Abstract
Treatment options for multiple myeloma (MM) have evolved significantly over the past four decades, improving overall survival (OS) through a deeper understanding of tumor biology and personalized treatment strategies. Key factors for individualizing therapy include eligibility for autologous stem cell transplantation (ASCT), disease risk stratification, patient frailty, performance status, age, and comorbidities, aiming to maximize disease control while minimizing toxicity. Current evidence supports triplet or quadruplet regimens incorporating an anti-CD38 monoclonal antibody, a proteasome inhibitor, an immunomodulatory drug, and dexamethasone, with or without ASCT, as initial therapy. Recent studies have explored whether specific regimen components can be omitted without compromising efficacy and whether maintenance therapy can be discontinued after sustained minimal residual disease (MRD) negativity for 12 months. This review examines the evolving recommendations and evidence guiding these treatment decisions.
Keywords
Evolution of treatment, Immunotherapy, Initial therapy, Multiple myeloma, Quadruple therapy
Introduction
The treatment landscape for multiple myeloma (MM) has transformed over the past four decades, significantly enhancing overall survival. Initially, melphalan and prednisone, introduced in the 1960s, offered a median overall survival (OS) of approximately 2.5 years [1]. Advances in understanding MM tumor biology, identifying drivers of disease heterogeneity, and developing targeted therapies have revolutionized patient care. Key milestones include the adoption of autologous stem cell transplantation (ASCT) in the 1980s, the approval of immunomodulatory drugs (IMiDs) such as thalidomide and lenalidomide in the early 2000s, and the introduction of proteasome inhibitors like bortezomib. More recently, anti-CD38 monoclonal antibodies, notably daratumumab and isatuximab, have improved treatment outcomes [2].
Modern MM management integrates induction therapy, ASCT when appropriate, consolidation chemotherapy, and maintenance therapy, tailored to individual patient needs. Personalizing care for newly diagnosed MM involves assessing ASCT eligibility, disease risk stratification, patient frailty, performance status, age, and comorbidities, with the goals of maximizing disease control, minimizing toxicity, and adapting treatment based on response [3,4].
The Evolution of Treatment
The treatment of MM began with melphalan and prednisone for patients ineligible for ASCT [5]. Subsequent regimens for ASCT-ineligible patients included melphalan-thalidomide [5], melphalan-lenalidomide [6], and melphalan-bortezomib [7].
ASCT-eligible patients received induction regimens such as vincristine, doxorubicin, and dexamethasone (VAD) or combinations like thalidomide-dexamethasone, bortezomib-dexamethasone, or lenalidomide-dexamethasone, followed by transplantation. The introduction of novel agents—thalidomide, lenalidomide, and bortezomib—enabled multi-drug regimens. ASCT-ineligible patients were treated with combinations like VMP (bortezomib, melphalan, prednisone) or VRd (bortezomib, lenalidomide, dexamethasone), while ASCT-eligible patients received regimens such as bortezomib-lenalidomide-dexamethasone, bortezomib-cyclophosphamide-dexamethasone [8], or bortezomib-thalidomide-dexamethasone [9].
The development of anti-CD38 monoclonal antibodies, particularly daratumumab, marked a significant advance. Both ASCT-eligible and -ineligible patients now benefit from regimens like daratumumab-VMP, daratumumab-lenalidomide-dexamethasone, and daratumumab-VRd. These regimens are increasingly similar across patient groups, as modern therapies do not impair stem cell collection, allowing uniform initial treatment strategies [10]. ASCT-eligible patients typically proceed to transplantation after induction, followed by maintenance with single-agent or combination regimens, such as lenalidomide-dexamethasone. Treatment decisions are guided by risk stratification, performance status, and frailty, with therapy intensity tailored to tolerability and disease response [3,4,11].
