Short Communication
Tumor angiogenesis, a hallmark of cancer, is a critical step in the tumorigenesis of solid cancers [1]. The process of tumor angiogenesis is orchestrated by a range of secreted factors, signaling pathways as well as nonendothelial cells [2]. Abnormal tumor vascular networks sometimes contribute to a decline in the efficacy of various therapies, such as chemotherapy, radiotherapy, and immunotherapy [3,4]. Anti-angiogenesis via targeting VEGF-mediated signaling has become one of the most promising therapies, which aimed at inhibiting VEGF activity to regress tumors by starvation [5]. In 2003, a clinical trial demonstrated that chemotherapy combined with bevacizumab (humanized neutralizing antibodies targeting VEGF) improved the clinical survival of metastatic colorectal cancer (CRC) patients [6]. After bevacizumab receiving the FDA (the Food and Drug Administration) approval [7], the FDA has approved various angiogenic inhibitors as cancer therapies. Unfortunately, emerging clinical evidence indicated that the benefit of these anti-angiogenic treatments have so far shown only modest clinical efficacy and remained to be suboptimal in patients who lack responses or acquire resistance.
Immune checkpoint inhibitors (ICIs) have shown a long-lasting clinical activity against a large number of malignancies [8], which re-start the anti-tumor immune responses of the host via inhibiting the negative regulatory immune signals of T cells [9]. In the last decade, cytotoxic T lymphocyte antigen 4 (CTLA-4) and the programmed cell death receptor 1 (PD-1) and its ligand, PD-L1 inhibitors have made remarkable progress in the clinical application of cancer immunotherapies [10]. Ipilimumab, an anti-CTLA-4 antibody, was granted FDA approval in 2011 and improved the overall survival of patients with advanced melanoma [11]. Furthermore, pembrolizumab and nivolumab, anti-PD-1 antibodies, were approved for the advanced melanoma treatment in 2014 [12,13]. However, the response rates of single antibody blocking PD-1 or CTLA-4 pathway remain low in a majority of patients [14,15]. To overcome this problem, a rationale for the combination of therapies with ICIs and conventional therapies such as chemotherapy, radiotherapy, and anti-angiogenesis therapy, started to be considered.
Previous evidence showed that angiogenesis played a key role in regulating tumor immune response and lead to resistance to ICIs [16]. VEGF family, which induces physiological and pathological angiogenesis [17], has been reported to suppress tumor immune response by enhancing T cell exhaustion by upregulating PD-L1, CTLA-4, TIM3 and LAG3 expression on T-cells [18]. Besides, VEGF promoted the expansion of T-regulatory cells (T-regs) and Myeloid-derived suppressor cell (MDSCs) and the infiltration of tumor-associated macrophages (TAMs) in tumors [19]. Conversely, the immune microenvironment is also able to affect the tumor angiogenesis. Huang et al. reported that IL-35 recruits monocytes via CCL5 and induces macrophages to promote angiogenesis through inducing CXCL1 and CXCL8 expression in pancreatic ductal adenocarcinoma cells [20]. CD11b+Gr-1+ MDSCs contributed to lung metastasis of breast cancer by inducing angiogenesis in a platelet-derived growth factor-BB dependent manner [21]. Therefore, the mutual interactions between tumor angiogenesis and immunosuppressive microenvironment may be a key cause of the failure of single anti-angiogenesis therapy or ICIs.
B7-H3 (B7 homolog 3 protein), also known as CD276, belongs to the B7-CD28 immune checkpoint family and takes part in cancer development and cancer immunity [22]. We have previously shown that B7-H3 is significantly upregulated in CRC tissue samples compared with normal adjacent tissues, and is positively associated with TNM stages [23]. Current research results also demonstrated that the upregulation of B7-H3 is closely related to lymph node metastasis in patients with CRC. Other groups have also shown that B7-H3 is overexpressed in multiple malignant tumors, and is associated with poor prognosis [24,25]. These results suggest that B7-H3 exerts crucial effects on tumor progression.
