Abstract
Background: Lost to follow-up (LTFU) among patients on antiretroviral therapy accounts for the most of all attrition. In Sub-Saharan Africa,there is a concern regarding high rates of LTFU and early mortality in antiretroviral therapy programs. Mortality and transferred out are the potential competing events for LTFU. Ignoring these events may give an invalid estimate by overestimating the probability of the occurrence of LTFU.
Objective: This study aims to assess the incidence and predictors of LTFU among adult HIV (Human Immunodeficiency Virus) patients who started antiretroviral therapy (ART) in Jigjiga Governmental Hospitals’ ART clinics between January 2015 and December 2021.
Methods: A multi-center Institution-based retrospective follow-up study has been conducted in Jigjiga Governmental Hospitals. Gray’s test was used to compare the cumulative incidence function (CIF) of LTFU across variable categories. A graphical examination of CIF for each category of variables, as well as the Schoenfeld residuals global test, validate the proportional sub-hazard assumption. We fitted both univariable and multivariable competing risk regression models. In the multivariable analysis, variables with p-values of 0.05 were considered statistically significant predictors of LTFU.
Result: A total of 842 clients were included in the study, and the LTFU incidence rate is 5.25 per 100 PYO. The participants’ median age ranged from 29 to 43 years. Those not disclosed their HIV status (aSHR=4.22; 95%CI (2.11-8.47)), those were a fair and poor level of recent adherence (aSHR=2.17; 95%CI (1.18-4.23)) and (aSHR=1.48; 95%CI (2.97-5.34)), patients with severe anemia (aSHR 4.58; 95% CI (1.28-16.39)) ambulatory functional status (aSHR 2.38; 95% CI (1.21-4.68)), patients who do not took cotrimoxazole prophylactic therapy (CPT) (aSHR 2.47; 95% CI (2.99-6.15)) were significant predictors of LTFU.
Conclusion: In this study, the incidence of LTFU was decreased with additional years on ART. Patients on ART who did not disclose their HIV status had poor levels of adherence, did not take CPT prophylaxis, on severe anemia and ambulatory functional status were at higher risk of LTFU. As a result, close monitoring and proper tracing mechanisms aimed at this higher-risk group would reduce AIDS (Acquired immunodeficiency syndrome)-related LTFU.
Keywords
Jigjiga-Ethiopia, HIV/AIDS, Antiretroviral therapy, Lost to follow-up, Cumulative incidence function, Competing risks regression
Abbreviations
AIDS: Acquired Immune Deficiency Syndrome; cART: Combination Antiretroviral Therapy; CD4: Cluster Differentiation Four; PLWH: People Living with HIV; BMI: Body Mass Index; cSHR: Crude Sub Hazard Ratio; aSHR: Adjusted Sub Hazard Ratio; CIFs: Cumulative Incidence Functions; CPT: Cotrimoxazole Preventive Therapy; HIV: Human Immune Deficiency Virus; INH: Isoniazid Preventive Therapy; LTFU: Lost To Follow Up; OIs: Opportunistic Infections; FS: Functional Status; TB: Tuberculosis; WHO: World Health Organization; FMOH: Federal Ministry of Health; HAART: Highly Active Antiretroviral Therapy
Introduction
In 2021, approximately 38.4 million people worldwide were living with HIV. Since the beginning of the epidemic, 84.2 million people have become infected with HIV, and 40.1 million have died from AIDS-related illnesses [1]. At the end of December 2021, around 28.7 million people were accessing ART, up from 7.8 million in 2010 [2]. According to the World Health Organization (WHO), the African region continues to be the most severely affected, accounting for nearly two-thirds of people living with HIV [3]. In Ethiopia by 2021, nearly 610,000 people were living with HIV/AIDS. The overall ART coverage is 78% of which adult ART coverage accounts for 76% [4].
ART lowers mortality and morbidity rates in HIV patients while improving their quality of life. The benefits also include preventing HIV transmission by suppressing HIV replication in people who already have the virus. Despite a significant reduction in HIV-related deaths as a result of widely available ART treatment, success remains critically dependent on regular patient follow-up in care [3,5,6].
The goals of ART are to lead and effectively suppress viral replication in order to prevent the development of HIV drug resistance, treatment failure, and lost follow-up. Adherence to ART and retention in HIV care program are critical for achieving optimal health outcomes in HIV patients [7,8]. Loss of follow-up from ART service has a pronounced negative impact on ART treatment outcomes. It can reduce the immunological benefits of ART and increase AIDS-related morbidity, mortality, and hospitalization, resulting in serious consequences such as drug toxicity, treatment failure due to poor adherence, and drug resistance [9,10].
A competing risk is an event that either impedes or modifies the occurrence of the primary event of interest. Classical survival analysis assumes non-informative or independent sampling (censoring), which means that subjects who remain in a given study at any given point in time have a similar future risk of event occurrence as those who are no longer being followed, as if subjects lost to follow up were random and thus non-informative [11,12]. Traditional survival analysis becomes unsuitable when the event of interest is neither censored nor observed, and as a result, the cumulative incidence of an event may be overestimated in the presence of competing risks. Because the assumption of non-informative censoring may be violated and event probability estimation is interpreted as occurring in a setting where competing events do not occur [13,14].
According to competing risk regression model results, the cumulative incidence in different countries was 7.9 per 100 person-year, 5.6 per 100 person-year, 11.6 per 100 person-year, and 10.9 per person-year, respectively [9,14-16]. Similar studies conducted in different parts of Ethiopia based on classical survival analysis reported the cumulative incidence ranging from 3.7 per 100 person-year up to 26.6 per 100 person-year [17-24].
Different studies showed that LTFU is associated with baseline sociodemographic factors like male sex [8,25-31] those whose age is younger 15-24 years [7,14,16,27,28,32,33] and those not having a committed partner [34], patients with no education [27,35,36], and unmarried [27,36], and clinical and treatment-related factors like CD4 count below 200 cells [8,19,37,38], BMI<18.5 kg/m2 [8,25,32], WHO stage I or II [8,14,15,38-41], anemia [42], fair/poor level of adherence and WHO stage III or IV [17,18,24,28,35], don’t take INH prophylaxis [16,17,22], BMI<18.5 kg/m2 [21,35], no CPT prophylaxis [16,43], ambulatory functional status [16,17,19,44] were significant predictors for LTFU.
