Abstract
Introduction: End-stage chronic kidney disease (CKD) poses a significant challenge to patients' mental health, frequently leading to anxiety, depression, and sleep disturbances. This study aimed to identify factors associated with psychiatric vulnerability among patients with end-stage renal disease at the Zou/Collines Departmental Hospital in Benin.
Methods: We conducted a cross-sectional study from January to June 2024, including patients with end-stage renal disease managed in the internal medicine department. In this study, psychiatric vulnerability was defined as the presence of anxiety, depression, or sleep disorders The primary outcomes were the presence of anxiety and depression, assessed using the Hospital Anxiety and Depression Scale (HADS). Statistical significance was set at p<0.05.
Results: A total of 110 patients were included (mean age: 47.19 ± 11.34 years; male-to-female ratio: 1.97:1). Notably, all participants were presented with at least one psychiatric disorder. The prevalence of sleep disturbances was 86.36%, while clinically significant anxiety was found in 29.09% and depression in 45.45% of patients. Factors significantly associated with these conditions included exposure to criticism and verbal abuse, unemployment, lack of income-generating activities, housing insecurity, food insufficiency, and perceived stigmatization related to inadequate care.
Conclusion: The high prevalence of psychiatric comorbidities in patients with end-stage renal disease highlights an urgent need for the integration of mental health services into routine nephrology care.
Keywords
Psychiatric disorders, Renal failure, Depression, Anxiety, Benin
Abbreviations
ACEIs: Angiotensin-Converting Enzyme Inhibitors; ARBs: Angiotensin II Receptor Blockers; CHD Z/C: Zou/Collines Departmental Hospital; CKD: Chronic Kidney Disease; ESS: Epworth Sleepiness Scale; HADS: Hospital Anxiety and Depression Scale; ISI: Insomnia Severity Index; KDIGO: Kidney Disease, Improving Global Outcomes; NSAIDs: Non-Steroidal Anti-inflammatory Drugs
Introduction
The end-stage of chronic kidney disease (CKD) is a major public health concern, both due to its prevalence and the substantial vulnerability it imposes on affected individuals [1]. Renal failure, a terminal stage of CKD, is characterized by the kidneys' inability to effectively eliminate waste products and maintain fluid homeostasis [1]. This condition disrupts physical, psychological, and emotional functions, often resulting in increased mortality and considerable healthcare costs [2].
Patients with advanced CKD frequently experience psychological distress, including anxiety, depression, and sleep disorders, stemming from abrupt changes in their personal, familial, and professional lives [3]. The prevalence of depression is high in CKD patients due to several factors, including uremic toxins, psychosocial symptoms, and economic challenges [4]. Anxiety is described as the feeling of fear, uncertainty, helplessness, and apprehension that an individual encounters when anticipating a threatening situation [5]. Existing studies have reported a high prevalence of these psychiatric symptoms in this population [6], which strongly influence treatment adherence and impact both quality of life and survival outcomes [7]. In patients with CKD, the frequency of these psychiatric disorders appears to be particularly high, with a reported prevalence of approximately 80% [8]. The burden is even more pronounced in patients undergoing hemodialysis, for whom studies show prevalences ranging from 48% to 95% [9,10].
In Benin, where the CKD burden is rising, the psychological consequences of the disease are still underexplored. Evidence suggests that early psychological intervention may alleviate emotional suffering and improve coping mechanisms among affected individuals [11]. This study aims to identify psychiatric vulnerability factors in patients with end-stage CKD managed at the Zou/Collines Departmental Hospital (CHD Z/C) in 2024.
Patients and Methods
This was a cross-sectional, descriptive, and analytical study conducted in the internal medicine department of the CHD Z/C (Benin), spanning the period from January 1 to June 30, 2024. The study included adult patients (≥18 years) with end stage of CKD (on or off hemodialysis) who gave informed consent and were capable of responding to structured interviews.
The primary outcome was the presence of psychiatric disorders, specifically anxiety and/or depression. Sleep quality disturbances, socioeconomic vulnerability, clinical parameters, and treatment variables were evaluated as independent factors.
CKD was defined according to the 2024 Kidney Disease, Improving Global Outcomes (KDIGO) guidelines as abnormalities of kidney structure or function, present for >3 months, with implications for health. CKD is classified based on GFR categories (G1-G5). End-stage CKD, or stage 5, is defined by a decrease in glomerular filtration rate below 15 ml/min/1.73 m2.