High Risk MM
High-risk multiple myeloma (HRMM) is defined by the International Myeloma Society-International Myeloma Working Group (IMS-IMWG) 2024 Consensus. Patients are classified as high-risk if they exhibit deletion 17p and/or TP53 mutation, translocations t(14;16) or t(14;20) with gain 1q and/or deletion 1p32, monoallelic deletion 1p32 with gain 1q, biallelic deletion 1p32, or high beta-2 microglobulin (>5.5 mg/dL) with normal creatinine (<1.2 mg/dL) (Table 1). Ongoing clinical trials are currently evaluating therapies targeting high-risk features, such as deletion 17p and TP53 mutations.
|
Criteria for High-risk Multiple Myeloma |
|
Del (17p) and/or TP53 mutation |
|
Translocations: t(14;16) or t(14;20) co-occurring with +1q and/or del(1p32) |
|
Monoallelic del(1p32) along with +1q, or biallelic del(1p32) |
|
High β2M (>5.5 mg/dL) with normal creatinine (<1.2 mg/dL) |
First Line Therapy
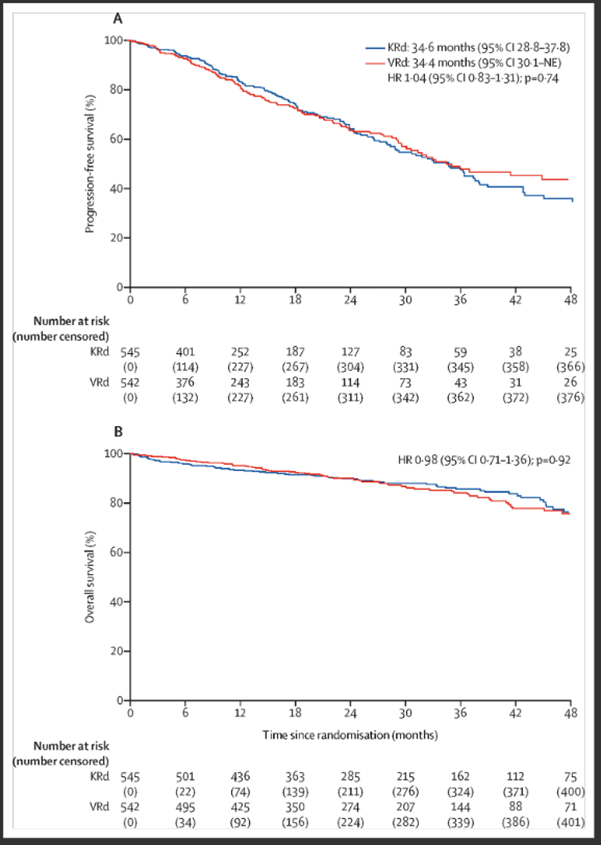
Initial therapy for newly diagnosed MM has advanced with triplet regimens, as demonstrated by the Southwest Oncology Group (SWOG) S0777 trial evaluating VRd (bortezomib, lenalidomide, dexamethasone), which improved progression-free survival (PFS) and OS [12]. Other triplet regimens include VTD (bortezomib, thalidomide, dexamethasone), associated with peripheral neuropathy, and CyBorD (bortezomib, cyclophosphamide, dexamethasone), linked to increased cytopenias. The ENDURANCE trial compared KRd (carfilzomib, lenalidomide, dexamethasone) with VRd (Figure 1), finding no PFS difference in standard-risk patients [13].
Figure 1. ENDURANCE trial: KRd vs. VRd in newly diagnosed MM.