Although many studies have been conducted on LTFU and its determinants, valid estimates of incidence and predictors of LFTU can be obtained if death and transfer out are considered as competing events (rather than counting those as censored), especially in poor clinical settings where death is common and alters the probability of the occurrence of LTFU. However, in most of these studies, death and transfer out, which are competing risks of LTFU, are frequently ignored, which can lead to misleading results. As a result, the purpose of this study was to estimate the incidence rate and identify the predictors of LTFU in Jigjiga city Governmental Hospitals ART Clinics, Eastern Ethiopia, by considering death and transfer out as competing events.
Methods
Study design and Setting
An institution-based retrospective follow-up study was conducted in Jigjiga city public hospitals in eastern Ethiopia between January 1, 2015, and December 31, 2021. Jigjiga is the capital city of the Ethio-Somali region, which is located 630 kilometers from Addis Ababa, Ethiopia's capital city. The study was conducted in three governmental hospitals in the city; these hospitals housed the city's only ART Centers. All of these hospitals offer HIV/AIDS interventions, such as free diagnosis, treatment, and patient follow-up. The research was carried out between July 1 and July 30, 2022. During the study period, there were a total of 2016 ART-attending patients in these hospitals, with 1816 of them being adults over the age of 15.
Population
All adult HIV-infected patients (Age ≥ 15years) who initiated ART at Jigjiga public hospitals (Karamara General Hospital, Sheik Hassen Yeberre Referral Hospital, and Ablelle Primary Hospital) from January 01, 2015, to December 31, 2021, were our study population. Adult HIV-positive patients who had at least one follow-up visit after starting ART were included in the study. HIV patients who transferred in with incomplete baseline data, whose medical charts were unavailable during the data collection period, and those with unknown ART initiation dates were excluded from the study.
Sample size determination and sampling method
The minimum required sample size (842) was calculated using Stata 14 software's survival analysis formula power cox command. The study participants' records were first filtered from the ART database based on their entry time to the follow-up, age, and inclusion criteria, and then we used stratified proportionate random sampling (among health facilities). Finally, we used SPSS Software to select the final sample size using a simple random sampling technique (computer-generated Random Sampling) (Figure 1).
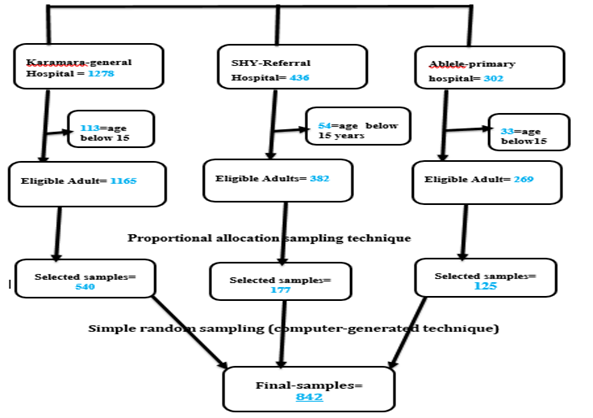
Figure 1. Thematic presentation of sampling procedure.
Variables definition
The primary outcome variable was time to LTFU, with death and transferred out considered as competing events. The predictor variables assessed were baseline socio-demographic factors like (sex, age, marital status, educational status, occupation, residence), baseline clinical and treatment-related factors like (BMI status at baseline, History of Tuberculosis (TB), Opportunistic infections, Hemoglobin level, Baseline CD4, Viral load, WHO clinical stage, Partner HIV status, Functional status, Co-morbidities) and ART adverse event, Regimen type at the start, Regimen substitution, Year of ART initiation, Registered phone number, OI prophylaxis (INH, CPT), Distance from the Hospitals), Disclosure status, Partner HIV status, Caregiver, Adherence level) were included.
Operational definitions
This study intended to determine the incidence of LTFU using seven-year data retrieved from medical record charts. LTFU is defined as not taking an ART refill for 3 consecutive months or longer after the last scheduled visit (from the last attendance for refill) not yet categorized as dead or transferred out [19]. The competing events were death and transferred out which is defined as patients recorded as dead on the patient’s exit form or whose outcome is recorded as death on the follow-up chart and patients formally transferred to another health facility respectively. A patient was classified as censored if he/she had still on follow-up at these hospitals at the end of the study period. Operationally the functional status was defined based on the ART guideline; Working FS: able to perform usual work inside or outside the home, Ambulatory FS: able to perform an activity of daily living, and bedridden FS: not able to perform an activity of daily living [45]. ART medication adherence is defined as the percentage of ART drug dosage calculated from a monthly total dose, and classified as good, fair, or poor. Therefore, good adherence was reported if equal to or greater than 95% or ≤ 3 doses missing per month, fair if 85–94% or 4–8 doses missing per month, or poor if less than 85% or ≥ 9 doses missing per month [46]. Disclosure in this study is defined as disclosure of the status that is being HIV positive to at least one individual. The caregiver is also defined as anyone who can support or assist an individual with HIV.
Data collection tool and procedure
ART patient data is stored in smart care as both a hard copy (chart) and a database. The ART follow-up forms served as the foundation for the data extraction checklist. The data extraction tool was carefully prepared from ART intake follow-up forms to ensure data quality. By randomly selecting and completing 42 sample chart reviews, we demonstrated consistency between data recording systems and the prepared checklist, resulting in minor modification to the data extraction checklist. Seven clinical nurses and health officers were recruited, as well as a data collector and three supervisors for each of the three hospitals.
Data processing and analysis
The Data was entered using Epi-data version 3.1 and then exported to Stata 14 software for analysis. Descriptive statistics including proportions, tables, and charts were done to describe the characteristics of the study participants. Non-parametric estimation of CIF was performed both graphically and using Gray’s test. After fitting the model, the proportional sub-distribution hazard assumption was also verified by using the plot of log (- log (1-CIF)) versus the log of time to failure for each covariate, by interacting each covariate with time (tvc) and using Schoenfeld residual test. Univariable competing risk regression analysis was fitted to identify factors associated with LTFU. Variables with a p-value of ≤ 0.2 in the univariable analysis were fit to the multivariable competing risk regression analysis once more. To express the strength of the association, the crude and adjusted sub-distribution hazard ratios were calculated, along with the corresponding 95% CI. Variables with a P-value of ≤ 0.05 were considered statistically significant in multivariable-analysis.