Assessment tools
Anxiety and depression: Evaluated using the Hospital Anxiety and Depression Scale (HADS) developed by Zigmond and Snaith [12]. This 14-item tool assesses anxiety and depression, with scores ≥11 reflecting definite psychiatric morbidity.
Sleep disorders: P0 via two standardized instruments:
- Insomnia Severity Index (ISI): A 7-item scale scored from 0 to 4; insomnia was defined by scores ≥15.
- Epworth Sleepiness Scale (ESS): Assessed daytime somnolence in eight routine situations; total scores range from 0 to 24.
Data collection and analysis
Interviews were conducted during scheduled consultations. Responses to questionnaires were documented directly, and clinical/biological data were extracted from patient records. Data entry and statistical analyses were performed using Epi Info v7.2.2.2. For descriptive analysis, quantitative variables were presented as means and standard deviations if the distribution was approximately normal, which was assessed visually (histograms) and by normality tests where appropriate. Categorical variables were reported as frequencies and percentages. Comparison of proportions was conducted using Pearson’s chi-squared test or Fisher’s exact test, depending on applicability. The measure of association was expressed as odds ratios (OR) with 95% confidence intervals. A p-value < 0.05 was considered statistically significant.
Ethical considerations
The study adhered to ethical principles outlined in the Declaration of Helsinki [13], with full respect for patient anonymity and confidentiality.
Results
Study population
A total of 129 patients met the inclusion criteria. Of these, 19 patients were excluded (16 due to difficulty expressing themselves and three due to incomplete forms). Therefore, analyses were performed on 110 patients representing a participation rate of 85.2%. Among the included patients, 20 (18.1%) were undergoing hemodialysis.
Sociodemographic and socioeconomic characteristics
The mean age of the study cohort was 47.19 ± 11.34 years, with an age range of 20 to 75 years. The 40–50-year age group was the most represented (31.6%). The cohort included 73 men (66.3%), corresponding to a male-to-female ratio of 1.97. Most participants were married (77.2%) and resided in urban areas (83.6%). Regarding education, 40.0% completed secondary schooling. The detailed sociodemographic and socioeconomic characteristics of the patients are presented in Table 1.
|
|
N=110 |
% |
|
Age groups (years) |
||
|
[20–30] |
9 |
8.9 |
|
[30–40] |
22 |
20.0 |
