Abstract
The proposed review reveals the pathogenesis of the malignant tumor process and the patterns of its initiation and development. It was established that the basis of the malignant tumor process is the physiological process of implantation of the embryo in the thickness of the decidual membrane of the uterus and the regulation of the invasive function of the trophoblast, created by evolution.
The determining role of the immune system in regulating these processes was revealed. The possibility of overcoming the malignant tumor process by blocking the key links of the body's immune response to the 37 kDa embryonic antigen has been revealed. The ways and methods of achieving this goal are indicated.
Keywords
Cancer and the Immune System, Cancer immunotherapy, Cancer treatment, Carcinogenesis, Embryo Antigens, Embryogenesis, Tumour antigens
Introduction
In natural conditions, a disease caused by a malignant tumor usually leads a tumor-bearing patient to death. The organism is not able to win it independently. The immune system demonstrates no defence abilities. The reason for this is that the potentially existing antitumor action of the effector block of immunoreactants of the immune system is blocked by its regulatory block, which ultimately manifests itself as a stimulating effect, as evidenced by the progressive course of the tumor process.
To date, there is no rational, scientifically based answer as to why such a paradoxical situation exists (if we look at it from the perspective of the body's interests), how it arose, and whether it has any biological sense.
Noting the extraordinary morphological and functional complexity of the immune response observed in the tumor process and the repeatability of its standard configuration in each case, we concluded that this situation could only be due to its evolutionary origin. This could not have happened within the tumor process, because it is not an object of evolution. This means that such a feature of the immune response to tumor antigens arose in the process of formation of some important physiological function of the body.
The following aims were set in the proposed work:
- To present a broad picture of modern ideas about the role of the immune system in the tumor process;
- Within this picture, describe the fundamental contradictions that impede the achievement of real progress in the fight against malignant diseases;
- To formulate a new broad understanding of the pathogenesis of the malignant tumor process;
- To identify possible new promising areas in the treatment of malignant neoplasms.
State of the Problem
In trying to consider the problem concerning the organism’s immune system's role in the malignant tumor process, it is necessary to understand its current state.
Today, the global community of oncoimmunologists thinks this function activation of immunosuppressive block to be a result of cancer cells activity. It means the cancer as an active subject:
- “constructs” its microenvironment [1];
- suppresses cellular antitumor mechanisms [2];
- “develops different strategies” to counteract the immune response of the organism [3];
- “robs” some normal physiological processes and uses them for its own purposes [4];
- “operates” immunoregulation systems CTLA-4 and Pd-L1 [5];
- “recruits” T-regulative cells in order to block the potential destructive antitumor action of the organism’s immune system [6];
- “counterattacks” [7], etc.
All the actions mentioned above and attributed to cancer cells are inherent in the field of social relations. Any extrapolation of these concepts to the field of objective biological processes and their use as arguments is a fundamental methodological mistake.
The cancer development is an objective pathological process having its own properties of initiation and development, the transformed cells excrescence and metastasis being no more than its manifestations.
Here it is appropriate to present a statement of Davydovski, a world authority in the field of pathology: “In oncology there are yet views on tumors as something independent on the organism, as a special race of cells. The organism is thought to mobilize its own resistance abilities against the cancer danger, the organism protects itself against an aggressive behavior of cancer cells, etc. This is how a subjective, anthropomorphous approach to biological problems is indicated”. He continues: “Tumors reflect an objective biological regularity having nothing to do with metaphysical ideas leading us to the conception of fight-defense”. This regularity can be studied only in general terms of biological processes [8].
Let’s also consider another phenomenon. We have in mind a discovery of a Scottish embryologist Beerd made at the beginning of the last century. He found a striking resemblance of trophoblast cells with malignant tumor cells.
Today, such basic characteristics of malignant cells are known as:
- Invasiveness, infiltrative nature of growth, and metastases;
- High proliferative capacity is not bounded by the Hayflick limit and provided with high telomerase activity;
- Vascular mimicry;
- Synthesis of proteolytic enzymes also inherent to trophoblast cells [9-11].