Quadruple Therapy Regimens
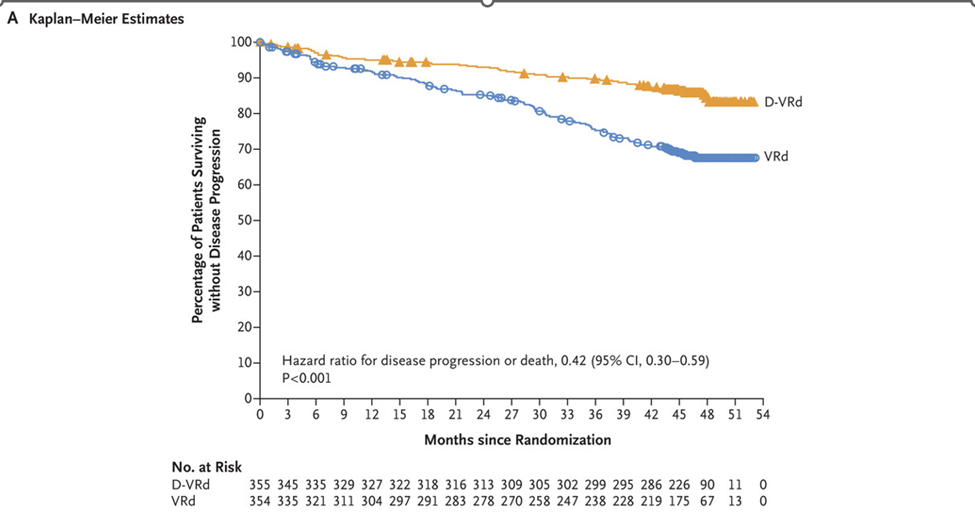
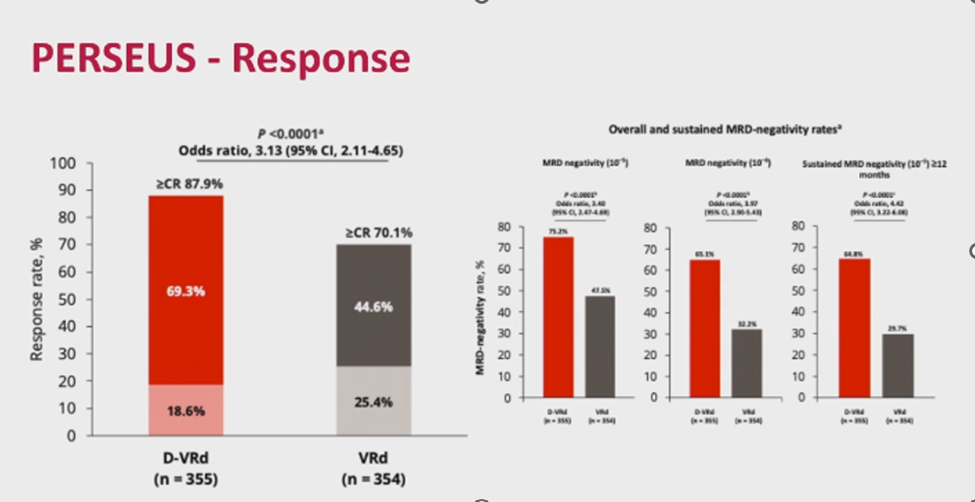
Quadruple therapy has become a cornerstone of MM treatment, particularly with daratumumab. The GRIFFIN trial demonstrated the efficacy of daratumumab-VRd, confirmed by the PERSEUS trial, which randomized ASCT-eligible patients (aged 18–70) to VRd versus daratumumab-VRd [2]. Induction was followed by ASCT, consolidation (two cycles of VRd or daratumumab-VRd), and maintenance (lenalidomide or daratumumab-lenalidomide). Patients achieving minimal residual disease (MRD) negativity after two years continued lenalidomide maintenance.
Daratumumab-VRd achieved an 88% complete remission (CR) rate, with MRD negativity at 75% (10-5 sensitivity) and 65.1% (with a 10-6 sensitivity, stringent exploratory analysis). Nearly two-thirds sustained MRD negativity for over one year, resulting in a 48-month PFS of 84.3% compared to 67.7% for VRd (Figures 2 and 3).
Figure 2. PERSEUS trial: progression-free survival: daratumumab-VRd vs. VRd in ASCT-eligible patients.
Other quadruple regimens, such as daratumumab-VTD, achieved a 64% very good partial response (VGPR) post-induction, while lenalidomide-based regimens like daratumumab-KRd and isatuximab-VRd showed VGPR rates of 84–90%, MRD negativity of 81%, and 3-year PFS of approximately 80% (Table 2).
