptomatic disease to viral pneumonia leading to acute respiratory distress syndrome (ARDS), multiorgan failure, and death. In addition to these risk factors, COVID-19 patients with cancer were shown to have poor outcomes in multiple studies. Hyperinflammatory response (cytokine storm) is one of the main features of severe disease and is also associated with poor outcomes including intensive care unit (ICU) and mortality in patients with COVID-19. Several potential factors might be associated to poor prognosis in patients with COVID-19 accompanying cancer. Higher development of cytokine storm, receiving chemotherapeutics and/or immunosuppressants, higher susceptibility to infections as well as other potential metabolic complications could be counted among these factors. Inflammatory cytokines are essential for the development and progression in cancer. Patients with malignancy and/
or receiving immunosuppressants are at high risk for complications, development of cytokine storm, and poor outcomes associated with COVID-19. Furthermore, recurrent/prolonged cytokine storm is an emerging potential life-threatening condition in patients with cancer or immunosuppressants beyond prolonged PCR positivity.
Keywords
COVID-19, SARS-Cov-2, Cytokine storm, Hyperinflammation, Cancer, IL-6
Introduction
At the end of 2019, the world faced a new disease, COVID-19 caused by SARS-Cov-2 and became a pandemic within few weeks. The fact that COVID-19 spreads very quickly and has significant morbidity and mortality had devastating effects all over the world. The clinical spectrum of COVID-19 ranges from asymptomatic disease to viral pneumonia leading to acute respiratory distress syndrome (ARDS), multiorgan failure, and death. Several clinical characteristics and comorbidities such as older age, male gender, and presence of diabetes mellitus were found to be associated with higher mortality in patients with COVID-19 so far [1]. In addition to these risk factors, COVID-19 patients with cancer were shown to have poor outcomes in multiple studies [2,3].
Hyperinflammatory response (cytokine storm) is one of the main features of severe disease, and also associated with poor outcomes including intensive care unit (ICU) requirement and mortality in patients with COVID-19 [4]. The cytokine storm is a condition of uncontrolled systemic hyperinflammation caused by the release of a large amount of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-18, interferon (IFN)-γ and tumor necrosis factor-alpha (TNF-α), leading to multi-organ failure and even death [5-7].
In this paper, we aimed to evaluate the course of COVID-19 in patients with cancer, the pathogenesis and treatment of COVID-19-associated cytokine storm in patients with cancer.
Immune Mechanism of Severe COVID-19 and COVID-19-Associated Cytokine Storm
In COVID-19, SARS-Cov-2 binds to angiotensin-converting enzyme type 2 (ACE2) receptor which is particularly expressed along with the virus S protein priming protease TMPRSS2 [8], leads to the release of viral ssRNA and binding to pattern recognition receptors (PRRs). Among the PRRs, three major receptors are involved in viral infections; Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [9]. Binding of SARS-Cov-2 viral ssRNA to TLR 7-8 stimulates nuclear factor-kappa B (NF-κB) with activation of the JAK-STAT pathway and production of several proinflammatory cytokines such as type 1 IFN (alpha and beta), IL-6, TNF-α and others [10]. IFN pathway is essential to eliminate viral RNA thereby termination of viral infection. Insufficient or delayed type 1 IFN response, especially in some circumstances such as immunosuppression, presence of cancer, or some genetic alterations (such as TLR 7 polymorphisms) causes defective clearance of viral RNA and leads to cytokine storm in patients with COVID-19. Low levels of type 1 IFN but higher levels of TNF-α and IL-6 produced by dendritic cells (DCs) infected with SARS-CoV have been revealed in one study and support this hypothesis [11]. Delayed type 1 IFN signaling was shown in mice with lethal SARS-CoV infection with the increase in viral titers and excessive inflammatory response [12]. This mechanism may explain higher frequency of cytokine storm s as well as poor outcomes in such patient groups.
Inflammasome and COVID-19-Associated Cytokine Storm
There is also another important pathway in the pathogenesis of COVID-19-associated cytokine storm. Recent evidences showed that SARS-CoV-2 also activates an intracellular multiprotein complex which is called ‘inflammasome’ after binding TLRs. Inflammasomes present in innate immune cells such as neutrophils, macrophages, and DCs and have an essential role in the host defense against microorganisms including viruses. The inflammasome is coordinated by the NLRP3 sensor (Nucleotide-binding oligomerization domain [NOD], Leucin e-rich Repeat, Pyrin domain, adaptor protein ASC, as well as the effector protein caspase 1) and also drives cleavage of pro-IL-1β by caspase-1, followed by the production of active IL-1β [13]. There are several rheumatologic conditions associated with inflammasome activation and higher IL-1β production such as Familial Mediterranean Fever (FMF), gout, adult-onset still disease (AOSD) [14] as well as hyperinflammatory circumstances such as macrophage activation syndrome (MAS), hemophagocytic lymphohistiocytosis (HLH) [15].
Viral genomic material and virus-induced Type I IFNs activates inflammasome and pyroptosis in many RNA virus infections [16]. Higher levels of IL-1α and IL-1β were shown in patients with COVID-19 compared to healthy controls [17]. Additionally, activation of NLRP3 inflammasome was observed in patients with severe COVID-19 with higher levels of inflammasome products such as caspase 1, IL-1β, and ILRIG 18 [18,19]. Considering the resemblance of inflammatory phenotype in HLH and MAS with COVID-19-associated cytokine storm, inflammasome and relevant cytokines are thought to have an important role in severe COVID-19. Immunopathogenesis and development of cytokine storm in COVID-19 was summarized in the Figure.