It proves the existence of two phenotype forms of invasive process:
- Physiological – trophoblast formation during the pregnancy; and
- Pathological – malignant tumor process.
The fundamental difference between them is that one of them is controlled, and the other is not controlled. Having understood the mechanisms regulating trophoblast invasiveness, we can also consider the malignant tumor a trophoblast growth homolog process. In addition to the common features mentioned above, there is one more feature that is exceptionally important for our investigation. We mean the parameters identity of all immune reactions [9-11] during embryogenesis and carcinogenesis, also including transcriptomes [12].
It leads to the conclusion that tumor cells and trophoblast cells possess a common antigen, determining the identity of immune reactions in both cases.
However, the modern oncoimmunology and oncology do not dare to draw such a conclusion. According to any literary evidence, the immune situation during embryogenesis is dependent on alloantigens of father’s origin, while personal neoantigens solve everything during the tumor process. It turns out that there is no common antigen for these two processes. We have a situation of a classical paradox.
Any paradox is always an alarm signal being a proof that something is wrong in our cognitive space [13].
Given that in our case the paradox is based on the problem of cancer, it becomes especially acute, and overcoming it has become an imperative task of today's theoretical oncoimmunology. However, it is not flexible enough in this direction. It would have to identify some regularities in the ocean of data on the manifestations of the tumor process, in which oncoimmunology is now drowning, and create hypotheses and theories based on them that would help solve the problem. However, we do not see sufficiently substantiated hypotheses and theories. We should not blame oncologists for this completely, because there is a general tendency to underestimate theoretical research with a simultaneous inclination to study details and details.
Davydovski wrote 60 years ago: “There is no other field in medicine as oncology, where contradictions are so demonstratively revealed between the wealth of factual material and chaos of ideas concerning tumor etiology and essence. No theory was yet created despite of numerous scattered facts being available” [8].
Claims against theoretical oncoimmunology are also found in a capital work concerning immunophenomenology of the malignant tumor process; it was published half a century later Davydovski: “Possessing a sufficient number of facts, with which our modern oncoimmunology is saturated, a shortage of new worldviews, new views on the problem, new theories should be underlined. The discovery of even extremely extraordinary processes and the establishment of the molecular mechanisms that ensure them do not compensate for this deficit” [7].
Unfortunately, the modern theoretical oncoimmunology pays no attention to the investigation of general mechanisms providing the formation and development of malignant tumor process, i.e. its pathogenesis. In fact, it turns out that this research area has completely dropped out from the field of its scientific interests, although the understanding of this process mechanism is the most important prerequisite for understanding of tumor process. The study of the pathogenesis of the malignant tumor process as a priority has been repeatedly pointed out by prominent oncologists such as Kavetsky, Serebrov [14,15] and others.
Today all the scientific forces of the world oncoimmunologist community aimed at the most detailed research of cellular and molecular changes during the tumor process; they hope to find there some keys to cancer problem solving. However, Mor underlines that “the description of any system’s isolated parts cannot help to understand this system’s properties as a whole” [16]. It was also Mendeleev’s opinion back in 1885 [see 8].
General Causes of Theoretical Oncoimmunology Lagging Behind Today’s Demands
We think such theoretical oncology lag is due to two reasons:
The first reason
Oncoimmunology as a branch of science was born, developed (and continues to develop) in frames of infectious process model. This situation has developed historically. Manifestations of cancer and infectious processes demonstrate some similarity.
The idea that the body's immune response to any antigen is always a "against" reaction, as in an infectious process, has been firmly established in the minds of researchers. This opinion became fixed as a dogma. This dogma worked all the time inhibiting the oncoimmunology development for more than 70 years. It distorted the idea concerning the role of immune system in cancer process. This dogma is still dominant in oncoimmunology.
It also concerns embryogenesis immunology.
More than forty years ago, Prehn and Outzen drew their attention to the fact that the infectious model was unsuitable for cancer studies. They compared the actions of the organism’s immune reaction to infective nature factors and its reaction to the tumor. As a result, they understood that these reactions were somewhat similar but had different bases. If, in the first case, we observe an antagonistic interaction of genetically different organisms, then in the second one, we see the interaction of the organism with deviant cells being natives of the same organism. It goes without saying immune reactions on these cells should be a special one. So, the carcinogenesis study in frames of the infectious immunology model (aggression and defence from aggression) is not promising.