Ethical consideration
We obtained ethical consent from the Institutional Review Board of the University of Gondar, Institute of Public Health. Since the study used an analysis of secondary data from patient charts; we received a waiver for informed consent. To keep privacy, names and other personal identifiers were not included in the data collection tool.
Result
Baseline socio-demographic characteristics
A total of 842 clients enrolled in ART care were included in the final analysis. Nearly half, 466 (53.34%) of the study participants were males. The median age of the participants was 35 (IQR=29-43) years. Of the total, 428 (50.83%) were married, and 270 (32.07%) participants had a primary level of education. The majority of subjects 759 (90.25%) had a caregiver and 673 (80.02%) study participants disclosed their HIV status (Table 1).
|
Variables |
Category |
Frequency |
Percentage (%) |
|
Sex |
Female Male |
466 376 |
55.34 44.66 |
|
Age |
15-24 25-34 35-44 ≥45 |
90 265 298 189 |
10.69 31.47 35.39 22.45 |
|
Marital status |
Single Married Divorced Others* |
121 428 190 103 |
14.37 50.83 22.57 12.23 |
|
Residence |
Urban Rural |
552 290 |
65.56 34.44 |
|
Distance from HF |
Below 5 km Above 5 km |
369 473 |
43.82 56.18 |
|
Educational status |
No education Primary Secondary College & university |
214 270 245 113 |
25.42 32.07 29.10 13.42 |
|
Occupation |
Unemployed Daily laborers Housewife Government employee Self-employee Others ** |
196 101 126 189 176 54 |
23.28 12.00 14.96 22.45 20.90 6.41 |
|
Religions |
Orthodox Muslim Others *** |
280 404 158 |
33.25 47.98 18.76 |
|
Year of ART Initiation |
Before COVID-19 After COVID-19 |
554 288 |
65.8 34.2 |
|
Disclosure status |
Disclosed Not disclosed |
673 168 |
80.02 19.98 |
|
Caregiver |
Yes No |
759 82 |
90.25 9.75 |
|
Others*: Separated, widowed; others**: Farmer, student; others***: Catholic, Protestant. |
|||
Clinical and treatment-related characteristics
About 240 (42.93%) participants were linked to care with a baseline CD4 count between 201-350 cells/ml. The median CD4 count was 292 (IQR=161-421 cells/ml), and 369 (43.82%) of the participants were WHO stage II, followed by WHO stage III & IV 244 (28.98%). Around 89.96% have been started with CPT prophylaxis and the majority (98.8%) of patients were screened for TB, of which 18.67% were positive (Table 2).
|
Variables |
Categories |
Frequency |
Percentage (%) |
|
Baseline CD4 count |
Below 200 cells 201-350 cells Above 350 cells |
275 255 292 |
33.45 31.02 35.52 |
|
Baseline WHO stage |
Stage I Stage II Stage III or IV |
229 369 244 |
27.20 43.82 28.98 |
|
Last known WHO stage |
Stage I Stage II Stage III or IV |
190 469 183 |
22.57 55.70 21.73 |
|
CPT prophylaxis (n=641) |
Yes No |
576 65 |
89.86 10.14 |
|
INH prophylaxis (n=579) |
Yes No |
487 92 |
84.11 15.89 |
|
OI at enrollment |
Yes No |
244 577 |
29.72 70.28 |
|
TB screening status at baseline |
Positive Negative |
144 667 |
18.67 81.33 |
|
Current TB status/last known/ |
Positive Negative |
99 743 |
11.76 88.24 |
|
Functional status (baseline) |
working Ambulatory Bed-ridden |
529 283 30 |
62.83 33.61 3.56 |
|
BMI |
<18.5 kg/m2 18.5-24.9 kg/m2 ≥ 25 kg/m2 |
206 510 126 |
24.47 60.57 14.96 |
|
Baseline adherence |
Good Fair poor |
485 207 150 |
57.60 24.58 17.81 |
|
last known adherence |
Good Fair poor |
541 210 90 |
64.33 24.97 10.70 |
Incidence of LTFU
Study subjects were followed for different periods of time with a median time of 28.7 months (IQR: 13.9-50.43) months. The minimum and maximum follow-up time was one month and 85.06 months respectively with a total observation time to be 2324.3 person-year. Among the 842 patients enrolled, 91 (10.81%) were dead, 122 (14.49%) had been LTFU, 114 (13.54%) were transferred out, furthermore 515 (61.16%) on treatment until December31, 2021 (Figure 2). The overall incidence of LTFU was 5.25 per 100 person-year (95% CI: 2.1–7.2). LTFU was highest in the second 12 months of ART follow-up, 7.4 per 100 person-year (95% CI: 5.4, 10.05).