B7-H3 has been reported to exert a costimulating effect on the proliferation and IFN-γ production of T cells [26,27]. By contrast, other studies have shown that B7- H3 plays an inhibitory role in T cell proliferation [28]. Although the immunologic function of B7-H3 remains controversial, B7-H3 has been a potential target for cancer immunotherapy. Du and colleagues generated chimeric antigen receptor (CAR) T cells targeting B7-H3 (B7-H3. CAR-Ts) and found that B7-H3.CAR-Ts controlled the growth of pancreatic ductal adenocarcinoma, ovarian cancer, and neuroblastoma in vitro and in orthotopic and metastatic xenograft mouse models [29]. In addition, B7- H3-deficient mice or mice treated with an antagonistic antibody to B7-H3 showed reduced growth of multiple tumors, which depended on NK and CD8+ T cells [30]. In ID8 ovarian cancer mouse models, B7-H3 expressed on tumor cells, but not host cells, had a dominant role in suppressing the function of CD8+ T cells [31]. Moreover, B7-H3 blockade, but not PD-1 blockade, prolonged the survival of ID8 tumor-bearing mice [31]. Therefore, B7- H3 may be a promising immune therapeutic target for tumors.
Aside from its immunologic function, B7-H3 has been reported to participate in multiple non-immunological functions in cancers, such as proliferation, metastasis, drug resistance and metabolism [32]. Previous reports from our group have shown that B7-H3 plays an important role in metabolism and chemoresistance in CRC [23,33]. Body of evidence has revealed that B7- H3 participates in the progression and metastasis of CRC, indicating that B7-H3 has become a new potential prognostic marker and therapeutic target for CRC [25]. More importantly, B7-H3 has been found to take part in the anti-angiogenesis in cancers. A prior study has shown that the expression of B7-H3 dramatically differs between physiological and pathological angiogenic vessels and is remarkably upregulated in the blood vessels of various human cancers [34]. In our current research, our data indicated that the expression level of B7-H3 is a positive association with CD31, a sensitive and specific endothelial marker for MVD in CRC tissue samples. Seaman et al. demonstrated that pyrrolobenzodiazepineconjugated anti-B7-H3-drug can target both angiogenic vessels and non-angiogenic vessels, showing promising antitumor activity while little toxicity [35]. Furthermore, a series of in vitro and in vivo experiments showed that B7-H3 on CRC cells upregulated VEGFA expression and angiogenesis by activating the NF-κB pathway. Therefore, B7-H3 may promote angiogenesis by upregulating the expression of VEGFA in CRC, showing its great prospect of a possible biomarker in personalized anti-angiogenic therapy.