|
Regimen |
Induction VGPR |
Best VGPR |
MRD |
PFS |
|
Dara-VTd |
65% |
84% |
65.1% |
83.7 months |
|
Dara-KRd |
88% |
98% |
81% |
3yr- 80% |
|
Isa-VRd |
77% |
- |
- |
3yr PFS 83% |
|
Isa-KRd |
84% |
90% |
82% |
NA |
Considerations for Older or Frail Patients
Treatment selection for MM must account for age, comorbidities (e.g., hypertension, diabetes, ischemic heart disease, and renal insufficiency), frailty, altered drug metabolism, social support, financial constraints, and mobility. The FIRST trial evaluated melphalan-free lenalidomide-dexamethasone in ASCT-ineligible patients, finding a 4-year PFS of 32.6% with continuous lenalidomide versus 14.3% with 18-month administration, with improved OS irrespective of duration [14]. The RVD-Lite study modified VRd for ASCT-eligible patients, using weekly bortezomib and reduced lenalidomide (15 mg) over a 5-week cycle, yielding outcomes comparable to the SWOG S0777 trial [15]. The MAIA trial demonstrated that triplet daratumumab-lenalidomide-dexamethasone was well-tolerated in older, ASCT-ineligible patients, achieving a 47% CR rate versus 25% for lenalidomide-dexamethasone, with improved PFS and a 7-year OS of 53% versus 39% [16].
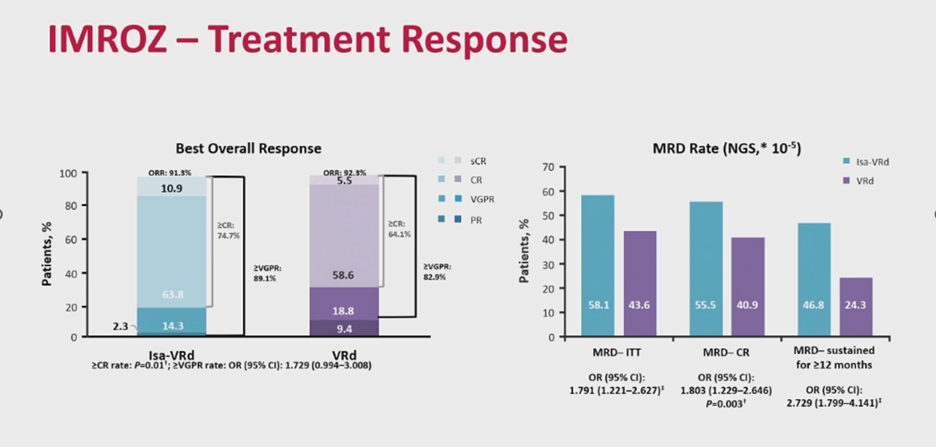
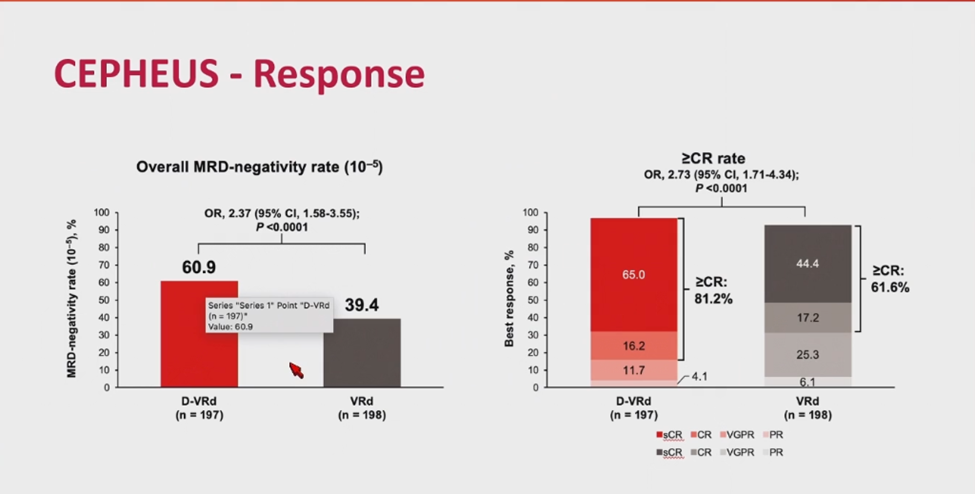
The success of D-Rd in MAIA prompted the development of quadruplet regimens, such as isatuximab-VRd (IMROZ) and daratumumab-VRd (CEPHEUS), to further enhance outcomes in transplant-ineligible patients. The IMROZ trial compared quadruplet isatuximab-VRd with VRd in ASCT-ineligible patients, reporting a 74% CR rate for isatuximab-VRd versus 64% for VRd, with 56% MRD negativity (sensitivity of 10-5) versus 40%, and sustained MRD negativity for over 12 months in 47% of isatuximab-VRd patients (Figure 4). Median PFS at 60 months was not reached, with a PFS of 63% [17]. The CEPHEUS trial similarly evaluated quadruplet daratumumab-VRd versus VRd, reporting 60.9% MRD negativity (sensitivity of 10-5) and an 81% CR rate for daratumumab-VRd versus 40% and 62% for VRd, with a 56-month PFS of 68% versus 49% (Figure 5) [18].