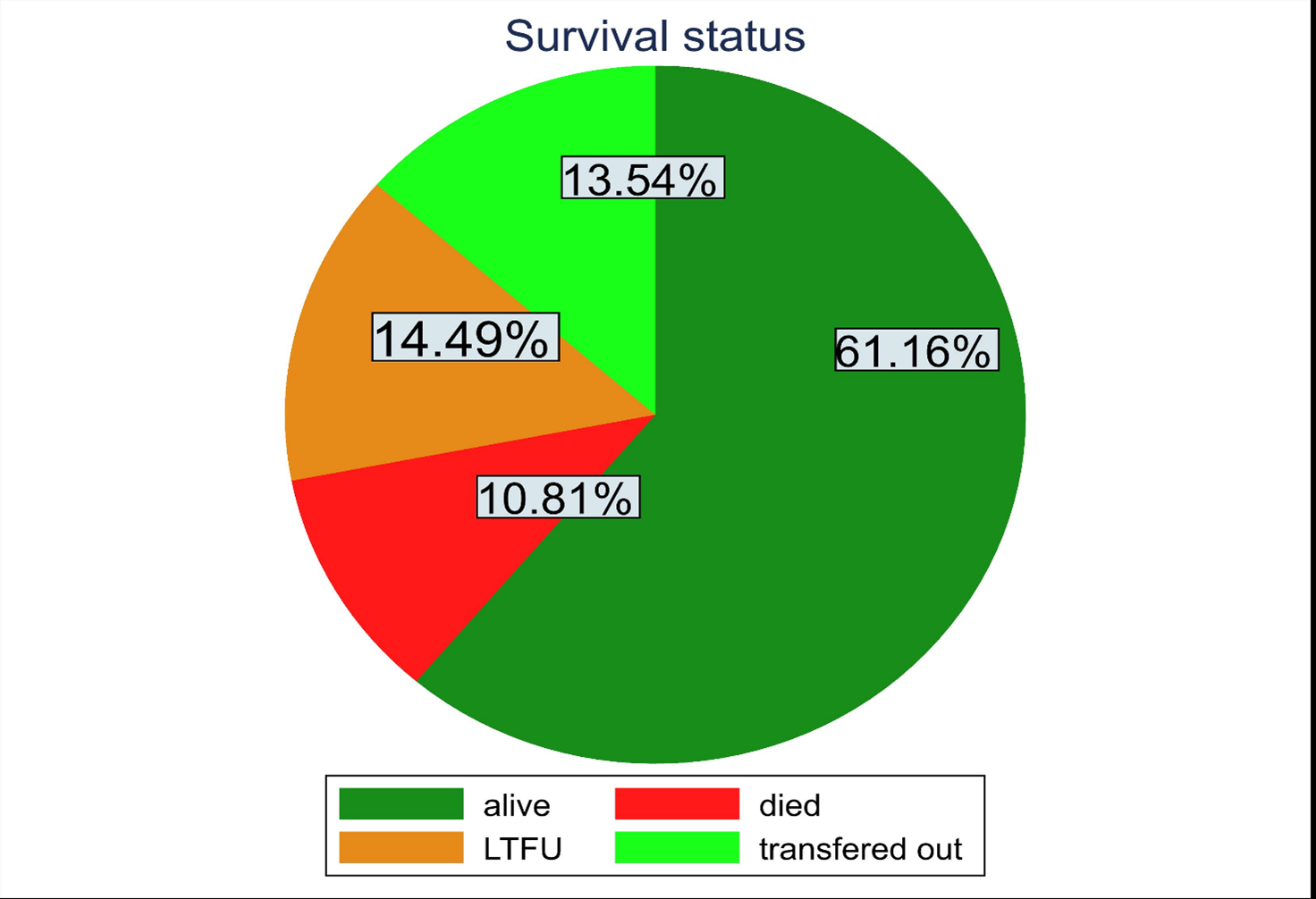
Figure 2. Pie chart proportion of survival status.
Non-parametric estimation of CIF of LTFU
CIFs across groups were checked statistically using Gray’s test (which is equivalent to the log-rank test of classical survival analysis) and graphically by plotting each predictor variables against failure time (Non parametrical checking).
Based on the result of the modified X2 test (Gray’s test), there was a significant difference in CIF among categories of age, marital status, occupation, education, IPT, CPT, Functional status, disclosure status, baseline WHO stage, caregiver, and type of regiment at start. Graphically, belonging to age 15-24, poor adherence level (baseline), non-disclosure status and WHO stage III &IV, male gender, and bedridden functional status were all risk factors for LTFU (Figure 3).
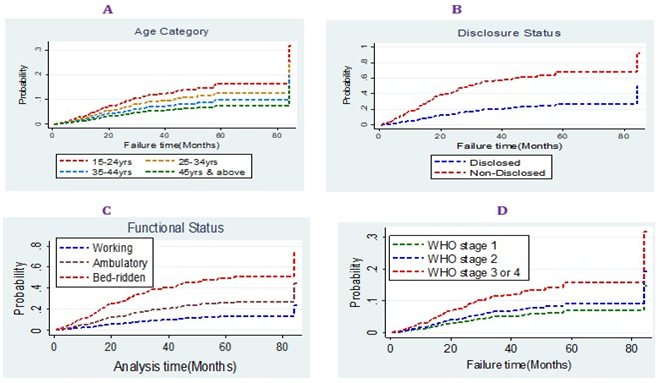
Figure 3. Graphical estimations of CIF among different categorical variables.
Modeling bivariable & multivariable competing risk regression model
After fitting a bivariable competing risk regression model almost all the predictor variables except educational status, occupation, opportunistic infection & INH prophylaxis, were crudely associated with LTFU at 0.2 level of significance. The Schoenfeld residuals and The Cox-Snell residuals (together with their Nelson-Aalen cumulative incidence function) were done to check the goodness fit test or model fitness (Figure 4). The sub-hazard proportionality assumption with the Schoenfeld residual of the global test was 0.3479, indicating no evidence for violation of the proportional sub-hazard assumption. Finally, those variables like: Functional status, disclosure status, hemoglobin, recent adherence level, and CPT prophylaxis, were found to be significant predictors for LTFU at 5% level of significance.
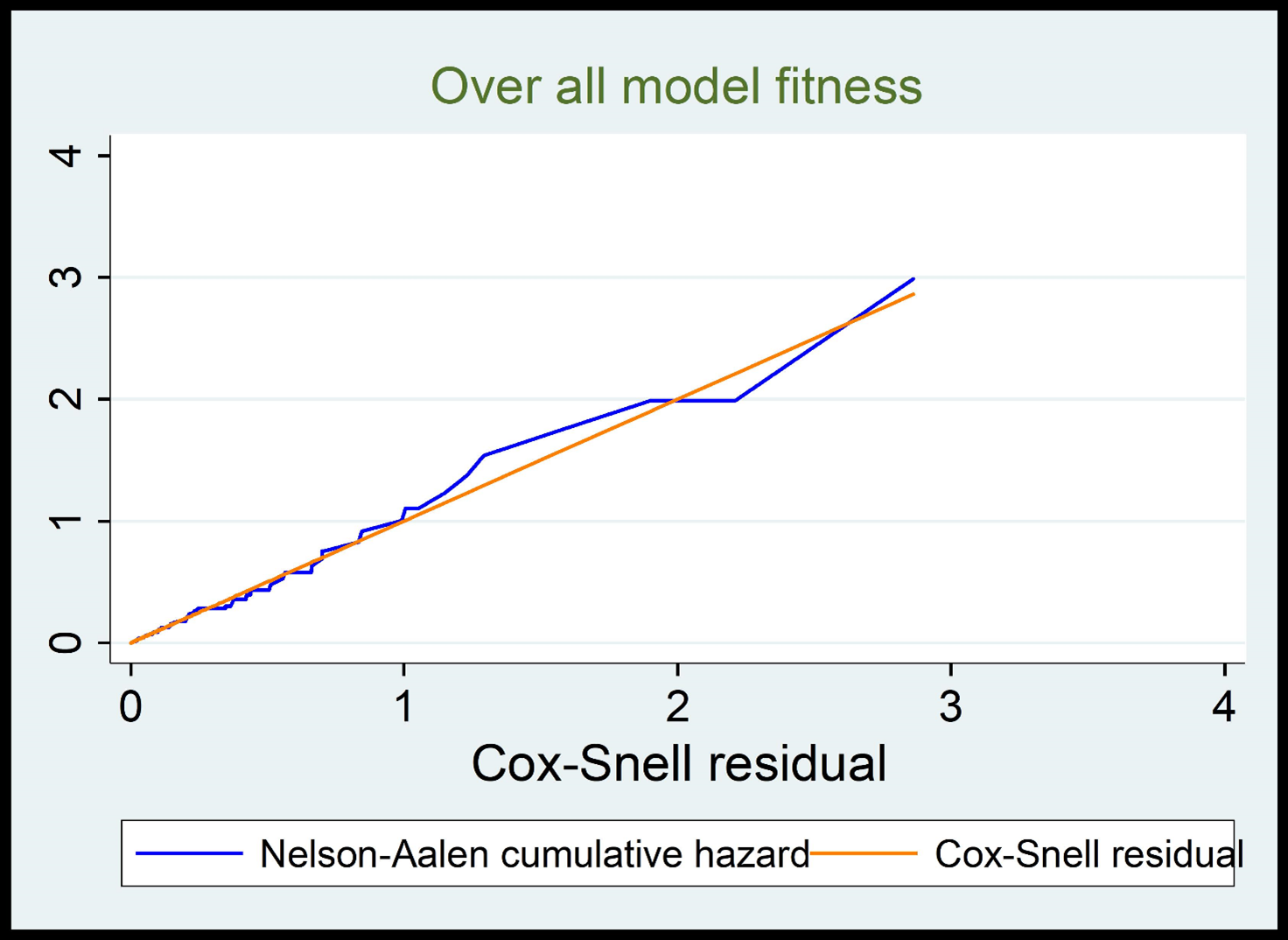
Figure 4. Cox Snell residual plot for model fitness.
In multivariable analysis disclosure status, adherence level (last known), CPT prophylaxis, functional status, hemoglobin level was statically significant for LTFU (p-value<0.05). The patients who did not disclose their HIV status can increase the sub-hazard of LTFU by 4.22 (asHR=4.22; 95%CI (2.11-8.47)) times compared with their counterparts. The sub-hazards of LTFU among the fair and poor level of recent adherence were 2.17(asHR=2.17; 95%CI (1.18-4.23)) and 2.27 (asHR=1.48; 95%CI (2.97-5.34)) times higher than those who have a good level of adherence respectively. The sub-hazard of LTFU is 2.47 higher among patients who do not take CPT prophylaxis 2.47 (asHR=2.47; 95%CI (2.99-6.15) compared with the counterparts. The sub-hazard of LTFU among patients with ambulatory functional status was 2.38 (asHR=2.38; 95%CI (1.21-4.68)) times higher compared with the patients in working functional status. The patient who had moderate and severe anemia increased the sub-hazard of LTFU by 2.22 (HR=2.22; 95%CI (1.09-4.52)) and 4.58 (asHR=4.58; 95% CI (1.28-11.39) times compared with that of those who had no anemia (Table 3).
