Abstract
Langerhans cell histiocytosis (LCH) is a rare, clonal, haematological disease of myeloid origin involving infiltration of neoplastic cells resembling Langerhans cells. The classification of histiocytoses has recently been revised. LCH belonging to the “L” group harbor BRAF mutations or other mutations involved in the MAPK pathway. We describe a case of a 73-year-old male presenting with LCH affecting the skin and concomitant chronic myelomonocytic leukaemia (CMML). NGS revealed TET2 and DNMT3A mutations in the bone marrow, which are well characterized mutations in CMML. In LCH skin lesions, TET2, DNMT3A, and a BRAF mutation, were found. The findings indicate potential coevolution of CMML and LCH from a common haematopoietic progenitor cell. The patient’s skin lesion were refractory to conventional skin treatment. Because skin manifestations in CMML are associated with aggressive disease progression the patient initiated azacitidine (AZA). This led to marked improvement of the debilitating skin affliction suggesting that AZA may be a novel effective treatment modality for LCH with refractory skin involvement.
Keywords
Histiocytoses, LCH, CMML, Azacitidine
Introduction
Langerhans cell histiocytosis (LCH) is a rare, clonal, haematological disease of myeloid origin involving infiltration of neoplastic cells resembling Langerhans cells in various tissues [1,2]. LCH cells express normal Langerhans cell markers such as CD1a, Langerin (CD207), and S100 [2,3]. LCH predominates in children and common manifestations are in bones and skin, although any organ can be involved [4,5]. The latest revision of the classification of histiocytoses by the Histiocyte Society divides histocytoses into five major groups based on clinical, histologic, immunophenotypic, and molecular characteristics [6]. In this classification LCH and Erdheim-Chester disease (ECD), belong to the so-called ”L” (Langerhans) group due to a common denominator in the convergence on clonal mutations in genes activating the MAPK pathway including BRAFV600E mutations present in about half of the cases [7-10]. Haematopoietic stem cells (HSC) give rise to dendritic cells and monocyte-derived cells and histiocytic skin manifestations can rarely present concomitant with clonal myeloid haematopoietic malignancies [6].
Here we present a case of 73-year-old male presenting with severe skin affection caused by LCH and concomitant chronic myelomonocytic leukaemia (CMML) who showed marked improvement of skin manifestations after initiation of 5-azacitidine.
Case Report
A 73-year old man was referred to additional dermatological evaluation because of recurrent papules, abscesses, and ulcerations involving axillae, groins, palms, and perianally, which had been present for a year. Initially the findings had been interpreted as hidrosadenitis suppurativa and treated with topical and oral antibiotics. A skin biopsy taken before referral described unspecific inflammation. The patient was admitted to hospital due to abdominal pain and fever. A CT scan revealed splenomegaly and a subcapsular haematoma in the right kidney. A complete blood count with differential showed hemoglobin of 11.6 g/dL, white blood cell count (WBC) of 12.9 k/µL, and a platelet count of 75 K/µL. Monocytes were elevated to 1.97 K/µL. C-reactive protein (CRP) was 243 mg/L. Clinically the patient improved on intravenous antibiotics and CRP levels normalized gradually. The spleen measured 18 cm on the longest axis with several hypodense areas suggestive of haemangiomas, lymphoma, or other malignancy. The haematological findings instigated a haematological work up. A bone marrow (BM) biopsy was performed, showing a hypercellular marrow with left-shifted and slightly dysmorphic granulopoiesis as well as elevated monocyte and megakaryocyte levels. CMML was suspected although criteria were not fully met. Next generation sequencing (NGS) of the bone marrow showed two different TET2 mutations and a DNMT3A mutation with an allelic ratio of 54%, 46%, and 44% respectively. The karyotype was normal. The patients’ multiple skin lesions were PET-positive, but the scan did not indicate lymphoma. Skin biopsies from the abdomen and groins revealed a hyperplastic, acanthotic, and reactive epithelium. In the epidermis and superficial dermis single or grouped, medium to large, cells with oval nuclei and varying numbers of nucleoli were identified. Some of the cells presented with inclusions. The cells were positive for CD1a, Langerin, S100, and BRAF (Figure 1). Proliferation based on Ki-67-staining was above 70%. Additionally, in the superficial dermis a mixed inflammatory infiltrate could be found including small T cells and polyclonal plasma cells. Low numbers of CD68 and CD14 positive mature monocytes as well as eosinophils and B cells were also present. Based on these finding the patient was diagnosed with LCH in the skin. Unfortunately, NGS on the skin biopsy failed due to inadequate quality of the formalin fixed paraffin embedded tissue. Revision of the previous skin biopsy, performed a year earlier, was in accordance with the LCH diagnosis and revision of the bone marrow revealed no neoplastic Langerhans cells confirmed by immunohistochemical staining for CD1a, Langerin, and BRAF.