|
[40–50] |
35 |
31.6 |
|
[50–60] |
34 |
30.8 |
|
[60–70] |
7 |
6.1 |
|
≥75 |
3 |
2.6 |
|
Gender |
||
|
Male |
73 |
66.3 |
|
Female |
37 |
33.6 |
|
Marital status |
||
|
Married |
85 |
77.2 |
|
Single |
15 |
13.6 |
|
Divorced |
5 |
04.5 |
|
Widowed |
5 |
04.5 |
|
Socioprofessional status |
||
|
Retailer |
36 |
32.7 |
|
Civil servant |
23 |
20.9 |
|
Craftsman |
15 |
13.6 |
|
Retired |
12 |
10.9 |
|
Housewife |
5 |
04.5 |
|
Customs declarant |
4 |
03.6 |
|
Student |
3 |
02.7 |
|
Breeder |
2 |
01.8 |
|
Unemployed |
10 |
09.1 |
|
Level of education |
||
|
None |
13 |
11.8 |
|
Primary |
23 |
20.9 |
|
Secondary |
44 |
40.0 |
|
Higher |
30 |
27.2 |
|
Residence |
||
|
Urban |
92 |
83.6 |
|
Rural |
18 |
16.3 |
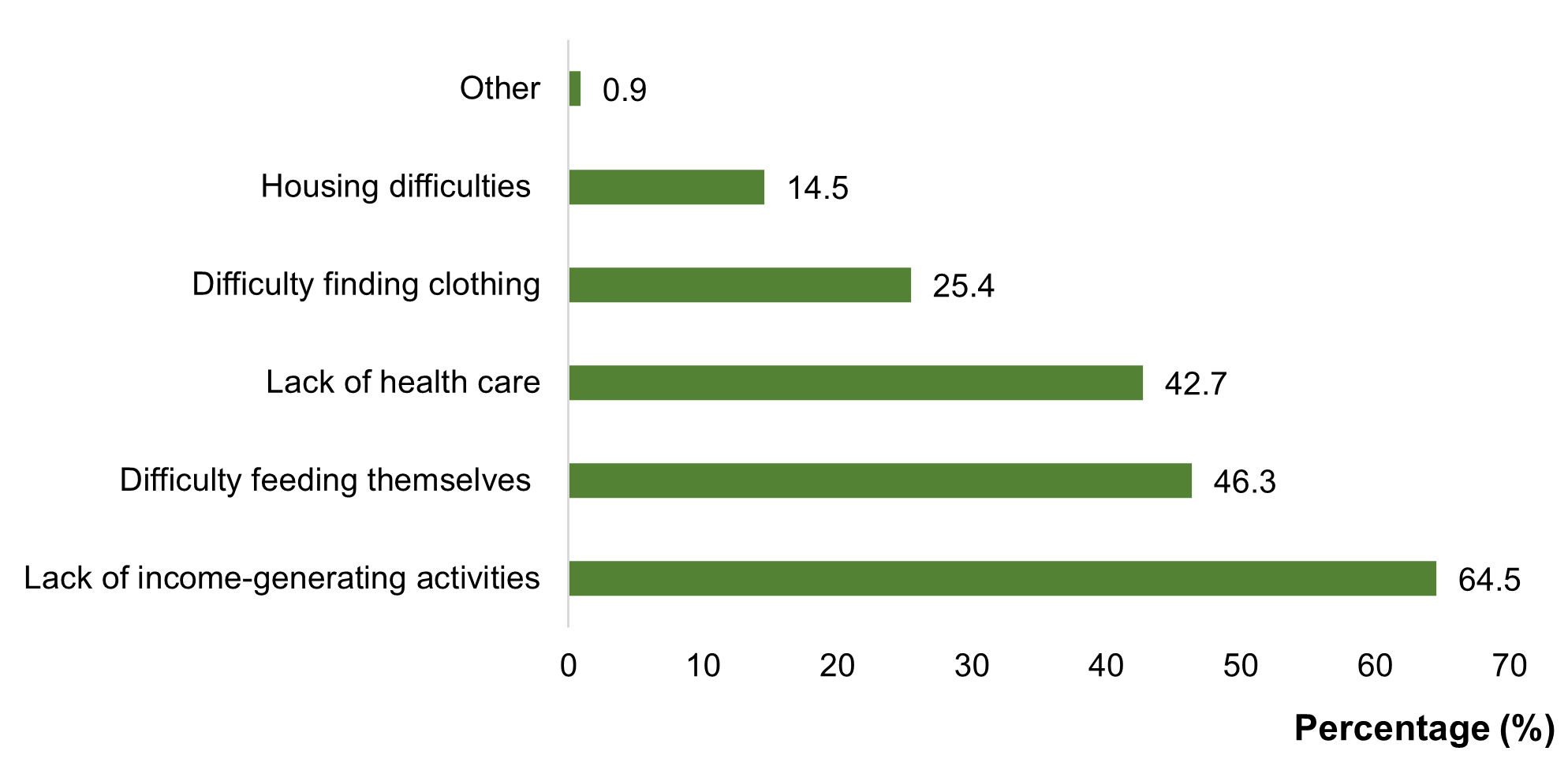
Socioeconomically, 64.5% of patients lacked an income-generating activity adapted to their health status. Furthermore, 46.3% reported food insufficiency, and 42.7% reported difficulties affording medical care (Figure 1). Socially, 44.5% of patients reported feeling rejected by their social circle, and 31.8% reported experiencing discriminatory behavior, with 17.2% of these instances occurring within their own family. Additionally, 13.6% reported exposure to criticism or insults related to their CKD, and 4.5% reported being stigmatized.
Figure 1. Economic consequences of ESKD at CHD Z/C in 2024
Clinical data and lifestyle
The clinical profile of the cohort revealed that 80.9% of patients had hypertension, 30.9% had diabetes, and 5.4% were carriers of the hepatitis B virus. Regarding lifestyle factors, 32.3% regularly consumed alcohol, 37.3% used herbal medicine, and 50.9% engaged in self-medication with non-steroidal anti-inflammatory drugs (NSAIDs). Consumption of fruits and vegetables was reported by 31.8% and 25.4% of patients, respectively, while only 5.4% participated in regular physical activity. Clinically, 69.1% of participants had elevated blood pressure at the time of examination. The mean body mass index (BMI) was 23.05 ± 5.46 kg/m², and 62.7% of patients had an elevated waist circumference.
Therapeutic data
In this study, 89 patients (80.91%) were receiving anti-hypertensive therapy. Among them, 80 (72.73%) were treated with calcium channel blockers, and 55 (50.00%) were receiving either angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs). Non-steroidal anti-inflammatory drugs (NSAIDs) were prescribed in 21 patients (19.09%). Additionally, 10 patients (9.09%) were treated with statins, and one patient was on hypnotic medication.