|
Variables |
Categories |
Censored (515) |
LTFU (122) |
Competing event (205) |
cSHR (95% CI) |
aSHR (95% CI) |
|
Sex |
Female Male |
304 211 |
53 69 |
109 96 |
1 1.56 (1.02-2.33) |
1 1.37 (0.75-0.52) |
|
Age |
15-24 25-34 35-44 ≥ 45 |
53 152 187 123 |
16 43 37 26 |
21 70 74 40 |
1.66 (0.83-3.34) 1.29 (0.73-2.27) 0.95 (0.53-1.69) 1 |
2.03 (0.65-6.33) 1.57 (0.77-3.22) 0.81 (0.36-1.82) 1 |
|
Residence |
Urban Rural |
374 141 |
71 51 |
107 98 |
1 1.44 (0.95-2.18) |
1 0.91 (0.49-1.66) |
|
Marital status |
Single Married Divorced Others |
62 283 117 53 |
21 45 30 26 |
38 100 43 24 |
2.09 (1.17-3.72) 1 1.65 (0.97-2.81) 2.37 (1.34-4.19) |
0.98 (0.39-2.46) 1 1.25 (0.59-2.63) 1.23 (0.43-3.56) |
|
Functional status |
Working Ambulatory Bedridden |
369 139 7 |
46 70 6 |
114 74 17 |
1 4.34 (2.82-6.67) 2.31 (0.82-6.49) |
1 2.38 (1.21-4.68)* 2.66 (0.70-10.04) |
|
BMI |
Below 18.5 18.5-24.9 Above 25 |
98 342 75 |
28 68 26 |
80 100 25 |
0.95 (0.56-1.61) 1 1.65 (1.02-2.72) |
0.78 (0.33-1.85) 1 1.63 (0.84-3.14) |
|
Care giver |
Yes No |
495 19 |
97 25 |
167 38 |
1 2.55 (1.53-4.24) |
1 0.59 (0.26-1.32) |
|
Disclosure status |
Yes No |
458 56 |
65 57 |
150 55 |
1 4.12 (2.73-6.19) |
1 4.22 (2.11-8.47)** |
|
TB-Screening status |
Positive Negative |
76 438 |
31 91 |
50 155 |
1.49 (0.93-2.39) 1 |
0.56 (0.25-1.25) 1 |
|
CD4 count (baseline) |
Below 200 cells 201-350 cells Above 350 cells |
115 158 226 |
53 43 23 |
107 54 43 |
2.14 (1.24-3.69) 2.46 (1.43- 4.23) 1 |
0.77 (0.31-1.89) 1.05 (0.49-2.22) 1 |
|
WHO stage (baseline) |
Stage one Stage two Stage 3 or 4 |
165 246 104 |
17 42 63 |
47 81 77 |
1 1.51 (0.79-2.86) 3.77 (2.02-7.05) |
1 0.99 (0.39-2.53) 2.08 (0.58-7.45) |
|
WHO stage (recent) |
Stage one Stage two Stage 3 or 4 |
119 325 71 |
15 59 48 |
56 85 64 |
1 1.52 (0.80-2.87) 3.45 (1.77- 6.73) |
1 0.83 (0.34-2.03) 0.49 (0.17-1.40) |
|
Hemoglobin level |
Severe anemia Moderate anemia Mild anemia No anemia |
26 135 126 226 |
20 59 21 21 |
10 71 51 72 |
7.81 (4.0-15.20) 3.18 (1.83-5.52) 1.64 (0.84-3.22) 1 |
4.58 (1.28-16.39)* 2.22 (1.09-4.52)* 1.24 (0.54-2.84) 1 |
|
Adherence level (baseline) |
Good Fair Poor |
358 99 58 |
49 41 32 |
78 67 60 |
1 2.39 (1.49-3.83) 2.02 (1.21-3.38) |
1 1.74 (0.79-3.86) 1.34 (0.62-2.89) |
|
Adherence level (recent) |
Good Fair Poor |
387 102 26 |
49 46 26 |
105 62 38 |
1 2.99 (1.89-4.75) 3.63 (2.11-6.25) |
1 2.17 (1.11-4.23)* 2.27 (2.97-5.34)* |
|
CPT prophylaxis |
Yes No |
357 45 |
67 12 |
152 8 |
1 1.43 (0.67-3.09) |
1 2.47 (2.99-6.15)* |
|
**= p value<0.01, *= p value<0.05 |
||||||
Discussion
In this study, we assessed the incidence and predictors of LTFU among adult HIV/AIDS patients under ART follow-up in Jigjiga Governmental Hospitals. A number of variables were used to explain the variation in the LTFU of HIV/AIDS patients using this competing risk regression model. In our study, we tried to assess socio-demographic, clinical, and treatment-related factors by using secondary data.