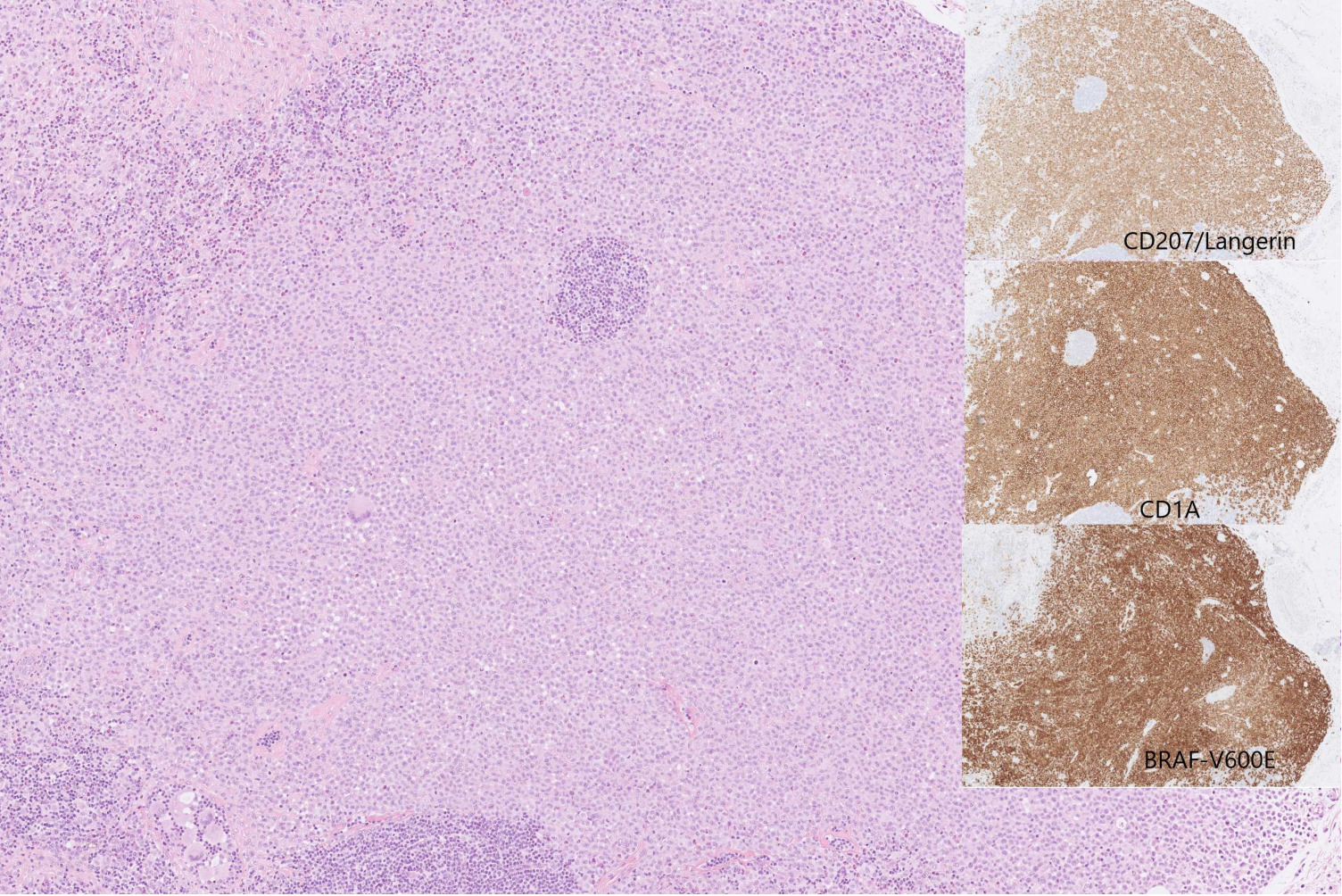
Figure 1. Histopathological examination. Skin biopsy heavily infiltrated by Langerhans cells with oval nuclei and moderately abundant cytoplasm surrounded by small lymphocytes, histiocytes and eosinofils. Small insert: immunohistochemical examination showed expression of CD1A, langerin (CD207), S100 (not shown), and BRAF-V600E.