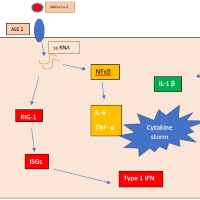
Figure: TLR: Toll-like Receptor, IFN: Interferon, IL-1β: Interleukin 1 Beta, IL-6: Interleukin 6, TNF-α: Tumor Necrosis Factor Alpha, ACE-2:Angiotensin Converting Enzyme 2, RIG-1: Retinoic acid-Inducible Gene I, ISG: Interferon-Stimulated Genes, NF?B: Nuclear Factor kappa B.
Cancer and COVID-19
Several potential factors might be associated to poor prognosis in patients with COVID-19 accompanying cancer. Higher development of cytokine storm, receiving chemotherapeutics and/or immunosuppressants, higher susceptibility to infections as well as other potential metabolic complications could be counted among these factors. In Mengyuan Dai et al. study in 2020, higher COVID-19 disease severity, higher secondary infection rate, and higher mechanical ventilation, as well as mortality rate, were observed in patients with COVID-19 accompanying cancer compared to the others [20]. In this study, higher mortality was also established among cancer patients, especially in patients with hematological cancer and receiving chemotherapeutics. Additionally, a higher mortality rate in patients who received immunotherapy compared to chemotherapeutics was a remarkable finding in the former study emphasize the crucial role of the underlying immune pathogenesis in COVID-19-associated cytokine storm. Beside the other risk factors such as genetic and demographic features, the level of immunosuppression may have important role in the development of COVID-19-associated cytokine storm in patients with cancer. Higher frequency of COVID- 19-associated cytokine storm in patients with hematological cancer compared to other malignancies as well as in patients with receiving chemotherapies compared to those had not, support this hypothesis. In another observational study from China mortality was two times higher in patients with cancer [21]. In this study, receiving anti-tumor treatment within 14 days had an increased risk of developing severe events (Hazard ratio [HR] = 4.079, 95% Confidence interval [CI]: 1.086– 15.322, p=0.037) [21]. Poor outcome in patients with COVID- 19-accompanying cancer compared to those had not was (39 % vs 8 %; p=0.0003, respectively) also established in Liang et al. study in 2020 [22]. Furthermore, anti-tumor treatment was also associated with severe disease course in this study (odds ratio [OR] 5·34, 95 % CI 1·80–16·18; p=0·0026).
Inflammatory cytokines are essential for the development and progression in cancer. High cytokine levels produced by tumor or stromal cells (IL-1β, IL-6, TNF, IL-8, IL-17 ) are associated with advanced stages of breast, prostate, and colon cancer [23,24]. Among these cytokines, IL-6 is important with its role in immune regulation and autoimmunity as well as cancer development. Blocking IL-6 is effective in several immunemediated diseases such as rheumatoid arthritis, AOSD, and large vessel vasculitis [25-27]. The mechanisms of IL-6 in tumorigenesis are promoting enhanced survival of cancer cells, increased angiogenesis, and inhibition of CD-8 T and NK cell functions [28-30]. The close association between IL-6 and cancer development was established in several cancers such as lymphomas, multiple myeloma, and solid tumors [31-33]. Moreover, promising results exist in several cancers such as myeloma and Castleman’s disease with IL-6 inhibitors such as tocilizumab [34,35]. Furthermore, the efficacy of IL-6 blocking was established in cytokine release syndrome (CRS) secondary to chimeric antigen receptor T cell (CAR-T cell) treatment in patients with hematological cancer [36]. These results emphasize the crucial role of IL-6 for the development of cancer, especially in hematological malignancies such as myeloma, lymphoma, and Castleman’s disease. IL-6 is also the main stimulator of STAT3, which has an important role in inflammation and oncogenesis together with NF-κB, which can activate the IL-6 amplifier [37]. This positive feedback loop is related to an increase in various proinflammatory cytokines and could be one of the main targets in the treatment of COVID-19 induced hyperinflammatory state. Higher cytokine levels are also shown during chemotherapy and radiotherapy in patients with cancer [38]. Additionally, it was shown that higher IL-6 and TNF-α levels secondary to cytostatic or targeted treatment were associated with higher mortality among hospitalized non-COVID-19 cancer patients [39]. The immune response triggered by antigens resulting from lysis of tumor tissue after cytotoxic therapy may explain the hyperinflammation as well as poor prognosis of COVID-19 in these patients. On the other hand, further studies are needed to clarify this issue.