Figure 4. IMROZ trial: isatuximab-VRd vs. VRd in ASCT-ineligible patients.
Figure 5. CEPHEUS trial: daratumumab-VRd vs. VRd.
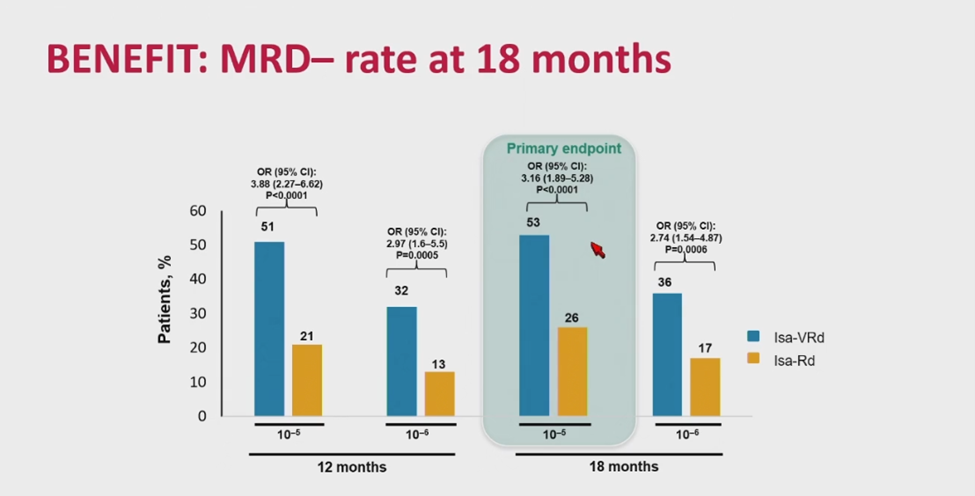
Given the IMROZ and CEPHEUS results, the BENEFIT trial subsequently compared isatuximab-VRd with isatuximab-Rd to investigate the therapeutic effect of bortezomib rather than isatuximab in a four drug regimen. Results thus far have found higher MRD negativity with the addition of bortezomib, suggesting that a fourth drug enhances response depth despite no PFS difference to date (Figure 6) [19].
Figure 6. BENEFIT trial: isatuximab-VRd vs. isatuximab-Rd.
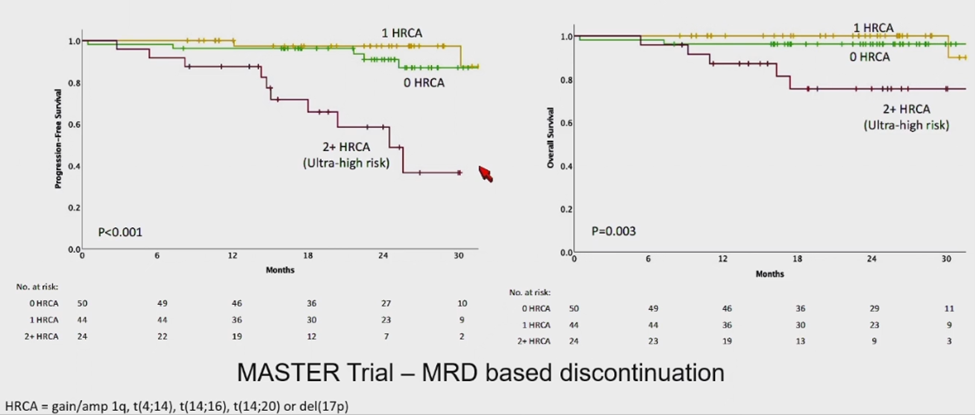
The optimal duration of MM therapy remains under investigation. Multiple meta-analyses have established that MRD negativity is a strong surrogate for both PFS and OS in MM. Sustained MRD negativity, particularly at 1 year, is highly predictive of long-term outcomes, supporting its use as a key endpoint in clinical trials and a goal in clinical practice. The MASTER trial evaluated therapy duration, intensity, and tumor biology, suggesting that patients with no or one high-risk abnormality may discontinue therapy after sustained MRD negativity for over 12 months. Patients with two or more high-risk abnormalities had worse PFS, indicating a need for prolonged treatment (Figure 7) [20]. However, while MRD negativity strongly correlates with OS, its role as a definitive surrogate requires further validation due to variability in single-time point assessments.
