Keywords
COVID-19, Vaccine, Cardiovascular adverse events, ACS, ED, Hypertensive urgency, Hypertensive emergency
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a global pandemic by the World Health Organization in March 2020 and since then, it has caused huge economic and social turmoil worldwide [1]. In an attempt to reduce the spread across the United States (U.S.) and worldwide, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) of different vaccines (BNT-162b2, mRNA-1273, and Ad26.COV2. S). Fear of adverse events has been a major impediment for widespread acceptance and utilization of available vaccines against the novel severe acute respiratory syndrome coronavirus (SARS-CoV2) [2,3]. As of November 2022, there have been a total of 650,810,290 vaccine doses administered and more than 267,476,279 people in the US have been fully vaccinated completing the primary series with either BNT-162b2 (Pfizer BioNTech), mRNA-1273 (Moderna), or Ad26.COV2.S (Janssen) [1]. Adverse events following immunization (AEFI) are rare and usually require studies of large population cohorts for detection [4]. The Centers for Disease Control and Prevention (CDC) reported that 9.2% of the general population had a documented adverse event after the COVID-19 vaccine [5]. However, data characterizing significant cardiovascular adverse events (CAEs) following vaccination against SARS CoV2 are sparse. In this study, we provide a unique real-world view into possible significant vaccine related CAEs by analyzing emergency department visits, hospitalizations, and intensive care unit admissions in the first ten days after vaccination.
Methods
We conducted a multi-center, retrospective study across the Cleveland Clinic enterprise (comprising a total of 11 hospitals in Northeast Ohio) between December 2020, and March 2021. All adult patients that presented to the emergency department (ED) within ten days after the first or the second dose of COVID-19 vaccination were included in the study. Possible CAEs were identified by the International Classification of Diseases, 10th Revision (ICD-10) codes and included: acute deep vein thrombosis or pulmonary embolism, acute heart failure exacerbation, arrhythmias, hypertensive urgency or emergency, and acute coronary syndrome. Basic demographics, presenting diagnosis, and admission to the hospital or intensive care units (ICU) were collected on consecutive patients. Further we conducted a literature review on PubMed of the United States National Library of Medicine using the following terms: Emergency Room or Department presentation and cardiovascular events; COVID-19 vaccine and cardiovascular events; COVID-19 vaccine and emergency department; COVID-19 vaccine and adverse events and emergency department; COVID-19 vaccine (as MeSH term: Medical Subject Headings) and Hospitalization (as MeSH term). The aim was to compare ED presentations with cardiovascular events post COVID-19 vaccine in our cohort to the general population reports on cardiovascular events presentation to the emergency rooms.
Results
We identified 1,842 patients that presented to the ED within 10 days after receiving the COVID-19 vaccine. The median age was 81 (IQR: 73-87) years. The median time to presentation after COVID-19 vaccine was 5 days.
The median length of stay in the hospital was 3 days in patients admitted after the first or the second dose.
Overall, 210 patients (11.4%) had possible CAEs, of which 138 (65.7%) were detected after the first dose and 72 (34.3%) after the second dose. In total, 103 patients required hospitalization after administration of the first vaccine dose due to possible CAE; of which 9 (8.7%) were admitted to the Intensive Care Unit (ICU). A total of 56 patients required hospitalization for a possible CAEs after the second vaccine dose administration, of which 4 (7.1%) patients required an ICU admission. Heart failure exacerbation (36%), hypertensive urgency/emergency and uncontrolled hypertension (19%), atrial fibrillation with rapid ventricular response (18%), non-ST elevation myocardial infarction (10%), ST-segment elevation myocardial infarction (2%), and acute DVT (4%), were amongst the most common causes of admission. Overall, death was observed in 19 (9.0%) of patients who developed possible CAEs during a 3-month follow-up period.