After the LCH diagnosis a new work-up, including BM biopsy, PET-CT scan, and MRI of the cerebrum, was performed. This led to diagnosis of CMML type 0 in the BM, but still no LCH cells were found in the BM. PET-CT did not reveal novel findings aside from regression of the previously described subcapsular haematoma in the kidney. Neither did MRI reveal any pathological findings including pituitary enlargement. Because skin manifestations in CMML may indicate aggressive disease progression the patient was recommended initiation of 5-azacitidine (AZA) treatment. Because of religious affiliation the patient did not wish to accept transfusion in case of severe cytopenias nor did he accept treatment with AZA. Despite of oral prednisolone, topical corticosteroid, and potassium permanganate baths, the skin involvement gradually worsened, particularly on the hands, and he developed external otitis. After approximately six months the patient accepted treatment with AZA. Following the first cycle of AZA the patient experienced significant improvement of skin lesions which continued to improve following subsequent cycles. Concomitant dermatological treatment was gradually reduced. Treatment was well tolerated without side effects. Platelet count decreased marginally and monocyte levels normalized. Following 8 cycles of AZA, a new PET-CT was performed showing significant reduction in FDG-positivity of the involved skin lesions. Splenomegaly was unchanged. A deeper surgical biopsy was taken from a skin area with continued FDG-positivity. Morphology and IHC was consistent with persisting LCH. NGS performed on fresh frozen tissue from the lesion identified the TET2 and DNMT3A mutations found in the BM. Allelic burden was between 4 and 8%. In addition a novel TET2 mutation was identified in the skin biopsy as well as a BRAF mutation just below detection limit. Reevaluation of NGS raw data from the BM revealed no BRAF mutation. The patient received a total of 12 courses of AZA and experienced significant improvement of the skin particularly of palms (Figure 2). Due to muscle aches, which the patient ascribed to AZA, treatment was stopped. Two months later the skin involvement was progressing and he is now treated with oral methotrexate.