Figure 7. MASTER trial: MRD response-adapted therapy.
|
Trial Name |
Year |
Phase & Design |
Population |
Interventions |
Key Outcomes |
Limitations |
|
SWOG S0777 [12] |
2017 |
Phase III, randomized |
NDMM, no intent for immediate ASCT, standard and high-risk |
VRd vs. Rd |
Median PFS: 43 months (VRd) vs. 30 months (Rd); OS: 75 months (VRd) vs. 64 months (Rd) |
Limited high-risk patient data; no ASCT arm |
|
MAIA [16] |
2019 |
Phase III, randomized |
NDMM, transplant-ineligible, median age 73, ~15% high-risk |
D-Rd vs. Rd |
7-yr PFS: 47% (D-Rd) vs. 25% (Rd); 7-yr OS: 53% vs. 39% |
Underrepresented high-risk patients; older population bias |
|
ENDURANCE [13] |
2020 |
Phase III, randomized |
NDMM, standard-risk, transplant-eligible, age 18-70 |
KRd vs. VRd |
No PFS difference (34.6 months KRd vs. 34.4 months VRd); KRd had higher cardiac toxicity |
Excluded high-risk patients; no ASCT in primary analysis |
|
MASTER [20] |
2023 |
Phase II, single-arm |
NDMM, post-ASCT, standard- and high-risk |
MRD response-adapted D-VRd |
Feasible to stop therapy after sustained MRD negativity (10-5) for 12 months in standard-risk; worse PFS in high-risk |
Single-arm design; small sample size |
|
PERSEUS [2] |
2024 |
Phase III, randomized |
NDMM, transplant-eligible, age 18-70, standard- and high-risk |
D-VRd vs. VRd |
48-month PFS: 84.3% (D-VRd) vs. 67.7% (VRd); 88% CR, 75% MRD negativity (10-5) |
Limited long-term OS data; complex regimen design |
|
IMROZ [17] |
2024 |
Phase III, randomized |
NDMM, transplant-ineligible, median age 68, standard- and high-risk |
Isa-VRd vs. VRd |
60-month PFS: 63% (Isa-VRd) vs. 45% (VRd); 74% CR, 39.6% MRD negativity (10-5) |
Limited high-risk subgroup analysis; older population |
|
CEPHEUS [18] |
2024 |
Phase III, randomized |
NDMM, transplant-ineligible or deferred, standard and high-risk |
D-VRd vs. VRd |
60.9% MRD negativity (10-5), 81% CR (D-VRd) vs. 40% MRD, 62% CR (VRd); 56-month PFS: 68% vs. 49% (VRd) |
Limited OS data; heterogeneous population |
|
BENEFIT [19] |
2024 |
Phase III, randomized |
NDMM, transplant-ineligible, median age 71, standard and high-risk |
Isa-VRd vs. Isa-Rd |
Higher MRD negativity (Isa-VRd); no PFS difference at interim analysis |
No PFS difference; limited high-risk data |
Conclusion
Selecting an initial therapy for newly diagnosed MM requires balancing risk stratification, frailty, and patient preferences.