Among the 20 patients undergoing hemodialysis, the mean duration of dialysis was 6.61 ± 5.02 years, with a minimum of six months and a maximum of 12 years. Hemodialysis was performed via a central venous catheter in 3 cases (15.00%). Most patients (16 individuals, 80.00%) received dialysis sessions three times per week.
When evaluating satisfaction with management, 70.91% of patients appreciated the therapeutic education provided, 55.45% felt that care staff showed sufficient interest in their case, while 39.09% expressed dissatisfaction with the quality of care.
Prevalence of psychiatric disorders
A key finding of the study was that all 110 patients (100%) presented with at least one psychiatric disorder, identified as either sleep disturbance, anxiety, or depression.
Prevalence of sleep disorders
Sleep disorders were identified in 86.3% of patients. The most common issues were non-restorative sleep (90.9%), difficulty initiating sleep (50.0%), and nocturnal awakenings (49.1%) (Table 2).
|
|
N=110 |
% |
|
Non-restorative sleep |
100 |
90.9 |
|
Difficulty falling asleep |
55 |
50.0 |
|
Night-time awakenings |
54 |
49.1 |
|
Insomnia |
12 |
10.9 |
|
Daytime sleepiness |
10 |
9.1 |
|
Irritability |
6 |
5.5 |
|
Hypersomnia |
4 |
3.6 |
Prevalence of anxiety
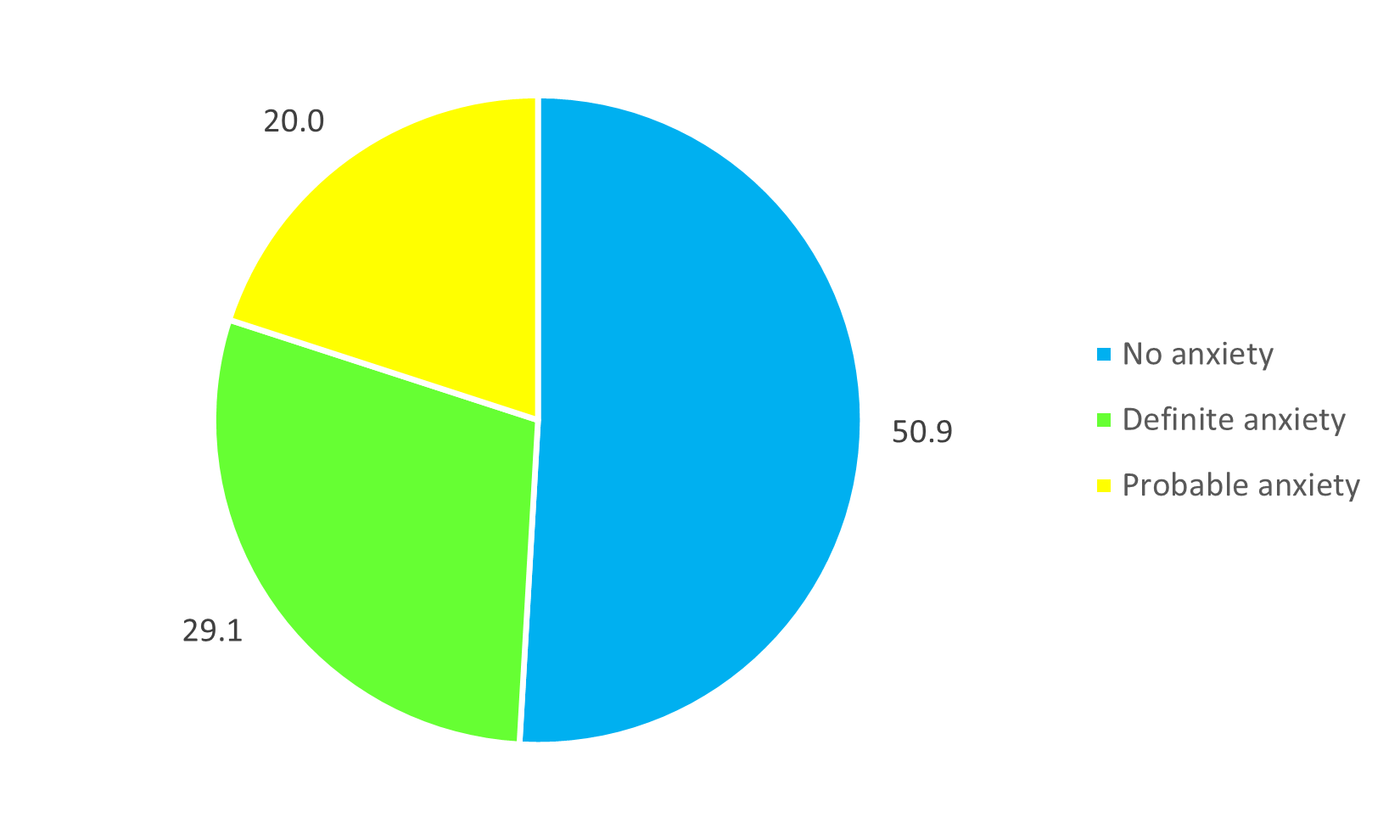
Based on the HADS assessment, 29.1% of subjects presented clinically significant anxiety (Figure 2).