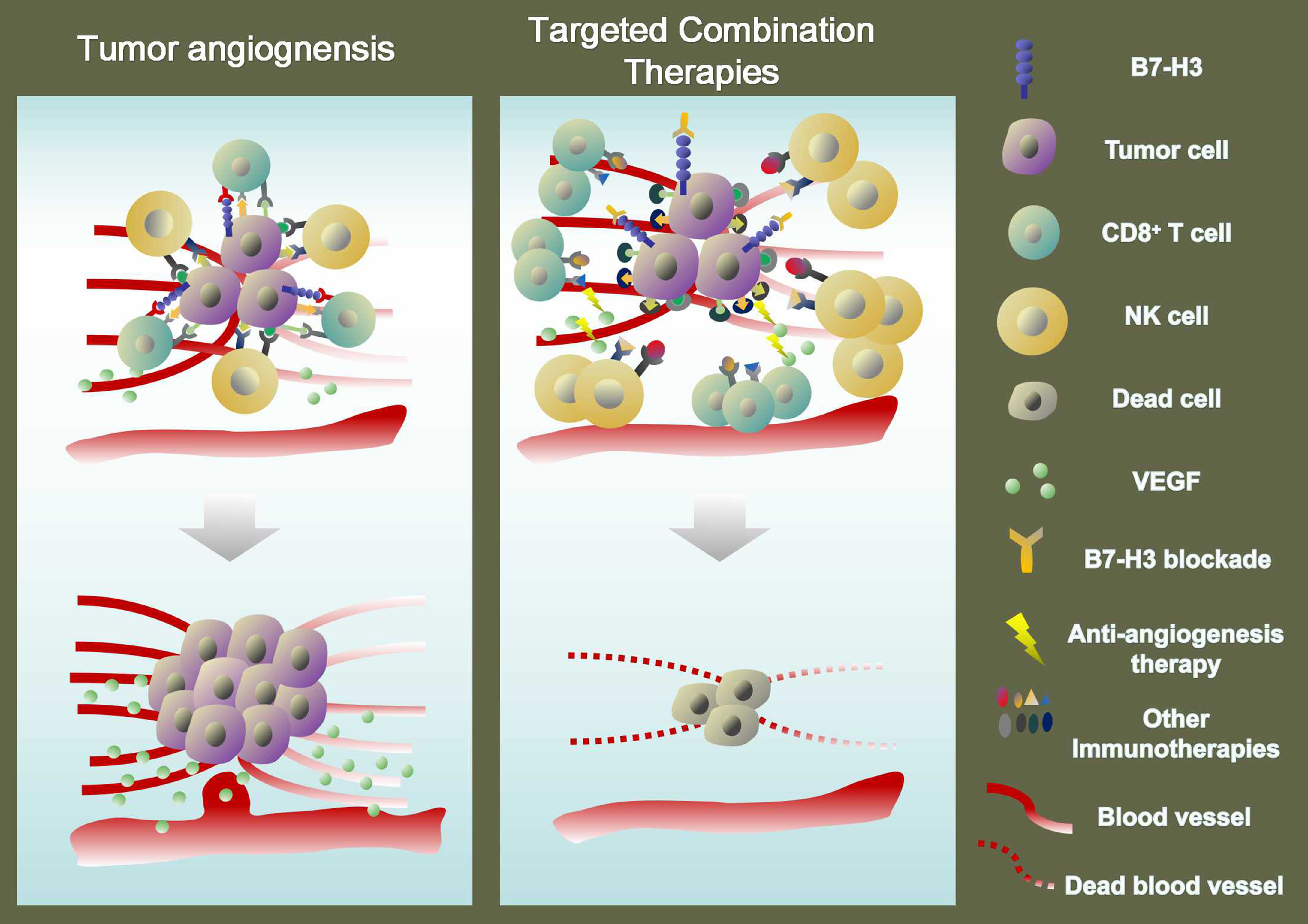
Nowadays, it is believed that combining an immune checkpoint inhibitor (ICI) with anti-angiogenesis would become a promising strategy, which can overcome the treatment resistance and finally improve patients’ prognosis [9]. We are aware that it seems to be a twoway street between anti-angiogenic therapies and immunotherapies, whose efficacy influence with each other [36]. Excessive levels of VEGF contribute to VEGFinduced immunosuppression in tumors [37]. The clinical success in the combination of VEGF inhibitors and ICI therapy attributed to the VEGF inhibitors, suppressing VEGF-induced immunosuppression and promoting an anti-tumor immune response [36]. Given that B7-H3 not only exerts crucial regulatory effects on the anti-tumor immune but also plays key roles in tumor angiogenesis, combination therapy with B7-H3 blockade and antiangiogenesis may be a particularly valuable option for the treatment of cancers. In our current research, we showed that the combination therapy of B7-H3 inhibitor 3E8 with bevacizumab inhibited the tumor growth, showing a more inhibitory effect on the MVD and VEGFA expression in mouse xenograft models (Figure 1). As such, it is expected that combination therapy with B7-H3 blockade and anti-angiogenesis is applied to the clinical treatment of tumors.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No.81802843, No.81672372).
Conflict of Interest
The authors declare no conflict of interest.
References
2. De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nature Reviews Cancer. 2017 Aug;17(8):457.
3. Mander KA, Finnie JW. Tumour angiogenesis, antiangiogenic therapy and chemotherapeutic resistance. Australian Veterinary Journal. 2018 Oct;96(10):371-8.
4. Tong L, Zhu G, Wang J, Sun R, He F, Zhai J.Suppressing angiogenesis regulates the irradiationinduced stimulation on osteoclastogenesis in vitro.Journal of Cellular Physiology. 2018 Apr;233(4):3429-38.
5. Rivera LB, Bergers G. Tumor angiogenesis, from foe to friend. Science. 2015 Aug 14;349(6249):694-5.
6. Jain RK. Normalizing tumor vasculature with antiangiogenic therapy: a new paradigm for combination therapy. Nature Medicine. 2001 Sep;7(9):987-9.
7. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New England Journal of Medicine. 2004 Jun 3;350(23):2335-42.
8. Gentzler R, Hall R, Kunk PR, Gaughan E, Dillon P, Slingluff Jr CL, Rahma OE. Beyond melanoma: inhibiting the PD-1/PD-L1 pathway in solid tumors. Immunotherapy. 2016 May;8(5):583-600.
9. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Molecular Cancer. 2019 Dec;18(1):60.
10. McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT, Watkins TB. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016 Mar 25;351(6280):1463-9.