Prehn and Outzen think a rational carcinogenesis study should be carried out on the models of normal reparative processes [17].
This methodological statement of the highest value was not heard until now.
The second reason
When analyzing the mechanisms of carcinogenesis and embryogenesis, oncoimmunologists and immunoembryologists do not take into account the existence of a huge amount of data concerning directly the problem of “immune system and cancer”; so no discovery of cancer mysteries becomes possible because:
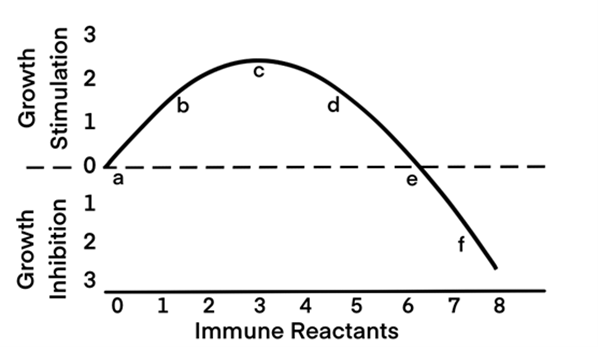
- Any immune reaction of moderate strength stimulates the function of the action object. It is correct for cancer and embryonic cells as well as for functions of all the cells of the organism. In 1970 Prehn who is now thought to be «the father of modern oncoimmunology» [18] demonstrated both in his fundamental theoretical investigation «An immunostimulation theory of tumor development» [19] and in his convincing experiment a weak immune response to stimulate directly (not indirectly) the tumor growth; a strong immune response inhibits such a growth and is able to stop it at all [20]. Prehn underlines such a dependence as a manifestation of a well-known general biological regularity: small doses of biologically active agents, including even toxic ones, stimulate the functions of biological systems, whereas large ones suppress them. This general biological regularity has been known for a long time. Everybody knows the statement of Paracelsus, a brilliant medieval physician: “Any substance is a poison, any substance is a remedy. The dose determines its action.” Prehn presents such a regularity concerning the effect of immune reaction on the course of cancer on the schedule, given here in Figure 1.
- The phenomenon of constitutive embryonic cell invasiveness is not taken into consideration. However, it is related to both embryogenesis and carcinogenesis processes. During the early embryogenesis period, the moving of embryo cell groups or individual cells relative to each other has a place in the intercellular matrix because of these cells' constitutive invasiveness. Such invasiveness is especially pronounced in the migration process of neutral crest rudiments in the embryo intercellular matrix to the place of their destination. Almost all the authors describing the mechanisms of neutral crest rudiments migration underline their striking similarity to mechanisms of malignant cells invasiveness [21-24].
- No data are taken for analysis obtained by the Coggin group and being of key importance for the tumor process disclosure. At the end of the 1960s, Coggin and colleagues paid attention to Prehn’s message informing embryoma to contain sometimes antigens cross-reacting with methylcholanthrene-induced tumour antigens. They carried out several investigations disclosing a number of exclusively important phenomena. The hamster vaccination by embryonic materials taken from hamsters, mice, and humans was found to prevent the development of malignant tumors induced later by viruses or chemical carcinogens in these objects [25-29].
Figure 1. Relationship between tumor stimulation or inhibition and the concentration and/or quality of the immune reactants. (Adapted from Prehn et al. [107]).
Only the material taken from embryos in the first half of pregnancy was shown to be effective. Different extracts obtained from embryos of different animals and from different tumor types demonstrate cross-reactivation.
We know that both embryos older than six decades and malignant tumors possess a common non-species-specific antigen. However, the significance of this fact was declared only at a symposium in 1983, the title of the report containing the name of the antigen [30].