Figure 2. Distribution of patients by type of anxiety at CHD Z/C in 2024.
Prevalence of depression
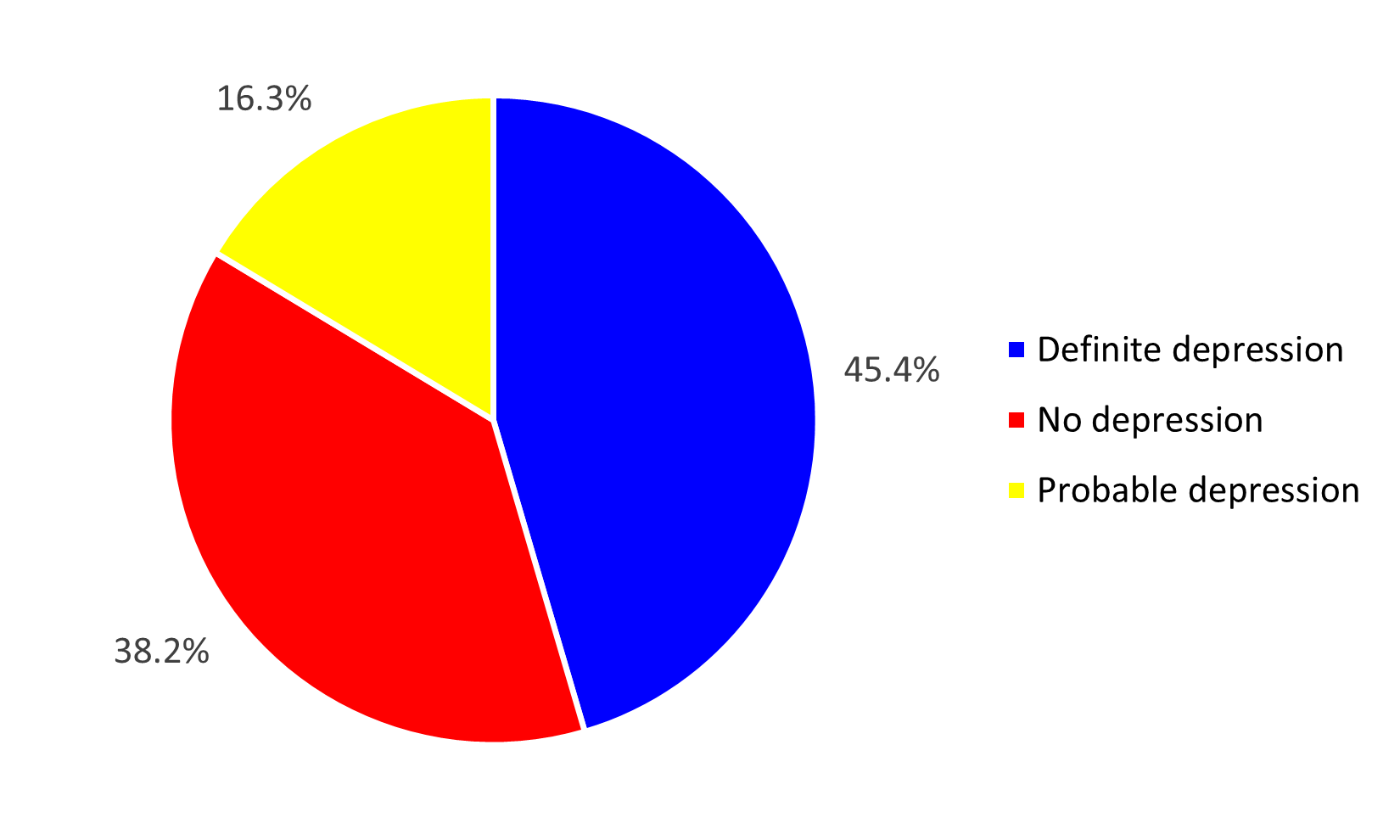
Clinically significant depression was identified in 45.4% (n=50) of the surveyed patients. Figure 3 illustrates the distribution of the prevalence of depression.