11. Koller KM, Mackley HB, Liu J, Wagner H, Talamo G, Schell TD, Pameijer C, Neves RI, Anderson B, Kokolus KM, Mallon CA. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biology & Therapy. 2017 Jan 2;18(1):36-42.a>
12. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J. Pembrolizumab versus ipilimumab in advanced melanoma. New England Journal of Medicine. 2015 Jun 25;372(26):2521-32.
13. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka- Warzocha E, Savage KJ. Nivolumab in previously untreated melanoma without BRAF mutation. New England Journal of Medicine. 2015 Jan 22;372(4):320- 30.
14. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New England Journal of Medicine. 2012 Jun 28;366(26):2443- 54.
15. Kyi C, Postow MA. Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy. 2016 Jun;8(7):821-37.
16. Liu D, Jenkins RW, Sullivan RJ. Mechanisms of resistance to immune checkpoint blockade. American Journal of Clinical Dermatology. 2019 Feb 13;20(1):41- 54.
17. Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306-9.
18. Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Research. 1996 Mar 1;56(5):1111-7.
19. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clinical Cancer Research. 2019 Sep 15;25(18):5449-57.
20. Huang C, Li Z, Li N, Li Y, Chang A, Zhao T, Wang X, Wang H, Gao S, Yang S, Hao J. Interleukin 35 expression correlates with microvessel density in pancreatic ductal adenocarcinoma, recruits monocytes, and promotes growth and angiogenesis of xenograft tumors in mice. Gastroenterology. 2018 Feb 1;154(3):675-88.
21. Hsu YL, Yen MC, Chang WA, Tsai PH, Pan YC, Liao SH, Kuo PL. CXCL17-derived CD11b+ Gr-1+ myeloidderived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Research. 2019 Dec 1;21(1):23.
22. Zang X, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proceedings of the National Academy of Sciences. 2003 Sep 2;100(18):10388-92.
23. Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, Liu C, Zhang G, Wang Z, Wang R, Lu H. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death & Disease. 2019 Apr 5;10(4):1-2.
24. Chen L, Xie Q, Wang Z, Shi L, Wu C, Jiang J. Assessment of combined expression of B7-H3 and B7- H4 as prognostic marker in esophageal cancer patients. Oncotarget. 2016 Nov 22;7(47):77237.
25. Ingebrigtsen VA, Boye K, Tekle C, Nesland JM, Flatmark K, Fodstad Ø. B7-H3 expression in colorectal cancer: nuclear localization strongly predicts poor outcome in colon cancer. International Journal of Cancer. 2012 Dec 1;131(11):2528-36.
26. Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-γ production. Nature Immunology. 2001 Mar;2(3):269-74.
27. Zhang GB, Chen YJ, Shi Q, Ma HB, Ge Y, Wang Q, Jiang Z, Xu Y, Zhang XG. Human recombinant B7-H3 expressed in E. coli enhances T lymphocyte proliferation and IL-10 secretion in vitro. Acta Biochimica Et Biophysica Sinica. 2004 Jun 1;36(6):430-6.
28. Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A. The B7 family member B7-H3 preferentially down-regulates T helper type 1– mediated immune responses. Nature Immunology. 2003 Sep;4(9):899-906.
29. Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, Tiruthani K, Mirlekar B, Michaud D, Greene K, Herrera SG. Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell. 2019 Feb 11;35(2):221-37.
30. Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, Park HJ, Jeong M, Chang SH, Kim BS, Xiong W. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Research. 2017 Aug;27(8):1034-45
31. Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, Li P, Lyu N, Sun T, Xie S, Guo L. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cellular & Molecular Immunology. 2019 Oct 14.
32. Nygren MK, Tekle C, Ingebrigtsen VA, Fodstad O. B7-H3 and its relevance in cancer; immunological and non-immunological perspectives. Frontiers in Bioscience (Elite edition). 2011;3:989-93.
33. Ma Y, Wang R, Lu H, Li X, Zhang G, Fu F, Cao L, Zhan S, Wang Z, Deng Z, Shi T. B7-H3 promotes the cell cyclemediated chemoresistance of colorectal cancer cells by regulating CDC25A. Journal of Cancer. 2020;11(8):2158.
34. Seaman S, Stevens J, Yang MY, Logsdon D, Graff- Cherry C, Croix BS. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007 Jun 12;11(6):539-54.
35. Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, Morris K, Szot C, Morris H, Swing DA, Tessarollo L. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017 Apr 10;31(4):501-15.
36. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nature Reviews Clinical Oncology. 2018 May;15(5):310.
37. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nature Reviews Clinical Oncology. 2018 May;15(5):325.