Immunosuppression and COVID-19
At the beginning of the pandemic , the prognosis and course of the COVID-19 in patients receiving immunosuppressants was one of the major concerns among immunocompromised patients. After this uncertainty, there has been growing evidence of poor outcomes including higher hospitalization, the need for ICU admission, and mortality in patients receiving immunosuppressants such as corticosteroids, azathioprine, and mycophenolate mofetil as well as several biologic therapies [40,41] . In Akama-Garren et al. study in 2021, prior immunosuppressive therapy (30 % vs 17 %; p=0.036) was associated with higher mortality in patients with COVID-19 [42]. Among biological drugs, rituximab has the highest risk for poor outcomes in COVID-19 both in patients with immunemediated diseases and hematological cancer [43]. Impaired antibody response and defective clearance of SARS-Cov-2 with immunosuppressants, especially rituximab are the main possible mechanisms of poor outcomes in these patients. In a large study of 1090 patients with inflammatory rheumatic diseases who were evaluated for COVID-19, those who received rituximab therapy developed more severe infections and needed longer hospitalization [43]. Chronic immune stimulation by prolonged SARS-Cov-2 RNA leads to higher production of proinflammatory cytokines and therefore development of severe disease course as well as cytokine storm in immunocompromised patients. Several case reports revealing poor outcome in patients receiving rituximab supported this hypothesis [44,45]. Although prolonged PCR positivity is well known among immunocompromised patients [46] there are few cases of symptomatic COVID-19 recurrence [47-51]. On the other hand, relapsing or prolonged cytokine storm in these patients is unclear. Recently we published a case of chronic lymphocytic leukemia patient receiving rituximab together chemotherapies who developed recurrent/prolonged cytokine storm after a dramatic response to steroid and tocilizumab [52]. While the patient had a good response to tocilizumab initially, after recurrence of cytokine storm high dose intravenous anakinra was not effective. These findings emphasize that recurrent/prolonged cytokine storm in patients with COVID-19 may occur especially in patients with cancer or on immunosuppression. Ye et al. study in 2020 also revealed a possible viral reactivation in 5/55 (9.1%) discharged patients previously diagnosed with COVID-19 [53]. Four of these five patients were symptomatic and also COVID-19 recurrence was associated with immunosuppression in this study. Moreover, recurrent COVID-19-associated cytokine storms in immunosuppressive patients was also described in other case reports [54,55]. In light of these results, close followup of immunocompromised patients, especially receiving rituximab and/or chemotherapeutics should be considered in patients with COVID-19.
Treatment of Cytokine Storm
Glucocorticoids
Glucocorticoids (GC) are nonselective immunosuppressive agents and effective against cytokine storms by inhibiting multiple inflammatory pathways including the expression of several proinflammatory cytokines and leukocyte migration [56]. These drugs are used in the first-line for the treatment of various hyperinflammatory conditions in resemblance to COVID-19-associated cytokine storm; such as HLH, MAS, and CRS associated with CAR-T cell therapy [57,58]. Initial data on the use of GCs from studies performed in China was conflicting and led to recommendations against the routine use of corticosteroids in World Health Organization guidelines due to results pointing to prolonged viral shedding and increased mortality in severe patients [59-61]. After the UK-based Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial reported its findings in favor of dexamethasone therapy among patients who required mechanical ventilation, further evidence supporting these conclusions has emerged. A metaanalysis of 7 randomized controlled trials (RCT) suggested that GCs decrease 28-day all-cause mortality in severe COVID-19 patients. These data had led the GCs to become the standard of care in patients who require hospitalization and/or need oxygen therapy.
However, GC therapy should always be used with caution due to possible side effects including secondary infection risk. Uncontrolled and prolonged GC use in patients with rheumatic and inflammatory diseases has shown to be associated with poor outcomes in some studies [62], most probably due to delayed immune response and prolonged viral shedding. Finally, there are no positive data on GC use in mild COVID-19 disease.
Anti-IL-6 therapy
IL-6 is a pleiotropic cytokine that is essential for inflammation and hematopoiesis and plays a central role in the immune response by the stimulation of acute-phase response; however, as mentioned above, it is one on the major contributors of chronic inflammation and cytokine storm. Tocilizumab, a humanized monoclonal antibody against IL-6 receptor (IL- 6R), has been shown to be effective in several conditions with excessive production of IL-6 such as systemic juvenile idiopathic arthritis and CRS. Several open-label or randomized controlled studies were initiated with tocilizumab and other biologic agents blocking IL-6 activity. One of the largest trials on tocilizumab, the RECOVERY trial (which included 4116 patients receiving respiratory support or invasive mechanical ventilation (IMV)) found that patients treated with tocilizumab (400 to 800 mg) have higher rates of discharge within 28-day (54% vs. 47%) and less likely to need IMV or die (38% vs. 33%) [63]. Although conflicting results were reported possibly due to inadequate methodology in several studies [64], the efficacy of tocilizumab on clinical improvement (31 more with improvement per 1000 patients) and death (32 fewer deaths per 1000 patients on 28-day) in severe COVID-19 were shown in a Cochrane meta-analysis which included 10 RCTs and a total of 7308 patients [65].