Figure 3. Distribution of patients by type of depression at CHD Z/C in 2024.
Factors associated with anxiety and depression
Statistical analysis identified several socioeconomic factors significantly associated with anxiety and depression, as detailed in Table 3.
Anxiety was associated with exposure to criticism or verbal abuse (OR=3.47; 95% CI: 1.03–11.68; p=0.033).
Depression was significantly associated with unemployment (OR=2.52; p=0.03), lack of income-generating activities (OR=2.34; p=0.03), housing insecurity (OR=5.18; p=0.02), family food insufficiency (OR=3.36; p<0.01), exposure to criticism or insults (OR=4.72; p=0.03), and prejudice regarding inadequate care (OR=4.64; p<0.01).
|
|
|
Vulnerability |
OR [ 95% CI] |
p |
|
|
Yes |
No |
||||
|
Anxiety |
|
|
|
|
|
|
Exposure to criticism and insults |
Yes |
11 |
4 |
3.47 [1.03–11.68] |
0.033 |
|
No |
42 |
53 |
1 |
||
|
Depression |
|||||
|
Exposure to criticism and insults |
Yes |
13 |
2 |
4.72 [1.01–22.12] |
0.03 |
|
No |
55 |
40 |
1 |
||
|
Unemployed |
Yes |
30 |
10 |
2.52 [1.07–5.95] |
0.03 |
|
No |
38 |
32 |
1 |
||
|
Lack of revenue-generating activity |
Yes |
49 |
22 |
2.34 [1.05–5.24] |
0.03 |
|
No |
19 |
20 |
1 |
||
|
Housing insecurity |
Yes |
14 |
2 |
5.18 [1.11–24.11] |
0.02 |
|
No |
54 |
42 |
1 |
||
|
Prejudice of poor and inadequate care |
Yes |
38 |
9 |
4.64 [1.92–11.85] |
<0.01 |
|
No |
30 |
33 |
1 |
||
|
Family food insufficiency |
Yes |
39 |
12 |
3.36 [1.47–7.66] |
<0.01 |
|
No |
29 |
30 |
1 |
||
Discussion
Our study is the first to investigate the psychiatric vulnerability of patients with end stage CKD at the CHD Z/C. As such, it provides a foundational basis for future research.
Psychiatric disorders related to dialysis
All 110 patients (100%) exhibited at least one psychiatric vulnerability. Sleep disorders were present in 95.5% of patients. This prevalence was similar to the 94.3% found among hemodialysis patients in Benin in 2022 [10].
Sleep disorders
Sleep disorders were identified in 86.3% of patients. The most common issues were non-restorative sleep (90.9%), difficulty initiating sleep (50.0%), and nocturnal awakenings (49.1%). Sleep disorders are highly prevalent in CKD but are often under-recognized [14]. Prevalence of approximately 80% has been reported in the literature [8]. Sleep disorders are associated with the activation of the renin–angiotensin–aldosterone system through pathways of hypoxemia and activated sympathetic activities, which result in endothelial dysfunction and lead to myocardial and renal damage [14]. The presence of sleep disorders in CKD can exacerbate the already heightened cardiovascular morbidity and mortality in these patients, as well as leading to daytime fatigue and reduced quality of life [14].
Anxiety
In our study, we identified psychiatric disorders within the study population. Specifically, 29.09% of patients presented with significant anxiety, a rate consistent with findings reported by Cukor et al. [15]. Patients with CKD have prevalence rates of anxiety disorders and anxiogenic symptoms of 19% and 43%, respectively [16]. The heightened susceptibility to anxiety in CKD is thought to be multifactorial. Postulated mechanisms include inflammatory processes secondary to uremic toxins, oxidative stress from increased cytokine production, microvascular brain damage, and the dysregulation of the renin–angiotensin system [16]. The clinical impact of this anxiety is substantial, adversely affecting quality of life and carrying significant prognostic weight. Furthermore, the literature reports that pre-dialysis CKD patients with elevated anxiety symptoms have a 60% higher risk of mortality, hospitalization, or requiring dialysis compared to those without an anxiety disorder [17].