It should be emphasized the animal vaccination is to be carried out not before the tumor process initiation, but during its latent period. This fact is significant because it indicates the path to the elaboration of prophylaxis approaches preventing the development of malignant tumors in individuals influenced by carcinogenesis factors. We can mention incidents at nuclear power plants or individuals with BRCA1 and BRCA2 gene mutations contributing to tumor development [31].
Shortly after the Coggin's results publication, similar investigations were carried out in many labs, including Medawar’s one, the results found by Coggin having been regularly confirmed [32-37]. As a summary it became clear the embryo cells of the first half of pregnancy as well as leukemia and other malignant cells to express a common non-species-specific antigen. This antigen is not expressed by mature embryo and adult cells, nor is it expressed in the course of inflammation and regeneration.
In 1983 at the Symposium concerning “Fetal Antigens and Cancer” Coggin presented his report entitled “Embryonic antigens in malignancy and pregnancy: common denominators in immune regulation” [30]. In his preface, the author underlines that more and more data appear proving the cells of the majority of malignant tumors to carry embryonic determinants (embryonic antigens). At the same time, true embryonic antigens are expressed on fetal and embryonic cells, neither their expression on cells of adult nor regenerating tissues being seen; the immunological role of embryonic antigens in intrauterine fetus development remains unknown.
Coggin also analyzes the results, having been obtained by his group in other labs. In particular, T-cytotoxic lymphocytes were shown to be generated as an answer to embryonic antigens expression by embryo cells. However, they do not damage the carriers of antigenicity because of simultaneous action of suppressive lymphocytes protecting embryo cells. When the inseparable connection exists between effector and suppressive lymphocytes, acting simultaneously, such a situation was named independently by Coggin and Medawar “an immunological coin”. At the end of this publication, there is a schematic image of the “coin’s” action mechanism proposed by these authors. It is seen the objective end result of the immune reaction (“coin”) on the embryo and the tumor is their growth promotion [30].
Both Coggin and Medawar interpret the mechanism of such an event from the position of dominant in that period (and, unfortunately, still dominant) idea that any immune reaction is an “anti-reaction”. They underline the immunosuppressive action of “the coin”. They think the promotion of tumor growth is due to the “coin’s” immunosuppressive action leading to the weakening of antitumor immune reaction which seems to hold back the tumor growth; during embryogenesis “the coin’s” activity weakens the attacking power of the maternal immune response [30].
In the following years, Coggin and his colleagues worked successfully, deepening the study of previously discovered phenomena and researching embryonic antibody biochemistry [38-49].
In 1983, in addition to the publication mentioned previously, which clearly underlined the existence of a common antigen in embryos and malignant tumors, another important event took place. Reports on the discovery of a high-affinity laminin receptor being expressed by malignant tumour cells came independently from three separate labs at one and a half-month intervals. Studying the cells of mammary gland carcinoma, the authors of the first publications found a substance with a high affinity for laminin, the main basal membrane component. According to the authors, this substance's expression promotes the initial interactions of tumor cells with vascular membranes, facilitates their invasion, and increases metastasis [50]. The authors of the second publication isolated an antigen molecule 67 kDa from melanoma cells; they think it facilitates the interactions of tumor cells with a basal membrane [51]. The third publication describes a receptor isolated from lymphosarcoma cells; it is possible that this receptor mediates the interactions of tumor cells with extracellular matrix [52]. These published works aroused interest in this direction and activated relevant studies. In the 1990s, several summaries of these researches were presented in several reviews [53-60].
It is revealed that increased laminin-receptor 67 kDa expression is seen with various forms of cancer affecting organs such as the mammary gland, large intestine, stomach, ovaries, endometrium, lungs, thyroid, and liver. The enhanced laminin-receptor expression is also demonstrated during the preneoplastic stage of tumor process development. The level of the laminin receptor expression correlates with the tumor aggressiveness. The 67 kDa receptor is shown to promote the increase of adhesiveness between tumor cells and laminin, their chemotactic approximation, and proteolytic enzyme synthesis in these cells. The laminin receptor 67 kDa is found to be a dimer; its corresponding gene codes it as a monomer with a molecular mass of 37 kDa [61].