Tocilizumab treatment was also reported to be efficient for patients with malignancy and severe COVID-19 in several cases. In the Table, we summarized the available reports of patients with malignancy and severe COVID-19 treated with tocilizumab and/or anakinra [52,66-72]. These findings were not surprising considering the role of IL-6 both in oncogenesis and COVID-19-associated cytokine storm and could be interpreted as the dominance of IL-6 in the inflammatory milieu of these patients. On the other hand, there are few data to evaluate the efficacy and safety of tocilizumab as well as other biological treatments since the patients with cancer were excluded from RCTs. Thus, only small observational studies and case reports exist in patients with cancer receiving biological treatments. This issue might be a research topic in future studies.
| Author | Age/Sex | Malignancy | Treatment for malignancy | Last treatment | COVID-19 associated cytokine storm treatment | |||
|---|---|---|---|---|---|---|---|---|
| Glucocorticoids | First line | Second line (in case of resistance) | Last reported status | |||||
| Bouchlarhem et al. [66] | 41/M | CML blast phase | HyperCVAD Nilotinib |
2 months ago | DXM 6 mg | Tocilizumab | discharge | |
| Lanceta et al. [67] | 65/M | Metastatic merkel cell carcinoma | None | none | Applied- dose not specified | Tocilizumab | discharge | |
| Bektas et al. [52] | 52/M | CLL | FCR | 30 days ago | MP 40 mg/day | Tocilizumab | Anakinra high dose iv. |
exitus |
| Innes et al. [91] | 53/M | CML blast phase - HSCT - GVHD | Glucocorticoids Cyclosporin A MMF |
ongoing | None | Tocilizumab | Ruxolitinib | discharge |
| Di Lorenzo et al. [68] | 69/M | Stage IV prostate cancer | LHRH agonism | ongoing | None | Tocilizumab | discharge | |
| Bonomi et al. [69] | 65/M | Metastatic lung adenocarcinoma | Carboplatin Pemetrexed Pembrolizumab |
ongoing | None | Tocilizumab | discharge | |
| Ranger et al. [70] | 52/M | CML | Imatinib | ongoing | None | Tocilizumab | discharge | |
| Zhang et al. [71] | 60/M | Myeloma | Thalidomide | ongoing | None | Tocilizumab | discharge | |
| Chaidos et al. [92] | 66/M | Myeloma | Lenalidomide | ongoing | None | tocilizumab | discharge | |
| 59/M | Myeloma | Bortezomib and panobinostat | ongoing | None | Tocilizumab | Tracheostomy in ICU | ||
| Villegas et al. [72] | 62/M | Diffuse large B cell lymphoma | 2nd line chemotherapy Not specified | ongoing | Applied- dose not specified | Tocilizumab | Anakinra low-dose sc. |
exitus |
| 50/F | Rosai-Dorfman syndrome | 3rd line chemotherapy - Not specified | ongoing | Applied- dose not specified | Tocilizumab | Anakinra low-dose sc. |
exitus | |
| 72/M | CLL | 1st line chemotherapy - Not specified | ongoing | Applied- dose not specified | Tocilizumab | Anakinra low-dose sc. |
exitus | |
| 82/M | Waldenstrom's macroglobulinemia | 2nd line chemotherapy Not specified | ongoing | Applied- dose not specified | Tocilizumab | Anakinra low-dose sc. |
exitus | |
| 56/F | Diffuse large B cell lymphoma | 1st line chemotherapy - Not specified | ongoing | Applied- dose not specified | Tocilizumab | Anakinra low-dose sc. |
exitus | |
| Anakinra high-dose: > 5 mg/kg/day iv.; Anakinra low-dose: 100-200 mg/day sc.; CLL: Chronic Lymphocytic Leukemia; CML: Chronic Myeloid Leukemia; HyperCVAD: Cyclophosphamide Vincristine sulphate Adriamycin Dexamethasone; DXM: Dexamethasone; FCR: Fludarabin Cyclophosphamide Rituximab; GVHD: Graft versus Host Disease; HSCT: Hematopoietic Stem Cell Transplantation; ICU: Intensive Care Unit; LHRH: Luteinizing Hormone Releasing Hormone; MP: Methylprednisolone | ||||||||
Table. Case reports of patients with malignancy and COVID-19 treated with anti-cytokine therapies in available literature (English).
It should be kept in mind that, anti-IL-6 therapy was effective only in severe and critically ill patients with COVID-19 in the studies mentioned. Tocilizumab was found to be associated with an increase in bacterial and fungal superinfections in COVID-19 patients in two different meta-analysis [73,74]. Therefore, anti-IL-6 therapies should be used with caution and patients should be followed in terms of secondary infections, considering the loss of acute-phase response after drug exposure.
Anti-IL-1 therapy
The rationale for anti-IL-1 treatment for COVID-19 can be summarized as the role of inflammasome and its products in the development of COVID-19 manifestations and cytokine storm which has been stated above. Anakinra is a recombinant human IL-1 receptor antagonist protein and approved by the European Medicine Agency for autoinflammatory conditions such as FMF, cryopyrin associated periodic syndromes, and AOSD [75]. It is used subcutaneously in standard doses, and the possibility of using high doses intravenously is one of its most important advantages. In addition to its subcutaneous use in standard doses (100-200 mg/kg), the possibility of using high doses (up to 5 mg/kg twice a day) intravenously is one of its most important advantages. In one of the first trials that evaluates the efficacy of anakinra in patients with mildmoderate COVID-19 pneumonia, the efficacy of low-dose subcutaneous administration could not be demonstrated [76]. However, in recent studies, encouraging data on the effectiveness and safety of anakinra both at standard and high doses is accumulating, when administered to severe and critical patients [77-81]. A meta-analysis of eight observational studies and one randomized controlled trial revealed that anakinra reduces the mortality risk in patients admitted to hospital with moderate to severe COVID-19 pneumonia, especially in the presence of hyperinflammation (CRP >100 mg/L) without increasing infection risk [82]. The safety and efficacy of anakinra were established in patients with secondary HLH due to rheumatological conditions, cancer and infection both in adults and children [15,83,84]. The short half-life (4-6 hours) of anakinra also has the advantage of rapid withdrawal in case of infections or other complications in critically ill patients [67].