Depression
Regarding depression, it emerged from our study that nearly half of our study population was significantly depressed. Our results are similar to data in the literature, which report a prevalence of 20% to 70% [18]. Similarly, Chih-Ken found that 47% of hemodialysis patients in his study met the DSM-IV criteria for a major depressive disorder [19]. Tsevi et al. observed a slightly higher prevalence (68.2%) than that of our series [20]. This difference could be explained by the tool used for assessing depression. Tsevi et al. [20] used the 13-item simplified version of the Beck Depression Inventory [21].
A lower prevalence of depression (22.67%) than ours was noted four years earlier by Djidonou in the same hemodialysis centers [6]. The increase in depressed patients observed in our investigation could be justified by a prevailing situation of distress linked to the difficulties in managing CKD, which was responsible for patient deaths, making the other patients more distressed. Chronic disease increases the risk of depression because it increases economic burden, reduces quality of life, and impairs activities of daily living [17]. More broadly, chronic disease is a well-established risk factor for depression, as it often impairs activities of daily living. While the specific mechanisms of depression in patients with CKD are numerous, they can be broadly categorized into behavioral and biological pathways [17].
Factors associated with the onset of psychiatric disorders
From a social perspective, the social environment plays an important role in the patients' experience. This role can be positive, strengthening the bonds between the individual and themselves on one hand, and between the individual and their entourage on the other. Conversely, the entourage, through its judgments, opinions, and prejudices, can have a negative effect on the patient's mental state, making them more fragile and more exposed to the onset of psychological disorders. Chronic diseases are poorly understood by society, which gives rise to various social interpretations. They are not well accepted by society, and affected patients are thus subject to criticism and sometimes insults.
Furthermore, nearly half of the sample had been rejected by their former friends. A deterioration of social relationships due to CKD has also been reported by Chiaranai, who noted the marital distress of patients [1]. Approximately 7 out of 10 patients reported having been victims of discriminatory behavior, more than half of which occurred within the family. Similarly, 1 in 10 patients had been insulted or criticized because of their CKD, and more than half of these instances occurred within the family. Nearly two out of ten people reported having had problems with someone because of the disease. The problems were primarily on the marital level, which is consistent with the findings of Chiaranai. Indeed, the patients in his series reported neglect from their spouse, who had allegedly opted for other companions [1]. These marital conflicts predominantly led to either divorce for married subjects or the loss of the romantic relationship for developing couples. In the latter case, our patients reported that even if the partner's support was present, the support from the in-laws was lacking and was instead replaced by discouragement of the relationship. This exposes young patients of both sexes to a life of involuntary celibacy.
From an economic perspective, faced with advanced CKD, the majority of patients sought help for their health condition. End-stage renal disease was accompanied by a loss of financial autonomy for the majority, due to a limitation of physical ability, which very often required asking for help. This assistance was dominated by financial aid (72.73%) and came mainly from the family (78.67%). This highlights the mutual support that prevails in African families.
The constraints related to CKD had forced patients to change their socioeconomic status, with an increase in the proportion of unemployed individuals. This observation was made in Togo by Sabi et al., who reported that the majority of patients in their series no longer carry out their previous activities [22]. Laborers, in particular, faced with physical limitations, were forced to find other, less strenuous activities. Physical limitation was also described by Chiaranai as a major problem in end-stage renal disease [1]. CKD and its replacement therapy are handicaps and impoverish patients. In this situation, patients found themselves with difficulties in meeting basic needs for themselves and their families, including food, housing, and healthcare.
Conclusion
This study confirms that psychiatric vulnerability, encompassing anxiety, depression, and sleep disorders, is a significant and highly prevalent comorbidity among patients with end-stage CKD. These conditions are not merely consequences of primary illness but are critical factors that severely impair quality of life and are associated with poorer clinical outcomes.
Therefore, our findings call for a shift towards a more holistic and integrated model of care. We recommend the implementation of systematic screening for these psychiatric disorders as a routine component of nephrology practice. Establishing accessible psychological support and collaborative psychiatric services within renal care units is essential to effectively manage these vulnerabilities and improve patient well-being.
References
2. Ganu VJ, Boima V, Adjei DN, Yendork JS, Dey ID, Yorke E, et al. Depression and quality of life in patients on long term hemodialysis at a nationalhospital in Ghana: a cross-sectional study. Ghana Med J. 2018 Mar;52(1):22–8.