Now, the idea is clearly formed that decreasing the expression of laminin receptors on tumor cells can reduce their invasive potential and metastases [62]. The blocking of the 37kDa/67kDa receptor was studied using the corresponding antibodies. Simultaneously, the search for alternative approaches permitting the blocking of this receptor expression by cancer cells was carried out with the help of low-molecular-weight compounds.
The next compounds have been found capable to join to the laminin receptor 37/67kDa:
- NSC47924 and hierarchically similar NSC479, NSC48478, NSC48861 [63-65].
- Small interferating RNAs and polysulfated glycans [66].
- EGCG – the main green tea catechin [67-69].
At the end of the 1990s, Coggin and colleagues found non-matured laminin receptor of 37kDa and an oncoembryonic antigen studied previously in their lab from the beginning of the 1970s to be identical compounds [70]. This fact shed more light on their previous data and combined two extensive data groups. This work demonstrates ILPR 37 kDa to be an immunogenic protein contrary to 67 kDa. Here we finish our short description of the most important oncoimmunology problems and achievements known today.
“Medawar’s paradox” and Mistakes on the Way to Understanding of Cancer Pathogenesis
First of all, we emphasize the embryo/fetus to express two antigens developing the immune reaction of the mother’s organism. We mean alloantigens of the father’s origin (they are expressed non-stop during the pregnancy) and an embryonic antigen ILRP 37 kDa being expressed only during the first half of pregnancy; there are no mentions of this antigen in any modern review concerning the immunology of pregnancy. The trophoblast as a physiological cancer homolog is a key to learning of cancer secrets, so we are to consider the embryogenesis course from its very beginning. In the process of its fragmentation and division a fertilized egg/zygote moves along the fallopian tube to the uterine cavity and gets there in the form of a blastocyst. Very soon, the blastocyst sticks to the decidual uterine membrane, realizing its contact with a maternal immune system and initiating a series of events, becoming fateful for the embryo. An idea is shared by a majority of immunoembryologists that at this moment the embryo as a carrier of father’s allogeneity is vulnerable to mother’s immune system attacks able to damage it; i.e., an immune conflict [71,72]. Such an opinion does not agree with real events, it even distorts the available facts. What do we see in reality? During a month, a female organism is preparing to accept a new life and to nurture it for a further nine months. And when such a meeting does not take place, she begins to prepare hard for her goal of becoming a mother again. Suddenly, we hear a statement that mother’s immune system attacks the embryo trying to harm it. From a logical point of view, it is a paradoxical situation.
This strange idea was born in 1953 after a report of Medawar. He attracted the attention of the scientific community to a phenomenon that seemed paradoxical; the fact is that any embryo is a peculiar allogeneic transplant in mother’s organism; however, it is not damaged by immune factors, contrary to allogeneic organ transplants which are always rejected by patient’s defence system. At first, Medawar proposed the opinion the mother’s immune system is unable to recognize embryonic antigens; such is the reason for the lack of response. This is preciously the reason of its non-injury. It goes without saying the embryo would be damaged if recognized. After it becomes soon clear the woman’s immune system still recognizes embryo’s alloantigens. According to the prevailing dogma, the embryo must be attacked. Here a real paradox appears: there is no damage in the presence of the damaging action. It was called “a Medawar paradox”. Intensive studies began aiming to understand how the task of allogeneic transplant incompatibility in the mother-fetus system had been solved; having answered this question, we can use our knowledge in practical transplantology. The intensity of this research may be illustrated by the eloquent title of a review, “The study of tissue incompatibility on the mother-fetus model”, published in 1968. More than a hundred of publications were reviewed and analyzed there. At the end of the review the author says the causes of the non-rejection of fetus remain unknown; further accumulation of factual material is necessary [73]. The Medawar’s paradox remains still an unsolved mystery in immunology. All efforts to find a way to overcome it have been unsuccessful during 70 years. We think such a state of problem is due to four mistakes which are intertwined in a tight knot and strengthen each other.