Efficacy of another anti-IL-1 option, canakinumab, humanized anti–interleukin-1β antibody was evaluated in COVID-19, albeit less often. A meta-analysis of available data (four observational studies, two RCTs) regarding the use of canakinumab on mild to severe COVID-19 pneumonia revealed lower mortality rate and decreased CRP levels in patients treated with canakinumab.
As a result, anti-IL-1 therapies can be used up to high doses in patients with hyperinflammatory response associated with COVID-19 who has comorbidities and an increased risk of secondary infections, based on the current data.
Janus kinase inhibitors
JAK/STAT pathway provides signal transduction from cytokines, growth factors, and hormones, to the nuclei of cells. JAK members are activated by multiple interleukin receptors including IL-2, IL-4, IL-7, IL-9, IL-15, IL-21, and IFN-γ receptors [85]. Considering the role of these cytokines in severe COVID-19, interruption of the JAK /STAT pathway was thought to be beneficial [86]. Baricitinib exhibits anti-inflammatory effects by reversible JAK inhibition and is approved by EMA for the treatment of rheumatoid arthritis and atopic dermatitis [87]. One of the largest RCTs on the efficacy of baricitinib, The Adaptive COVID-19 Treatment Trial 2, enrolled 1033 patients and revealed that treatment with baricitinib was superior to placebo in reducing time to recovery and was associated with fewer serious adverse events (AEs), although 28-day mortality was similar between groups (5.1% with baricitinib and remdesivir vs. 7.8% with remdesivir alone). Another RCT which included 1525 patients (COV-BARRIER) found that baricitinib 4 mg/day up to 14 days reduces 28- day and 60-day all-cause mortality (8% vs. 13% and 10% vs. 15%, respectively). The frequency of serious AEs, secondary infections, and thromboembolic events were similar between groups in both studies. Increased rate of serious side effects on long-term treatment for rheumatoid arthritis such as increased rate of serious infections (including zoster zona) or venous thrombosis was not observed in COVID-19 studies [88,89], possibly due to short term treatment in COVID-19. A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib in onco-hematological patients with COVID19 is still in progress [90]. However, particularly in immunosuppressed or cancer patients, the possibility of these AEs should be kept in mind during treatment with JAK inhibitors.
This paper has some limitations. Most of the studies about biological therapies are consisted of small retrospective observational studies and/or case reports due to exclusion of cancer patients from RCTs was the main limitation. Lack of comparative data with the biological treatments in patients with COVID-19 accompanying cancer was another limitation.
Conclusion
Patients with malignancy and/or receiving immunosuppressants are at high risk for complications, development of cytokine storm, and poor outcomes associated with COVID-19. Furthermore, recurrent/prolonged cytokine storm is an emerging potential life-threatening condition in patients with cancer or immunosuppressants beyond prolonged PCR positivity. Revealing the common pathways of inflammation for immune-mediated diseases, oncogenesis, and COVID- 19-associated cytokine storm is crucial for the development of targeted therapies in patients accompanying cancer. Tocilizumab may be a good option in the treatment of cytokine storm in patients with cancer due to the crucial role of IL-6 both in cancer development and COVID-19-associated cytokine storm. Further studies including new therapeutic strategies are needed to improve outcomes in these patients.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out work described in this article.
Conflicts of Interest
Authors declare no conflicts of interest.
References
2. Arellano-Llamas AA, Vela-Ojeda J, Hernandez-Caballero A. Chronic Lymphocytic Leukemia in the SARS-CoV-2 Pandemic. Current Oncology Reports. 2022 Jan 21:1-5.
3. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discovery. 2020 Jun 1;10(6):783-91.
4. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmunity Reviews. 2020 Jun 1;19(6):102537.
5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. Journal of Infection. 2020 Jun 1;80(6):607-13.
6. Gustine JN, Jones D. Immunopathology of Hyperinflammation in COVID-19. The American Journal of Pathology. 2021 Jan 1;191(1):4-17.
7. Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine. 2020 Jul 1;180(7):934-43.
8. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine. 2020 May;26(5):681-7.
9. Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. 2008 Sep 1;43(3):336-41.
10. Chau AS, Weber AG, Maria NI, Narain S, Liu A, Hajizadeh N, et al. The longitudinal immune response to coronavirus disease 2019: chasing the cytokine storm. Arthritis & Rheumatology. 2021 Jan;73(1):23-35.
11. Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, et al. Chemokine up-regulation in SARS-coronavirus–infected, monocyte-derived human dendritic cells. Blood. 2005 Oct 1;106(7):2366-74.
12. Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host & Microbe. 2016 Feb 10;19(2):181-93.
13. López-Reyes A, Martinez-Armenta C, Espinosa-Velázquez R, Vázquez-Cárdenas P, Cruz-Ramos M, Palacios-Gonzalez B, et al. NLRP3 inflammasome: the stormy link between obesity and COVID-19. Frontiers in Immunology. 2020 Oct 30;11:2875.
14. Dinarello CA, van der Meer JW. Treating inflammation by blocking interleukin-1 in humans. Seminars in Immunology. 2013 Dec 15;25(6):469-84.