The debate over whether quadruplet therapy should be universally adopted or tailored based on patient risk and frailty is ongoing. Trials such as PERSEUS [2] and IMROZ [17] demonstrate that quadruplet regimens, like daratumumab-VRd and isatuximab-VRd, significantly improve MRD negativity and PFS compared to triplet regimens. However, the added toxicity, including infections and cytopenias, may outweigh benefits in standard-risk or frail patients. For instance, the BENEFIT trial [19] showed that adding bortezomib to isatuximab, lenalidomide, and dexamethasone (Isa-VRd) improved MRD negativity but did not enhance PFS at the time of reporting, suggesting that triplet regimens like isatuximab-Rd may be sufficient for some patients. A risk- and frailty-adjusted approach, guided by dynamic frailty assessments and cytogenetic risk, may optimize outcomes while minimizing toxicity [11].
The BENEFIT trial [19] highlights the implications of omitting proteasome inhibitors in MM regimens. While isatuximab-Rd achieved robust responses in transplant-ineligible patients, adding bortezomib (Isa-VRd) deepened responses, particularly in patients with high-risk cytogenetics. This suggests that proteasome inhibitors remain critical for certain subgroups, but their omission may be feasible in patients with standard-risk disease or those unable to tolerate bortezomib’s side effects, such as peripheral neuropathy. Alternative strategies, such as doublet or triplet regimens without proteasome inhibitors, may be considered for frail patients, balancing efficacy with tolerability [9].
Pivotal trials such as MAIA, PERSEUS, and IMROZ have shaped treatment guidelines, recommending D-Rd for transplant-ineligible patients and daratumumab-VRd for transplant-eligible patients. These trials emphasize personalized medicine, tailoring therapy to transplant eligibility, disease risk, and frailty. The incorporation of MRD negativity as a treatment goal reflects its prognostic value, influencing both clinical practice and trial design.
Looking ahead, the integration of emerging therapies such as bispecific antibodies and chimeric antigen receptor T-cell (CAR-T) therapy into frontline treatment protocols holds significant promise, particularly for patients with high-risk cytogenetics. Bispecific antibodies, which simultaneously engage T cells and MM-specific antigens like B-cell maturation antigen (BCMA) or G-protein coupled receptor family C group 5 member D (GPRC5D), have shown impressive response rates in relapsed/refractory MM and are now being evaluated in newly diagnosed patients to achieve deeper and more durable responses. For instance, early-phase trials are exploring bispecifics as part of induction regimens to eliminate residual disease early, potentially reducing the need for prolonged maintenance. Similarly, CAR-T therapy, currently approved for later-line treatment, is under investigation for frontline use in high-risk MM, where its ability to target and eradicate malignant plasma cells could address unmet needs. These therapies could shift the treatment paradigm by enabling MRD-driven, finite-duration strategies, though their efficacy, safety, and optimal sequencing in frontline settings await confirmation from ongoing clinical trials.
Beyond novel therapies, advancements in genomic profiling and dynamic risk assessment are positioned to revolutionize MM management. High-throughput sequencing and single-cell analysis could identify actionable mutations and immune profiles, enabling precision medicine approaches that tailor therapy to each patient’s molecular and clinical characteristics. For example, real-time monitoring of disease evolution may guide adaptive treatment strategies, optimizing outcomes while minimizing toxicity. These innovations, combined with patient-centered care models, have the potential to transform MM into a chronic, manageable condition with sustained remission for a growing number of patients.
Future advancements, including genomic and immune profiling and ex vivo drug screening, may further refine treatment decisions. Regular response assessments, particularly for depth of response, are essential to adjust therapy and determine treatment duration.
Abbreviations
MM: Multiple Myeloma; NDMM: Newly diagnosed Multiple Myeloma; ASCT: Autologous Stem Cell Transplantation; MRD: Minimal Residual Disease; VAD: Vincristine, Doxorubicin, Dexamethasone; VMP: Bortezomib, Melphalan, Prednisone; VRd: Bortezomib, Lenalidomide, Dexamethasone; IMS: International Myeloma Society; IMWG: International Myeloma Working Group; SWOG: Southwest Oncology Group; HRMM: High-Risk Multiple Myeloma; VTD: Bortezomib, Thalidomide, Dexamethasone; PFS: Progression-Free Survival; OS: Overall Survival; CR: Complete Response; CyBorD: Bortezomib, Cyclophosphamide, Dexamethasone; KRd: Carfilzomib, Lenalidomide, Dexamethasone; VGPR: Very Good Partial Response
Footnotes
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
There is no conflict of interest.