The first mistake (a comprehensive one)
The immune reaction is interpreted unilaterally as a damaging “anti-reaction”, its ability to cause a stimulating effect being ignored. It is a dogma mentioned at the beginning of this review. However, since the first decade of the last century, many publications have appeared proving that the immune reaction may also have a stimulating effect.
The second mistake
The immune reaction against the allogeneic embryo is considered not as an immune system reaction as a whole, but as a sum of reactions of its separate elements able to compete with each other. However, the immune system acts as an indivisible whole; the properties of its integral action may be quite opposite to our idea, which was developed as a result of a simple summation of the activities manifested by its individual elements. Immunologists consider the activation of effector lymphocytes by embryonic alloantigens as a reaction of the whole mother’s immune system. From here, a conception is developed concerning the attack and mother-fetus immune conflict. Effector lymphocytes (they are always activated during the pregnancy) are thought to form the hostile micro-environment being damaging for the embryo [74]. Simultaneously, T-regulatory/suppressory lymphocytes soften this effect without its complete elimination.
We emphasize again – it is a mistake to think the integral immune system becomes divided into opposite parts, the properties of integral maternal immune system reaction on the embryo remains unknown.
The third mistake
Equal sign is put between events developing after allogeneic organ transplantation and embryogenesis-accompanying immune situation. It is an oversight of Medawar who has not noticed a principal difference between an artificially created situation appearing after organ transplantation and a natural situation developed in the course of embryogenesis evolution. As a result, the fetus non-rejection is considered as a transplantology problem [75], as a violation of its classical rules [76]; so during seven decades the researchers try to put embryogenesis in the Procrustean bed of these rules.
However, a question should be asked: what is immune system’s objective task during the pregnancy?
Does this task consist in the rejecting of the embryo, such a conception being derived from the immune attack theory? Of course, it is a wrong idea! It is a radical contradiction to the laws of nature. The wise evolution process has been working for millions of years, aiming to elaborate a number of mechanisms contributing as much as possible to embryo development. Undoubtedly, the functions of the elements of the immune system were also coordinated so that the overall result of their action was the stimulation of the development of the embryo. It is, namely, an immunostimulating action that explains all the advantages of allogeneic pregnancy over syngeneic one. It is appropriate here to quote Prehn’s work: “In a certain period, next to the development of anti-transplantation immune reactions, immune mechanisms prevail promoting the development of embryo cells” [77].
Our PRESENT views were previously demonstrated in some publications of Beaman and colleagues: “The pregnancy is a tumor model, it is not a transplantation model” [78] and “Immune response during the pregnancy and tumor process is active and supporting the growth of placental and tumor cells, it is not a response destroying them” [79]. Unfortunately, they did not give any further development of this theme, and they also gave no recommendations based on their point of view. Several more authors also revised Medawar’s conception [80-82].
Our conclusion is the following: there is no mother’s immune system attack against the embryo, there is no immune conflict between the mother and the embryo, and no tolerance is needed, so Medawar’s paradox finishes its existence.
Let’s be fair: researchers are advocating for the usefulness of immune reactions in embryos, even for their necessity; however, they also declare that the first necessity is tolerance creation [83-85].
The forth mistake
Violation of the principles for overcoming paradoxes:
Any paradox problem has always two sides. Firstly, a certain fact appears suddenly; secondly, there are a number of generally accepted ideas, theories, conceptions, giving no possibility to explain such a fact. To overcome the paradox, we are to verify, first of all, the reliability of the fact existence, correctness of its discovery. If we have no doubt about it, we have to analyze carefully and impartially the reality of ideas and theory which are unable to explain this fact [13].
In our case, the reality of the fact concerning the fetus safety from the mother’s immune system is absolute, so it is necessary to analyze the reality of ideas concerning the damaging nature of immune reaction.
However, all the immunoembryologists adhere firmly to the theory that the immune reaction to be of damaging character. So they strive to study all the details of paradox-creating situation hoping to find there a key for overcoming it [86].