15. Eloseily EM, Weiser P, Crayne CB, Haines H, Mannion ML, Stoll ML, et al. Benefit of anakinra in treating pediatric secondary hemophagocytic lymphohistiocytosis. Arthritis & Rheumatology. 2020 Feb;72(2):326-34.
16. Malireddi R, Kanneganti TD. Role of type I interferons in inflammasome activation, cell death, and disease during microbial infection. Frontiers in Cellular and Infection Microbiology. 2013 Nov 12;3:77.
17. Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell host & Microbe. 2020 Jun 10;27(6):883-90.
18. Rodrigues TS, de Sá KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. Journal of Experimental Medicine. 2021 Mar 1;218(3).
19. Bryant C. COVID-19 stokes inflammasomes. Journal of Experimental Medicine. 2021 Mar 1;218(3).
20. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discovery. 2020 Jun 1;10(6):783-91.
21. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Annals of Oncology. 2020 Jul 1;31(7):894-901.
22. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. The Lancet Oncology. 2020 Mar 1;21(3):335-7.
23. Do HT, Lee CH, Cho J. Chemokines and their receptors: multifaceted roles in cancer progression and potential value as cancer prognostic markers. Cancers. 2020 Feb;12(2):287.
24. Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpé S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clinical Cancer Research. 2004 Nov 1;10(21):7157-62.
25. Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, et al. Therapeutic benefit of blocking interleukin‐6 activity with an anti–interleukin‐6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double‐blind, placebo‐controlled, dose‐escalation trial. Arthritis & Rheumatism. 2002 Dec;46(12):3143-50.
26. Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. New England Journal of Medicine. 2017 Jul 27;377(4):317-28.
27. Castañeda S, Martínez-Quintanilla D, Martín-Varillas JL, García-Castañeda N, Atienza-Mateo B, González-Gay MA. Tocilizumab for the treatment of adult-onset Still’s disease. Expert Opinion on Biological Therapy. 2019 Apr 3;19(4):273-86.
28. Karakasheva TA, Lin EW, Tang Q, Qiao E, Waldron TJ, Soni M, et al. IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Research. 2018 Sep 1;78(17):4957-70.
29. Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, et al. Cancer-associated fibroblasts affect intratumoral CD8+ and FoxP3+ T cells via IL6 in the tumor microenvironment. Clinical Cancer Research. 2018 Oct 1;24(19):4820-33.
30. Gotthardt D, Putz EM, Straka E, Kudweis P, Biaggio M, Poli V, et al. Loss of STAT3 in murine NK cells enhances NK cell–dependent tumor surveillance. Blood, The Journal of the American Society of Hematology. 2014 Oct 9;124(15):2370-9.
31. Gadó K, Domján G, Hegyesi H, Falus A. Role of interleukin‐6 in the pathogenesis of multiple myeloma. Cell Biology International. 2000 Apr;24(4):195-209.
32. Kurzrock R, Redman J, Cabanillas F, Jones D, Rothberg J, Talpaz M. Serum interleukin 6 levels are elevated in lymphoma patients and correlate with survival in advanced Hodgkin's disease and with B symptoms. Cancer Research. 1993 May 1;53(9):2118-22.
33. Altundag O, Altundag K, Gunduz E. Interleukin-6 and C-reactive protein in metastatic renal cell carcinoma. Journal of Clinical Oncology. 2004 Dec;23(5):1044-1045.
34. Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, et al. A phase I, open-label study of siltuximab, an anti–IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clinical Cancer Research. 2013 Jul 1;19(13):3659-70.
35. Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, et al. A phase 2 multicentre study of siltuximab, an anti‐interleukin‐6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. British Journal of Haematology. 2013 May;161(3):357-66.
36. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Review of Clinical Immunology. 2019 Aug 3;15(8):813-22.
37. Hirano T, Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020 May 19;52(5):731-3.
38. Ansems M, Span PN. The tumor microenvironment and radiotherapy response; a central role for cancer-associated fibroblasts. Clinical and Translational Radiation Oncology. 2020 May 1;22:90-7.
39. Stoll JR, Vaidya TS, Mori S, Dusza SW, Lacouture ME, Markova A. Association of interleukin-6 and tumor necrosis factor-α with mortality in hospitalized patients with cancer. Journal of the American Academy of Dermatology. 2021 Feb 1;84(2):273-82.
40. Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the Rheumatic Diseases. 2021 Jul 1;80(7):930-42.
41. Zhang M, Bai X, Cao W, Ji J, Wang L, Yang Y, et al. The Influence of Corticosteroids, Immunosuppressants and Biologics on Patients With Inflammatory Bowel Diseases, Psoriasis and Rheumatic Diseases in the Era of COVID-19: A Review of Current Evidence. Frontiers in Immunology. 2021:2571.
42. Akama‐Garren EH, Li JX. Prior immunosuppressive therapy is associated with mortality in COVID‐19 patients: A retrospective study of 835 patients. Journal of Medical Virology. 2021 Oct;93(10):5768-76.
43. Avouac J, Drumez E, Hachulla E, Seror R, Georgin-Lavialle S, El Mahou S, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. The Lancet Rheumatology. 2021 Jun 1;3(6):e419-26.