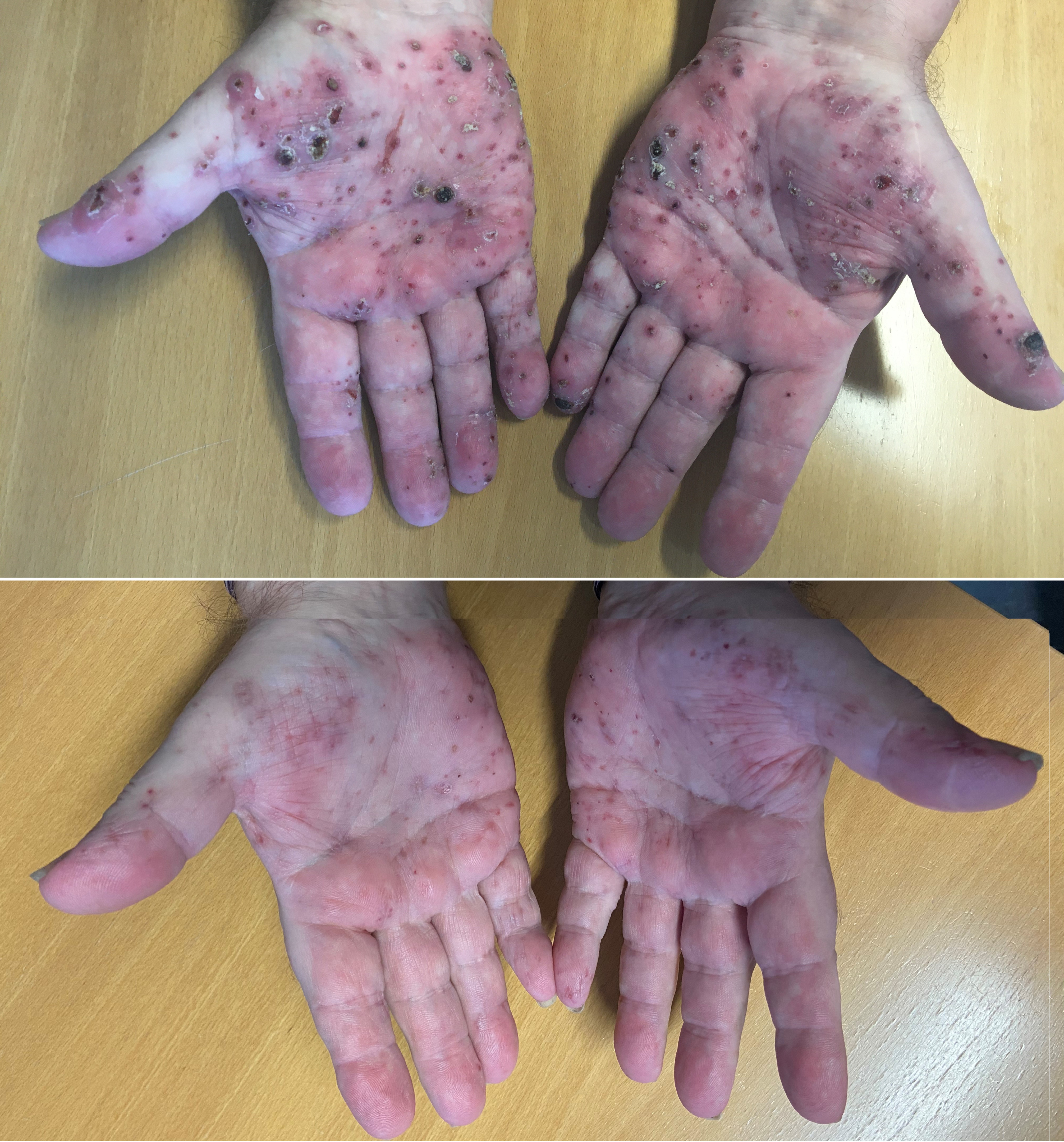
Figure 2. Remission of palmar Langerhans cell histiocytoses skin manifestations during treatment with 5-azacitidine (AZA). The patient initiated treatment with AZA in August 2020. Top picture: August 2020, prior to therapy. Bottom picture: December 2020, following 5 courses of AZA.
Discussion
Skin involvement of CMML is seen in approximately 10% of patients and has been linked to disease progression and leukaemic transformation [11]. Furthermore, histiocytic disorders have been associated with concomitant clonal haematopoietic disease. A Dutch study identified three patients with histocytic sarcoma, indeterminate histiocytosis, and ECD bearing NRAS or KRAS mutations that were also present in the patients’ concomitant CMML or AML [12]. In another report, both TET2 and BRAF mutations were found in a patient with acute myelomonocytic leukaemia and mixed histiocytosis, and TET2 was found to precede the BRAF mutation.[13] In the present report, the patient was diagnosed with LCH and CMML concomitantly, thus representing a case of LCH associated with a myeloproliferative/myelodysplastic disorder. Despite of progression of the skin involvement, bone marrow function remained stable. NGS revealed TET2 and DNMT3A mutations in the BM, which are well characterized mutations in CMML. TET2 mutations have been associated with favorable outcomes in CMML in the absence of ASXL1 mutations [14]. DNMT3A, on the other hand, has been associated with inferior outcomes [15]. Following 8 cycles of AZA a larger skin excision biopsy from an area with continued PET positive activity confirmed the continued presence LCH in the skin. NGS performed on the skin biopsy revealed the same mutations found in the BM and an additional TET2 and BRAF mutation.