The estimated Incidence rate for LTFU was 5.25 per 100-person year observation. It is similar to the finding of a study conducted in different places in Ethiopia [18,21,23] Zimbabwe [14] and Zambia [47]. This might be due to the similarity in the quality of care given according to ART guidelines for HIV/AIDS patients across these different Hospitals since all study areas were in eastern African regions, have almost consistent guidelines prepared by WHO.
However, the current finding is higher than the findings in Debre Markos [24], Kenya [13], Republic of Democratic Congo [48]. This may be due to the study areas being located around the capital city of the region and the majority of the respondents were rural residents, so the flow of the population around the city is somehow low. In addition, the area is located at a small distance from the Ethio-somaliland border, and many of the long-track drivers and emigrants have joined the city most of the time. This also could be due to variations in study design, patient follow-ups, and definitions of LTFU is defined when a patient is lost for at least 90 days since some studies defined LTFU as 180 days [32,40].
The current finding is lower than the findings in Myanmar [37], Guinea-Bisaw [8], South- Africa [34], Cameroon [29], Nigeria [30], Uganda [42], Malawi [33], Ethiopia (mekelle, Jigjiga, Pawi, Gondar) [9,19,20,23]. The difference might be due to variations in study design and the operationalization of terms, as we know that our model is a competing risk regression model, majority of the preceding studies were classical survival analysis, so basically, it makes overestimate the incidence rate. Patients who were not taking CPT were more likely to be lost from ART care and this result is consistent with the study conducted in Myanmar [37], Gondar [16], and Tepi [43]. This might be due to the fact that CPT, given for the prevention of many opportunistic infections such as pneumocystis pneumonia, toxoplasmosis, bacterial infections, and diarrheal diseases [5] results from the patient feels better and intend to attend their schedule of ART follow-up accordingly. In contrast, patients who are not taking CPT, are more vulnerable to many opportunistic infections and finally, they may have such diseases and either they may develop drug toxicity due to drug-drug interaction or they may prefer to go to Traditional healers (Holly water) by discontinuing such burden of drugs and finally end up with LFTU or Death [10,31].
Patients who were in ambulatory functional status increased the risk of LTFU, this finding is in agreement with the study conducted in Indonesia [44], and different studies in Ethiopia [16,17,19,30]. This may be due to ambulatory patients being more likely to be LTFU could be due they become poor performance status at the initiation and due to the social, economic, and financial influences that are caused by their inability to work, this may lead to enabling to staying to the care [17]. The sub-hazard of LFTU was higher among patients with a fair and poor level of adherence, this is in agreement with studies in Gunie-Bisaw [8], Zimbabwe [14], Uganda [41], Ethiopia [17,18,31], this may be due to that the patients with the poor level of adherence for ART drug become interrupt the treatment schedule which leads to resisting to the virus. This treatment interruption make fail to suppress the viral load and become prone to poor treatment outcome like treatment failure, this leads the patient to lose hope for a good prognosis, and He/she prefers to lost from care after all the outcome turned to death [4,7].
The patients with severe and moderate anemia status were more likely to LFTU compared with their counterparts, this finding is in agreement with studies conducted in Mynamar [37], Togo [40], Mizan-Teferi [22], the possible reasons for this finding might be the azidothymidine (AZT) based regimen have an interruption on bone marrow function and it becomes resolved as time goes and can be treatable as easy, but the patients who develop anemia due to this AZT based regimen lack their trust about the treatment and they hesitate to continue the care [21]. The patient with severe anemia at baseline hope that immediate resolution by ART treatment and if the expectation is not soon, then they try other medication like traditional healer and they become lost from the care. Patients who did not disclose their HIV/AIDS status were 2.27 times more at the risk of lost from the treatment program as compared to their counter parts. This study is similar with studies conducted in South Africa [37], Tepi [43], Oromiya region [28]. This might be due to that patients may remain on treatment much more if they disclose their status and they had someone else to share their ideas and feelings about the treatment and the diseases condition. Since staying on treatment needs intensive support and care from different parts of the community as well [3]. This study has some limitations because of the retrospective nature of the study, which lacks completeness of some potentially important predictors (patient information) like substance use. Since the study uses baseline socio-demographic and clinical-related factors, there may be a change of these variables (change of exposure variable) after a time.
Conclusion
In this study, the incidence of LTFU was one person lost from twenty patients within a one-year follow-up time. For examining the predictor variables of LTFU, competing risk regression analysis was done considering death and transferred out as competing events. After all, patients on ART who disclosed their HIV status, not taking CPT prophylaxis, being ambulatory functional status, those have moderate to severe anemia at baseline, and have a poor level of adherence to ART were at higher risk for LTFU. Therefore, giving more consideration and close follow-up of these high-risk groups could reduce the rate of LTFU.
Competing Interests
All the authors declared that they have no competing interest.