Literature search retrieved the titles of more than 1,700 articles, of which less than 30 abstracts were selected for relevance. Only one article specifically studied patients presenting to the ED with cardiovascular adverse events after the COVID-19 vaccination [6]. Another article did report the incidence of cardiovascular adverse events on patients presenting to the ED following vaccination, however, did not specifically focus on these cardiovascular adverse events only [7].
Discussion
Our study describes possible CAEs following the COVID-19 vaccination that required presentation to the ED, hospital admission, and ICU admission. Of the 1,843 patients presenting to the ED post-COVID 19 vaccination, 159 (8.6%) had CAE leading to hospital admission and 13 (0.7%) required a higher level of care in the ICU because of their cardiovascular symptoms and presentation. Close to 75.7% of the total population presenting to the ED post-COVID-19 vaccination with CAE required hospitalization and/or ICU admission.
Other studies have investigated the incidence of cardiovascular adverse events following the COVID-19 vaccination in the general population, using databases such as Vigibase [8], VAERS [9] or IEMS [10]. To our knowledge, our present study would be the second one to specifically investigate the rates of cardiovascular adverse events leading to emergency room visits and hospitalizations following COVID-19 vaccination. We noted a larger proportion of patients presenting to the ED after the first vaccination dose, compared to the second dose. Similar results were found on a retrospective study in patients vaccinated with either of two mRNA vaccines who presented to the ED in Korea [6]. Patients with heart failure exacerbation and coronary artery disease presented more frequently after the second dose, while Arrhythmias and hypertensive emergencies were more common after the first dose (Figure 1A). The most frequent arrhythmia in our cohort of patients who presented to the ED within 10 days of vaccination was atrial fibrillation with rapid ventricular response at 76% (Figure 1A). Similar findings were reported by Jeet Kaur et al., who analyzed the frequency of cardiovascular adverse events after COVID-19 vaccination from the VigiBase database of the World Health Organization (WHO) finding that atrial fibrillation was one of the most common serious arrhythmias reported after the COVID 19 vaccine [8].