44. Tepasse PR, Hafezi W, Lutz M, Kühn J, Wilms C, Wiewrodt R, et al. Persisting SARS‐CoV‐2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. British Journal of Haematology. 2020 Jul;190(2):185-8.
45. Lancman G, Mascarenhas J, Bar-Natan M. Severe COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. Journal of Hematology & Oncology. 2020 Dec;13(1):1-3.
46. Niyonkuru M, Pedersen RM, Assing K, Andersen TE, Skov MN, Johansen IS, et al. Prolonged viral shedding of SARS-CoV-2 in two immunocompromised patients, a case report. BMC Infectious Diseases. 2021 Dec;21(1):1-5.
47. Niess H, Börner N, Muenchhoff M, Khatamzas E, Stangl M, Graf A, et al. Liver transplantation in a patient after COVID‐19–Rapid loss of antibodies and prolonged viral RNA shedding. American Journal of Transplantation. 2021 Apr;21(4):1629-32.
48. Paybast S, Shahrab F, Hejazi SA. Recurrence of COVID-19 in a Patient With NMO Spectrum Disorder While Treating With Rituximab: A Case Report and Review of the Literature. The Neurologist. 2021 Nov;26(6):281.
49. He F, Luo Q, Lei M, Fan L, Shao X, Hu K, et al. Successful recovery of recurrence of positive SARS-CoV-2 RNA in COVID-19 patient with systemic lupus erythematosus: a case report and review. Clinical Rheumatology. 2020 Sep;39(9):2803-10.
50. Murillo-Zamora E, Trujillo X, Huerta M, Ríos-Silva M, Aguilar-Sollano F, Mendoza-Cano O. Symptomatic SARS-COV-2 reinfection: healthcare workers and immunosuppressed individuals at high risk. BMC Infectious Diseases. 2021 Dec;21(1):1-5.
51. Gulati K, Prendecki M, Clarke C, Willicombe M, McAdoo S. COVID-19 reinfection in a patient receiving immunosuppressive treatment for antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatology .2021 Jun;73(6):1091-1092
52. Bektaş M, Özdemir G. Protracted or recurrent COVID-19 associated cytokine storm in a patient with chronic lymphocytic leukemia receiving rituximab-based chemotherapy. Clinical Immunology. 2022 Mar; 236:108936.
53. Ye G, Pan Z, Pan Y, Deng Q, Chen L, Li J, et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. Journal of Infection. 2020 May 1;80(5):e14-7.
54. Rodríguez-Espinosa D, Broseta Monzó JJ, Casals Q, Piñeiro GJ, Rodas L, Vera M, et al. Fatal SARS-CoV-2 reinfection in an immunosuppressed patient on hemodialysis. Journal of Nephrology. 2021 Aug;34(4):1041-3.
55. Reuken PA, Stallmach A, Pletz MW, Brandt C, Andreas N, Hahnfeld S, et al. Severe clinical relapse in an immunocompromised host with persistent SARS-CoV-2 infection. Leukemia. 2021 Mar;35(3):920-3.
56. Strehl C, Ehlers L, Gaber T, Buttgereit F. Glucocorticoids—All-Rounders tackling the versatile players of the immune system. Frontiers in Immunology. 2019 Jul 24;10:1744.
57. Jamilloux Y, Gerfaud-Valentin M, Henry T, Sève P. Treatment of adult-onset Still’s disease: a review. Therapeutics and Clinical Risk Management. 2015;11:33.
58. Behrens EM, Koretzky GA. Review: cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatology. 2017;69:1135–1143.
59. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. Journal of Allergy and Clinical Immunology. 2020 Jul 1;146(1):110-8.
60. Lu X, Chen T, Wang Y, Wang J, Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Critical Care. 2020 Dec;24(1):1-4.
61. Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, Harris E, Bannister P, Rigby I, Horby P. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ. 2020 May 26;369.
62. FAI2R/SFR/SNFMI/SOFREMIP/CRI/IMIDIATE consortium and contributors. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Annals of the Rheumatic Diseases. 2021 Apr;80(4):527-38.
63. Horby PW, Campbell M, Staplin N, Spata E, Emberson JR, Pessoa-Amorim G, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Medrxiv. 2021 May 1;397(10285):1637-1645..
64. Angriman F, Ferreyro BL, Burry L, Fan E, Ferguson ND, Husain S, et al. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. The Lancet Respiratory Medicine. 2021 Jun 1;9(6):655-64.
65. Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, et al. Interleukin‐6 blocking agents for treating COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews. 2021 Mar 18;3(3):CD013881.
66. Bouchlarhem A, Es-Saad O, Haddar L, Lamzouri O, Elaidouni G, Mimouni H, et al. Special case of a patient in the blast phase of chronic myeloid leukemia successfully treated with tocilizumab during critical SARS-CoV-2 infection. Journal of International Medical Research. 2022 Mar;50(3):03000605221082875.
67. Lanceta J, Toprak M, Rosca OC. Merkel cell carcinoma presenting as a malignant pleural effusion post‐COVID‐19 hospitalization: A case report and literature review. Diagnostic Cytopathology. 2022 Jan;50(1):E37-41.
68. Di Lorenzo G, Buonerba L, Ingenito C, Crocetto F, Buonerba C, Libroia A, et al. Clinical characteristics of metastatic prostate cancer patients infected with COVID-19 in South Italy. Oncology. 2020;98(10):743-7.