Authors’ Contributions
GSA: conception of the research idea, study design, data collection, analysis and interpretation, and manuscript write-up. KAG, AML, FMA, and YMC: analysis and interpretation, manuscript write-up, Writing – review & editing, visualization. All authors have read, validate, and approved the final manuscript.
Acknowledgments
I would like to express my deepest appreciation and special admiration for Jigjiga city Governmental Hospital administrative bodies and chart room experts for their cooperation as well as their permission to access this ART data. We are also thankful to the health professionals who work in the ART clinic and ART data clerk managers for giving relevant information. Finally, we would like to thank the data collectors and the supervisor for their tolerance and commitment to the data collection and research writing process.
Availability of Data and Materials
Please contact the author for further dataset requests.
References
2. UNAIDS. “Global HIV statistics,” Fact Sheet – WORLD AIDS DAY 2021 Global, 2021, no. June, pp. 1–6.
3. GLOBAL AIDS UPDATE, EXECUTIVE SUMMARY SEIZING THE MOMENT, 2020.
4. UNAIDS and AIDSinfo, “Country factsheets Mozambique 2020 HIV and AIDS Estimates Adults and children living with Country factsheets Mozambique 2020 HIV testing and treatment cascade People living with HIV Coverage of adults and children,” UNAIDS, pp. 1–6, 2021.
5. "UNAIDS data 2021," 2021.
6. Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PloS One. 2009 Jun 4;4(6):e5790.
7. Ndiaye B, Ould-Kaci K, Salleron J, Bataille P, Bonnevie F, Choisy P, et al. Incidence rate and risk factors for loss to follow-up in HIV-infected patients from five French clinical centres in Northern France–January 1997 to December 2006. Antiviral Therapy. 2009 May;14(4):567-75.
8. Hønge BL, Jespersen S, Nordentoft PB, Medina C, da Silva D, da Silva ZJ, et al. Loss to follow-up occurs at all stages in the diagnostic and follow-up period among HIV-infected patients in Guinea-Bissau: a 7-year retrospective cohort study. BMJ Open. 2013 Oct 1;3(10):e003499.
9. Assemie MA, Muchie KF, Ayele TA. Incidence and predictors of loss to follow up among HIV infected adults at Pawi General Hospital, northwest Ethiopia : competing risk regression model. BMC Research Notes. 2018;11:287.
10. Ayele W, Mulugeta A, Desta A, Rabito FA. Treatment outcomes and their determinants in HIV patients on Anti-retroviral Treatment Program in selected health facilities of Kembata and Hadiya zones, Southern Nations, Nationalities and Peoples Region, Ethiopia. BMC Public Health. 2015 Dec;15:1-3.
11. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. American Journal of Epidemiology. 2009 Jul 15;170(2):244-56.
12. Mohammad KA, Fatima-Tuz-Zahura M, Bari W. Fine and Gray competing risk regression model to study the cause-specific under-five child mortality in Bangladesh. BMC International Health and Human Rights. 2017 Dec;17(1):1-8.
13. M. C. N, "University of Nairobi School of Mathematics," no. 25, 2020.
14. Zingoni ZM, Chirwa T, Todd J, Musenge E. Competing risk of mortality on loss to follow-up outcome among patients with HIV on ART: a retrospective cohort study from the Zimbabwe national ART programme. BMJ Open. 2020 Oct 1;10(10):e036136.
15. Odafe S, Idoko O, Badru T, Aiyenigba B, Suzuki C, Khamofu H, et al. Patients’ demographic and clinical characteristics and level of care associated with lost to follow‐up and mortality in adult patients on first‐line ART in Nigerian hospitals. Journal of The International AIDS Society. 2012 Apr;15(2):17424.
16. Teshale AB, Tsegaye AT, Wolde HF. Incidence and predictors of loss to follow up among adult HIV patients on antiretroviral therapy in University of Gondar Comprehensive Specialized Hospital: A competing risk regression modeling. PloS One. 2020 Jan 24;15(1):e0227473.
17. Mekonnen N, Abdulkadir M, Shumetie E, Baraki AG, Yenit MK. Incidence and predictors of loss to follow-up among HIV infected adults after initiation of first line anti-retroviral therapy at University of Gondar comprehensive specialized Hospital Northwest Ethiopia, 2018: retrospective follow up study. BMC Research Notes. 2019 Feb 28;12(1):111.
18. Gebremichael MA, Gurara MK, Weldehawaryat HN, Mengesha MM, Berbada DA. Predictors of Loss to Follow-Up among HIV-Infected Adults after Initiation of the First-Line Antiretroviral Therapy at Arba Minch General Hospital, Southern Ethiopia: A 5-Year Retrospective Cohort Study. BioMed Research International. 2021 Nov 11;2021.
19. Wubshet M, Berhane Y, Worku A, Kebede Y, Diro E. High loss to followup and early mortality create substantial reduction in patient retention at antiretroviral treatment program in north-west Ethiopia. International Scholarly Research Notices. 2012;2012.
20. Seifu W, Ali W, Meresa B. Predictors of loss to follow up among adult clients attending antiretroviral treatment at Karamara general hospital, Jigjiga town, Eastern Ethiopia, 2015: a retrospective cohort study. BMC Infectious Diseases. 2018 Dec;18(1):1-8.
21. Dessu S, Mesele M, Habte A, Dawit Z. Time until loss to follow-up, incidence, and predictors among adults taking ART at public hospitals in Southern Ethiopia. HIV/AIDS-Research and Palliative Care. 2021 Feb 17:205-15.
22. Berheto TM, Haile DB, Mohammed S. Predictors of loss to follow-up in patients living with HIV/AIDS after initiation of antiretroviral therapy. North American Journal of Medical Sciences. 2014 Sep;6(9):453.
23. Gezae KE, Abebe HT, Gebretsadik LG. Incidence and predictors of LTFU among adults with TB/HIV co-infection in two governmental hospitals, Mekelle, Ethiopia, 2009-2016: survival model approach. BMC Infectious Diseases. 2019 Dec;19(1):1-9.
24. Birhanu MY, Leshargie CT, Alebel A, Wagnew F, Siferih M, Gebre T, Kibret GD. Incidence and predictors of loss to follow-up among HIV-positive adults in northwest Ethiopia: a retrospective cohort study. Tropical Medicine and Health. 2020 Dec;48:78.
25. Mutasa-Apollo T, Shiraishi RW, Takarinda KC, Dzangare J, Mugurungi O, Murungu J, et al. Patient retention, clinical outcomes and attrition-associated factors of HIV-infected patients enrolled in Zimbabwe's National Antiretroviral Therapy Programme, 2007-2010. PloS One. 2014 Jan 29;9(1):e86305.