Ethical statement
The study was reviewed and approved by the institutional review boards (IRBs) of participating sites.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [MN], upon reasonable request.
References
2. Sonneveld P, Dimopoulos MA, Boccadoro M, Quach H, Ho PJ, Beksac M, et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024 Jan 25;390(4):301–13.
3. Kumar SK. What is the ideal approach-doublet, triplet, or quadruplet(s)? Hematology Am Soc Hematol Educ Program. 2024 Dec 6;2024(1):551–60.
4. Miller HL, Sharpley FA. Frail Multiple Myeloma Patients Deserve More Than Just a Score. Hematol Rep. 2023 Feb 21;15(1):151–6.
5. Facon T, Darre S. Frontline treatment in multiple myeloma patients not eligible for stem-cell transplantation. Best Pract Res Clin Haematol. 2007 Dec;20(4):737–46.
6. Palumbo A, Falco P, Corradini P, Falcone A, Di Raimondo F, Giuliani N, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA--Italian Multiple Myeloma Network. J Clin Oncol. 2007 Oct 1;25(28):4459–65.
7. Sandecká V, Pour L, Špička I, Minařík J, Radocha J, Jelínek T, et al. Bortezomib-based therapy for newly diagnosed multiple myeloma patients ineligible for autologous stem cell transplantation: Czech Registry Data. Eur J Haematol. 2021 Oct;107(4):466–74.
8. Moreau P, Hulin C, Macro M, Caillot D, Chaleteix C, Roussel M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016 May 26;127(21):2569–74.
9. Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010 Dec 18;376(9758):2075–85.
10. Gozzetti A, Ciofini S, Simoncelli M, Santoni A, Pacelli P, Raspadori D, et al. Anti CD38 monoclonal antibodies for multiple myeloma treatment. Hum Vaccin Immunother. 2022 Nov 30;18(5):2052658.
11. Mian H, Wildes TM, Vij R, Pianko MJ, Major A, Fiala MA. Dynamic frailty risk assessment among older adults with multiple myeloma: A population-based cohort study. Blood Cancer J. 2023 May 10;13(1):76.
12. Ludwig H, Delforge M. Multiple myeloma: new treatments gain momentum. Lancet. 2017 Feb 4;389(10068):480–2.
13. Kumar SK, Jacobus SJ, Cohen AD, Weiss M, Callander N, Singh AK, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020 Oct;21(10):1317–30.
14. Facon T, Dimopoulos MA, Dispenzieri A, Catalano JV, Belch A, Cavo M, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018 Jan 18;131(3):301–10.
15. Richardson PG, Durie BG, Rosiñol L, Mateos MV, Dispenzieri A, Moreau P, et al. Clinical perspectives on the optimal use of lenalidomide plus bortezomib and dexamethasone for the treatment of newly diagnosed multiple myeloma. Haematologica. 2023 Nov 1;108(11):2894–912.
16. Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021 Nov;22(11):1582–96.
17. Facon T, Dimopoulos MA, Leleu XP, Beksac M, Pour L, Hájek R, et al. Isatuximab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2024 Oct 31;391(17):1597–609.
18. Usmani SZ, Facon T, Hungria V, Bahlis NJ, Venner CP, Braunstein M, et al. OA-63 Daratumumab+ bortezomib/lenalidomide/dexamethasone in patients with transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: results of the phase 3 CEPHEUS study. Clinical Lymphoma Myeloma and Leukemia. 2024 Sep 1;24:S288–9.
19. Leleu X, Hulin C, Lambert J, Bobin A, Perrot A, Karlin L, et al. Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized phase 3 BENEFIT trial. Nat Med. 2024 Aug;30(8):2235–41.
20. Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma (MASTER): final report of the multicentre, single-arm, phase 2 trial. Lancet Haematol. 2023 Nov;10(11):e890–e901.