69. Bonomi M, Maltese M, Brighenti M, Muri M, Passalacqua R. Tocilizumab for COVID-19 Pneumonia in a Patient With Non–Small-cell Lung Cancer Treated With Chemoimmunotherapy. Clinical Lung Cancer. 2021 Jan;22(1):e67.
70. Ranger A, Haji R, Kaczmarski R, Danga A. Interleukin‐6 Blockade Treatment for COVID‐19 associated Cytokine Release Syndrome in a Patient with Poorly Controlled Chronic Myeloid Leukaemia. British Journal of Haematology. 2020 Aug;190(3):e128-e130.
71. Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Advances. 2020 Apr 14;4(7):1307.
72. Villegas C, Poza M, Talayero P, Teller JM, Zafra D, Garcia C, Vera E, Hidalgo M, Lopez N, Cuellar C, Zamanillo I. IL-1R blockade is not effective in patients with hematological malignancies and severe SARS-CoV-2 infection. Annals of Hematology. 2020 Dec;99(12):2953-6.
73. Satyanarayana G, Enriquez KT, Sun T, Klein EJ, Abidi M, Advani SM, et al. Coinfections in Patients With Cancer and COVID-19: A COVID-19 and Cancer Consortium (CCC19) Study. Open Forum Infectious Diseases. 2022 Feb 14;9(3):ofac037.
74. Peng J, Fu M, Mei H, Zheng H, Liang G, She X, Wang Q, Liu W. Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID‐19 patients: A systematic review and meta‐analysis. Reviews in Medical Virology. 2021 Sep 24:e2295.
75. Agency, E.M. Kineret (anakinra). SUMMARY OF PRODUCT CHARACTERISTICS. April 10, 2022]; Available from: https://www.ema.europa.eu/en/documents/product-information/kineret-epar-product-information_en.pdf.
76. Tharaux PL, Pialoux G, Pavot A, Mariette X, Hermine O, Resche-Rigon M, et al. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. The Lancet Respiratory Medicine. 2021 Mar 1;9(3):295-304.
77. Huet T, Beaussier H, Voisin O, Jouveshomme S, Dauriat G, Lazareth I, et al. Anakinra for severe forms of COVID-19: a cohort study. The Lancet Rheumatology. 2020 Jul 1;2(7):e393-400.
78. Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology. 2020 Jun 1;2(6):e325-31.
79. Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. Journal of Allergy and Clinical Immunology. 2020 Jul 1;146(1):213-5.
80. González-García A, García-Sánchez I, Lopes V, Moreno-Arrones OM, Tortosa-Cabañas M, Elías-Sáenz I, et al. Successful treatment of severe COVID-19 with subcutaneous anakinra as a sole treatment. Rheumatology. 2020 Aug 1;59(8):2171-3.
81. Navarro‐Millán I, Sattui SE, Lakhanpal A, Zisa D, Siegel CH, Crow MK. Use of anakinra to prevent mechanical ventilation in severe COVID‐19: a case series. Arthritis & Rheumatology. 2020 Dec;72(12):1990-7.
82. Kyriazopoulou E, Huet T, Cavalli G, Gori A, Kyprianou M, Pickkers P, et al. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. The Lancet Rheumatology. 2021 Oct 1;3(10):e690-7.
83. Bami S, Vagrecha A, Soberman D, Badawi M, Cannone D, Lipton JM, et al. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatric Blood & Cancer. 2020 Nov;67(11):e28581.
84. Wohlfarth P, Agis H, Gualdoni GA, Weber J, Staudinger T, Schellongowski P, et al. Interleukin 1 receptor antagonist anakinra, intravenous immunoglobulin, and corticosteroids in the management of critically ill adult patients with hemophagocytic lymphohistiocytosis. Journal of Intensive Care Medicine. 2019 Sep;34(9):723-31.
85. Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunological Reviews. 2009 Mar;228(1):273-87.
86. Chen G, Wu DI, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of Clinical Investigation. 2020 May 1;130(5):2620-9.
87. Agency, E.M. Olumiant (Baricitinib). Available from: https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf.
88. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nature Reviews Rheumatology. 2017 Apr;13(4):234-43.
89. Kim H, Brooks KM, Tang CC, Wakim P, Blake M, Brooks SR, et al. Pharmacokinetics, pharmacodynamics, and proposed dosing of the oral JAK1 and JAK2 inhibitor baricitinib in pediatric and young adult CANDLE and SAVI patients. Clinical Pharmacology & Therapeutics. 2018 Aug;104(2):364-73.
90. Moreno-González G, Mussetti A, Albasanz-Puig A, Salvador I, Sureda A, Gudiol C, et al. A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2021 Dec;22(1):1-4.
91. Innes AJ, Cook LB, Marks S, Bataillard E, Crossette-Thambiah C, Sivasubramaniam G, Apperley J, Milojkovic D. Ruxolitinib for tocilizumab-refractory severe COVID-19 infection. British Journal of Haematology. 2020 Aug;190(4):e198-e200.
92. Chaidos A, Katsarou A, Mustafa C, Milojkovic D, Karadimitris A. Interleukin 6-blockade treatment for severe COVID-19 in two patients with multiple myeloma. British Journal of Haematology. 2020 Jul;190(1):e9-e11.