26. Agaba PA, Meloni ST, Sule HM, Agbaji OO, Sagay AS, Okonkwo P, et al. Treatment outcomes among older human immunodeficiency virus-infected adults in Nigeria. Open Forum Infectious Diseases. 2017 Apr 1;4(2):ofx031.
27. Alvarez-Uria G, Naik PK, Pakam R, Midde M. Factors associated with attrition, mortality, and loss to follow up after antiretroviral therapy initiation: data from an HIV cohort study in India. Global Health Action. 2013 Dec 1;6(1):21682.
28. Megerso A, Garoma S, Eticha T, Workineh T, Daba S, Tarekegn M, et al. Predictors of loss to follow-up in antiretroviral treatment for adult patients in the Oromia region, Ethiopia. HIV/AIDS-Research and Palliative Care. 2016 Apr 26:83-92.
29. Bekolo CE, Webster J, Batenganya M, Sume GE, Kollo B. Trends in mortality and loss to follow-up in HIV care at the Nkongsamba Regional hospital , Cameroon. BMC Research Notes. 2013;6(1):512.
30. Odafe S, Torpey K, Khamofu H, Ogbanufe O, Oladele EA, Kuti O, et al. The pattern of attrition from an antiretroviral treatment program in Nigeria. PLoS One. 2012 Dec 13;7(12):e51254.
31. Carriquiry G, Fink V, Koethe JR, Giganti MJ, Jayathilake K, Blevins M, et al. Mortality and loss to follow‐up among HIV‐infected persons on long‐term antiretroviral therapy in Latin America and the Caribbean. Journal of The International AIDS Society. 2015 Jan;18(1):20016.
32. Hassan AS, Mwaringa SM, Ndirangu KK, Sanders EJ, de Wit TF, Berkley JA. Incidence and predictors of attrition from antiretroviral care among adults in a rural HIV clinic in Coastal Kenya: a retrospective cohort study. BMC Public Health. 2015 Dec;15:1-9.
33. Tweya H, Oboho IK, Gugsa ST, Phiri S, Rambiki E, Banda R, et al. Loss to follow-up before and after initiation of antiretroviral therapy in HIV facilities in Lilongwe, Malawi. PloS One. 2018 Jan 26;13(1):e0188488.
34. Mberi MN, Kuonza LR, Dube NM, Nattey C, Manda S, Summers R. Determinants of loss to follow-up in patients on antiretroviral treatment, South Africa, 2004-2012: a cohort study. BMC Health Services Research. 2015 Dec;15(1):1-1.
35. Bucciardini R, Fragola V, Abegaz T, Lucattini S, Halifom A, Tadesse E, et al. Retention in care of adult HIV patients initiating antiretroviral therapy in Tigray, Ethiopia: a prospective observational cohort study. PloS One. 2015 Sep 4;10(9):e0136117.
36. Meloni ST, Chang C, Chaplin B, Rawizza H, Jolayemi O, Banigbe B, et al. Time-dependent predictors of loss to follow-up in a large HIV treatment cohort in Nigeria. InOpen forum infectious diseases 2014 Sep 1 (Vol. 1, No. 2). Oxford University Press.
37. Thida A, Tun ST, Zaw SK, Lover AA, Cavailler P, Chunn J, et al. Retention and risk factors for attrition in a large public health ART program in Myanmar: a retrospective cohort analysis. PLoS One. 2014 Sep 30;9(9):e108615.
38. Onoka CA, Uzochukwu BS, Onwujekwe OE, Chukwuka C, Ilozumba J, Onyedum C, et al. Retention and loss to follow-up in antiretroviral treatment programmes in southeast Nigeria. Pathogens and Global Health. 2012 Mar 1;106(1):46-54.
39. Khumalo PG, Chou YJ, Pu C. Antiretroviral treatment attrition in Swaziland: a population-based study. Epidemiology & Infection. 2016 Dec;144(16):3474-82.
40. Saka B, Landoh DE, Patassi A, d'Almeida S, Singo A, Gessner BD, et al. Loss of HIV-infected patients on potent antiretroviral therapy programs in Togo: risk factors and the fate of these patients. Pan African Medical Journal. 2013;15(1).
41. Kiwanuka J, Mukulu Waila J, Muhindo Kahungu M, Kitonsa J, Kiwanuka N. Determinants of loss to follow-up among HIV positive patients receiving antiretroviral therapy in a test and treat setting: A retrospective cohort study in Masaka, Uganda. PLoS One. 2020 Apr 7;15(4):e0217606.
42. Nuwagira E, Lumori BA, Muhindo R, Kanyesigye M, Amir A, Muyindike W, et al. Incidence and predictors of early loss to follow up among patients initiated on protease inhibitor-based second-line antiretroviral therapy in southwestern Uganda. AIDS Research and Therapy. 2021 Dec;18(1):1-9.
43. Shaweno T, Asefa H, Gesesew HA. Time to Attrition and Associated Factors among Adults Enrolled In Pre-Anti-Retroviral Therapy Care in Tepi General Hospital, Ethiopia. Austin Journal of HIV/AIDS Research. 2018;5(1):1041.
44. Ambarwati RD, Wardani HE, Tama TD. Functional Status and Incidence of Loss to Follow-up after Antiretroviral Therapy Initiation. KnE Life Sciences. 2021 Mar 25:312-21.
45. Participant Manual. National Comprehensive HIV Prevention, Care and Treatment Training for Healthcare Providers. 2021.
46. Participant Manual. National Comprehensive HIV Prevention, Care and Treatment Training for Healthcare Providers. 2017.
47. Zhou J, Tanuma J, Chaiwarith R, Lee CK, Law MG, Kumarasamy N, et al. Loss to followup in HIV-infected patients from Asia-Pacific region: results from TAHOD. AIDS Research and Treatment. 2012 Feb 22;2012.
48. Akilimali PZ, Musumari PM, Kashala-Abotnes E, Kayembe PK, Lepira FB, Mutombo PB, et al. Disclosure of HIV status and its impact on the loss in the follow-up of HIV-infected patients on potent anti-retroviral therapy programs in a (post-) conflict setting: A retrospective cohort study from Goma, Democratic Republic of Congo. PloS One. 2017 Feb 7;12(2):e0171407.