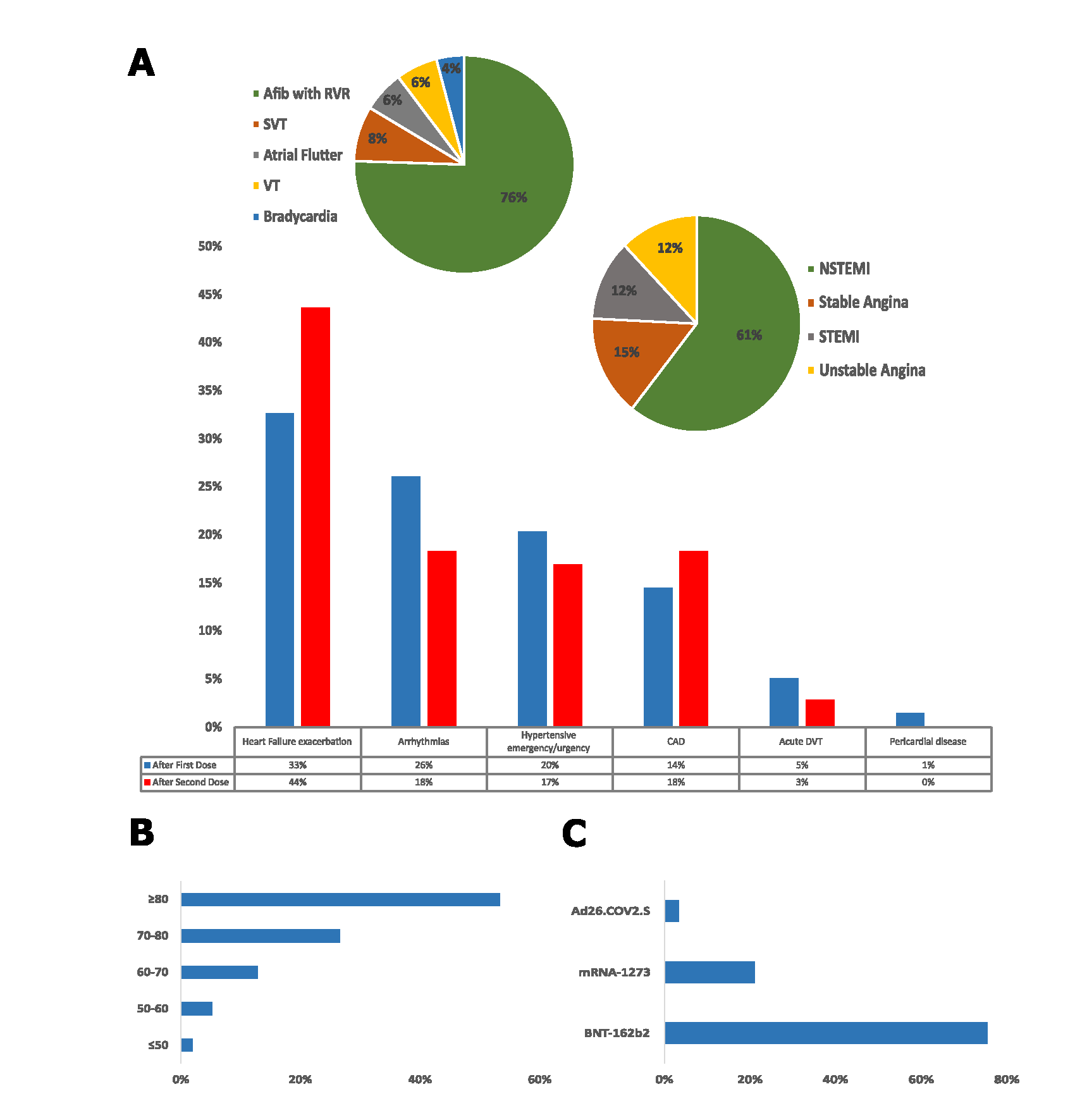
Figure.1: Cardiovascular events among patients presenting to the emergency department within ten days of COVID-19 vaccination. A: Percentage of cardiovascular-related adverse events after the first (blue) and the second (red) doses of the COVID-19 vaccine. Pie charts show the relative frequencies of different arrhythmias and coronary arteries diseases. B: Distribution of cardiovascular-related adverse events among different age groups (years). C: Frequencies of cardiovascular-related adverse events among different vaccine types.
Afib with RVR: Atrial Fibrillation with Rapid Ventricular Response; SVT: Supraventricular Tachycardia; VT: Ventricular Tachycardia; NSTEMI: Non ST Elevation Myocardial Infarction; STEMI: ST Elevation Myocardial Infarction; CAD: Coronary Artery Disease; and DVT: Deep Vein Thrombosis.
In order to put our data into context, we looked in the literature to understand the typical rates of presentation for cardiovascular events in a typical ER population, independent of vaccination for SARS-CoV2. Raisi-Estabragh et. al. reported more than 20.6 million cardiovascular emergency room presentations from the Nation Emergency Department Sample (NEDS). The most common presenting ER diagnoses were uncontrolled hypertension and hypertensive crisis (16.1%), atrial fibrillation and atrial flutter (10.0%), acute myocardial infarction (8.9 %), heart failure (7.2 %), and DVT (3.7%) [11]. These prevalence’s were all very similar to our post COVID-19 vaccination ED cohort, except for a higher prevalence of heart failure exacerbation at 36% reported in our cohort. This is likely explained by the older age population in our cohort with a median age of 81 (IQR: 73-87) years, compared to the NEDS cohort reported by Raisi-Estabragh with a median age of 67 (IQR: 54-78) (Figure 1B). Raisi-Estabragh et al. also reported that cardiovascular presentation to the emergency department led to an average of 50.7% hospital inpatient admissions [11]. Our cohort had a higher admission rate at 75.7%, again very likely due to the fact our cohort is mainly composed of an older patient population. Although our study was not designed to investigate causality, the fact that the rates of possible CAEs are similar in our vaccinated study population compared to the unvaccinated general population is reassuring and lends credence to the safety of current vaccines.