3. Nasr M, Ammar MH, Khammouma S, Dhia NB, Ghachem A. L’hémodialyse et son impact sur la qualité de vie. Néphrologie & thérapeutique. 2008 Feb 1;4(1):21–7.
4. Nath M, Agarwal A. New insights into the role of heme oxygenase-1 in acute kidney injury. Kidney Res Clin Pract. 2020 Dec 31;39(4):387–401.
5. Goh ZS, Griva K. Anxiety and depression in patients with end-stage renal disease: impact and management challenges - a narrative review. Int J Nephrol Renovasc Dis. 2018 Mar 12;11:93–102.
6. Djidonou A, Tognon Tchegnonsi F, Ahoui S, Ataïgba INE, Houndefandan M, Hers D, et al. Prevalence and factors associated with depression among chronic renal failure patients receiving hemodialysis at public health centers in Benin. Le Bénin Médical. 2015;59:15–20.
7. Kimmel PL, Peterson RA. Depression in end-stage renal disease patients treated with hemodialysis: tools, correlates, outcomes, and needs. Semin Dial. 2005 Mar-Apr;18(2):91–7.
8. Muller ME, Heinzer R, Pruijm M, Wuerzner G, Burnier M. Troubles du sommeil chez des patients présentant une insuffisance rénale chronique [sleep disorders in patients with chronic renal insufficiency]. Rev Med Suisse. 2012 Feb 29;8(330):458–61.
9. Coulibaly N, Yattara H, Baya B, Diallo D, Sy S, Coulibaly SD, et al. Prévalence et Facteurs Associés à l’Insomnie chez l’Hémodialysé Chronique au CHU du Point G (Bamako): Prevalence and factors associated to insomnia in chronic hemodialysis patients at the CHU du Point G (Bamako). HEALTH SCIENCES AND DISEASE. 2022 May 27;23(6):12–4.
10. Ahoui S, Agbetou M, Hounsinou F, Vinasse A, Eteka E, Houeto N, et al. Facteurs associés aux troubles du sommeil chez les hémodialysés chroniques : une étude transversale analytique au Centre national hospitalier et universitaire Hubert Koutoukou Maga de Cotonou, Benin. Ann Afr Med. 2025;18(3):e6199–210.
11. Augusto CR, Krzesinski JM, Warling X, Smelten N, Étienne AM. Intérêt des interventions psychologiques en dialyse: étude exploratoire. Néphrologie & thérapeutique. 2011 Jul 1;7(4):211–8.
12. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983 Jun;67(6):361-70.
13. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013 Nov 27;310(20):2191–4.
14. yons OD. Sleep disorders in chronic kidney disease. Nat Rev Nephrol. 2024 Oct;20(10):690–700.
15. Cukor D, Coplan J, Brown C, Friedman S, Newville H, Safier M, et al. anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008 Jul;52(1):128–36.
16. Huang CW, Wee PH, Low LL, Koong YLA, Htay H, Fan Q, et al. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: A systematic review and meta-analysis. Gen Hosp Psychiatry. 2021 Mar-Apr;69:27–40.
17. Kim DS, Kim SW, Gil HW. Emotional and cognitive changes in chronic kidney disease. Korean J Intern Med. 2022 May;37(3):489–501.
18. Cukor D, Coplan J, Brown C, Friedman S, Cromwell-Smith A, Peterson RA, et al. Depression and anxiety in urban hemodialysis patients. Clin J Am Soc Nephrol. 2007 May;2(3):484–90.
19. Chen CK, Tsai YC, Hsu HJ, Wu IW, Sun CY, Chou CC, et al. Depression and suicide risk in hemodialysis patients with chronic renal failure. Psychosomatics. 2010 Nov-Dec;51(6):528–528.e6
20. Tsevi MY, Salifou S, Sabi AK, Noto-Kadou-Kaza B, Amekoudi EY, Dassa SK. Depression in patients on chronic hemodialysis at the Sylvanus Olympio University Hospital of Lomé (Togo). The Pan African Medical Journal. 2016 Sep 27;25:26.
21. Beck AT, Rial WY, Rickels K. Short form of depression inventory: cross-validation. Psychol Rep. 1974 Jun;34(3):1184–6.
22. Sabi KA, Noto-Kadou-Kaza B, Amekoudi EY, Tsevi CM, Mahamat HA, Kossidze K, et al. Vécu et représentation des malades souffrant d’IRC sous dialyse au Togo. Néphrologie & Thérapeutique. 2015 Sep 1;11(5):309.