To our knowledge, this is the first report of a patient with LCH with primary skin involvement and concomitant CMML who was treated with AZA. The patient had debilitating skin involvement, which improved significantly on AZA. A previous report described a CMML patient that developed skin eruptions, splenomegaly, and lymphadenopathy following 36 cycles of AZA [16]. Punch biopsies revealed an indeterminate dendritic cell tumor and NGS found a KRAS mutations in the skin lesions, which had previously been found in the bone marrow.
In conclusion, we present a patient with potential coevolution of CMML and LCH sharing several mutations suggesting a common haematopoietic progenitor cell which is in concordance with findings by others. We further propose that AZA may be a novel effective treatment modality for LCH with refractory skin involvement.
Conflicts of Interest
DLH: research grant from Novartis and Alexion, conference admission from EUSA pharma.
The remaining authors have no conflicts of interest to declare.
Author Contributions
All authors have contributed to the manuscript, read the manuscript, and approved its submission to the Journal of Clinical Haematology.
References
2. Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, et al. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med. 1994;331(3):154-60.
3. Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12(1):71-81.
4. Guyot-Goubin A, Donadieu J, Barkaoui M, Bellec S, Thomas C, Clavel J. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000-2004. Pediatr Blood Cancer. 2008;51(1):71-5.
5. Kobayashi M, Tojo A. Langerhans cell histiocytosis in adults: Advances in pathophysiology and treatment. Cancer Sci. 2018;109(12):3707-13.
6. Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672-81.
7. Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116(11):1919-23.
8. Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700-3.
9. Emile JF, Diamond EL, Hélias-Rodzewicz Z, Cohen-Aubart F, Charlotte F, Hyman DM, et al. Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood. 2014;124(19):3016-9.
10. Diamond EL, Durham BH, Haroche J, Yao Z, Ma J, Parikh SA, et al. Diverse and Targetable Kinase Alterations Drive Histiocytic Neoplasms. Cancer Discov. 2016;6(2):154-65.
11. Mathew RA, Bennett JM, Liu JJ, Komrokji RS, Lancet JE, Naghashpour M, et al. Cutaneous manifestations in CMML: Indication of disease acceleration or transformation to AML and review of the literature. Leuk Res. 2012;36(1):72-80.
12. Kemps PG, Hebeda KM, Pals ST, Verdijk RM, Lam KH, Bruggink AH, et al. Spectrum of histiocytic neoplasms associated with diverse haematological malignancies bearing the same oncogenic mutation. J Pathol Clin Res. 2021;7(1):10-26.
13. Durham BH, Roos-Weil D, Baillou C, Cohen-Aubart F, Yoshimi A, Miyara M, et al. Functional evidence for derivation of systemic histiocytic neoplasms from hematopoietic stem/progenitor cells. Blood. 2017;130(2):176-80.
14. Patnaik MM, Zahid MF, Lasho TL, Finke C, Ketterling RL, Gangat N, et al. Number and type of TET2 mutations in chronic myelomonocytic leukemia and their clinical relevance. Blood Cancer J. 2016;6(9):e472.
15. Patnaik MM, Barraco D, Lasho TL, Finke CM, Hanson CA, Ketterling RP, et al. DNMT3A mutations are associated with inferior overall and leukemia-free survival in chronic myelomonocytic leukemia. Am J Hematol. 2017;92(1):56-61.
16. Loghavi S, Curry JL, Garcia-Manero G, Patel KP, Xu J, Khoury JD, et al. Chronic myelomonocytic leukemia masquerading as cutaneous indeterminate dendritic cell tumor: Expanding the spectrum of skin lesions in chronic myelomonocytic leukemia. J Cutan Pathol. 2017;44(12):1075-9.