Case et. al. analyzed vaccine adverse events on patients 18 years or older presenting to the ED within 30 days after the first dose (In Vaccination Window) or second dose (Fully Vaccinated) of COVID-19 vaccine and also found no significant association between vaccinations and rate of presentations for coronary artery disease, heart failure, or conduction abnormality [7].
Fear of adverse events, especially cardiovascular adverse events, following vaccination against SARS-CoV 2 has limited public health efforts to increase vaccination rates in the United States despite the fact vaccines have reduced COVID-19 cases and severe illness in populations with high vaccine coverage [12]. Our study is a purely descriptive assessment of cardiovascular event rates in an elderly population presenting to the ED. As such, it cannot be used to make assumptions about the causality of vaccination and Cardiovascular events. Moreover, our data may not be generalizable to a younger population or to demographic areas beyond Northeast Ohio and the United States. Nevertheless, our study provides important observational evidence that possible cardiovascular adverse events following vaccination are not contributing to increased emergency department visits. These types of observations are an important tool for public health care providers who have to deal with skepticism and misinformation about the consequences of vaccines against SARS CoV2.
References
2. Feleszko W, Lewulis P, Czarnecki A, Waszkiewicz P. Flattening the Curve of COVID-19 Vaccine Rejection-An International Overview. Vaccines (Basel). 2021 Jan 13;9(1):44.
3. Shih SF, Wagner AL, Masters NB, Prosser LA, Lu Y, Zikmund-Fisher BJ. Vaccine Hesitancy and Rejection of a Vaccine for the Novel Coronavirus in the United States. Frontiers in Immunology. 2021 Jun 14;12:558270.
4. Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, et al. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine. 2002 Dec 13;21(3-4):298-302.
5. Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. “First Month of COVID-19 Vaccine Safety Monitoring — United States, December 14, 2020–January 13, 2021,” MMWR Surveillance Summaries. 2021;70(8):283-8.
6. Oh TH, Woo SH, Hong S, Lee C, Lee WJ, Jeong SK. Clinical Features of Patients Presenting to the Emergency Department With Cardiovascular Adverse Reactions After COVID-19 mRNA Vaccination. Journal of Korean Medical Science. 2022 Mar 7;37(9):e73.
7. Case BC, Rosenfeld B, Shea C, Rappaport H, Zhang C, Medranda GA, et al. Implications of COVID-19 Vaccination on Hospital Encounters and Outcomes. The American Journal of Cardiology. 2022 May 1;170:105-11.
8. Jeet Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, Yadav D, et al. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. International Journal of General Medicine. 2021 Jul 27;14:3909-27.
9. Kumar A, Shariff M, Bhat V, DeSimone C, Deshmukh A. Atrial fibrillation after vaccination for COVID-19: analysis of the vaccine adverse event reporting system. Journal of Interventional Cardiac Electrophysiology. 2022 Oct;65(1):1-2.
10. Sun CLF, Jaffe E, Levi R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Scientific Reports. 2022 Apr 28;12(1):6978.
11. Raisi-Estabragh Z, Kobo O, Elbadawi A, Velagapudi P, Sharma G, Bullock-Palmer RP, et al. Differential Patterns and Outcomes of 20.6 Million Cardiovascular Emergency Department Encounters for Men and Women in the United States. Journal of the American Heart Association. 2022 Oct 4;11(19):e026432.
12. Christie A, Henley SJ, Mattocks L, Fernando R, Lansky A, Ahmad FB, et al. Decreases in COVID-19 Cases, Emergency Department Visits, Hospital Admissions, and Deaths Among Older Adults Following the Introduction of COVID-19 Vaccine - United States, September 6, 2020-May 1, 2021. Morbidity and Mortality Weekly Report. 2021 Jun 11;70(23):858-64.