A Way from the Damaging “Anti-Reaction” to the Idea of “Regulation”
Now, in immunology (and in biology in general), little attention is paid to the regulator function of the immune system, belonging to the main systems regulating the organism’s life activity together with hormonal, nervous, and circulatory ones: the immune system interacts with all of them. Not so long ago, autoantigen expression by cells was considered dangerous; such an expression increases the risk of damage to the immune system. So the autoimmune lymphocytes are usually removed during their development in the thymus or later are deeply repressed. It is a way to eliminate the autoantigens system from “the game”.
Let’s consider the situation, including autoantigen and the organism’s reaction to it through the matrix of evolution. It is known the main evolution principle is selection followed by useful traits fixing. And the autoantigen phenomenon has passed through the crucible of evolution. So it is a useful situation. What is its usefulness?
The body's immune system is a structure that consists of three inextricably linked reactant blocks: antigen reception and processing block, effector and regulatory block. The effector block actions include both functional stimulation of the antigen carrier and this carrier destruction. At the same time, the regulative block defines the strength of the effector one depending on a situation needs. The action of three blocks, i.e. immune system’s action, is antigen-specific one.
Based on the above consideration, let’s analyze the situation with autoantigens in more detail.
Then such a hypothetical picture appears. Autoantigens carriers are cells which perform certain functions in the body. Each immunoantigen receives its own system having three blocks. The power of immune action is established which ensures the optimal level for the function of the autoantigenicity carrier. And when the body's need for this function changes in one direction or another, it is recorded by the analyzers of the body's homeostatic system, and from there a signal is sent to the immune system function regulation mechanisms. As a result, the functional level of the autoantigenicity carrier changes according to the situation's requirements. Such block systems and their antigen-specific function appear and work only during embryogenesis period, as various body systems are formed. No such systems appear during later stages of development. This process is gradual. For example, the antigens of the main histocompatibility complex begin to express themselves not from the very beginning of embryogenesis, but with a delay. In cows, this process begins on the 120th day of pregnancy being earlier blocked at the post-transcriptional level [87-89].
Recently, a detailed review has appeared concerning the study of immunoregulation functions of lipid system cells, digestive organs, skin, and muscles [90]. The main idea of this publication is to state a fact of somatic cells regulation by organism’s immune system. The opinions of the authors are fully consistent with our vision outlined above and concerning the expression of cell-specific autoantigens by the cells of our organism and the role of these autoantigens in cell functions regulation. The authors emphasize on the T-regulatory cells participation in this process. However, T-regulatory cells do not act directly on the object of regulation.
They are only one of the three blocks of immunoreactants of the body's immune system, thanks to their coordinated action, the optimal level of function of the cells listed above is established and maintained.
Let’s consider another bright example. As mentioned above, in the early 1970s, Coggin et al. found the embryo from the very beginning of pregnancy to its middle to express a non-species-specific antigen; animal vaccination by this antigen protects them from the development of induced malignant tumors. It was called transplantation antigen or tumor rejection antigen. We do not agree with the interpretation: the term “transplantation antigens” is usually used to indicate histocompatibility antigens, the antigen discovered by these authors is not like that. It is the non-mature laminin-receptor 37 kDa [70]. The fact that it is expressed only in the first half of pregnancy indicates its connection with some process that occurs during this period. Such a process is the invasion of trophoblast cells into the thickness of the decidual membrane and the formation of an intimate connection between the embryo and the mother's body. And the 37 kDa laminin receptor is an autoantigen through which immunoregulation of trophoblastic function occurs. This is its role in the intrauterine development of the fetus.
The fact that Soggin and his colleagues did not notice this function of the 37 kDa laminc receptor (embryonic antigen) [30] in no way diminishes the absolutely invaluable value of the objective data obtained by them for understanding the processes of carcinogenesis. We paid great attention to immunostimulation to underline its importance in regulating organisms' functions. Now we are ready to consider embryonic antigens being a common denominator for embryos and malignant tumor. Here is the key to the secrets of cancer.
Let’s continue a step-by-step description of carcinogenesis.
Any embryo or blastocyst that reaches the uterine cavity must attach to the mother’s organism as a source of its life support. For this purpose, it must penetrate into the decidual membrane of the uterus and implant there.
A lot of works concerns the study of implantation mechanisms, in particular the role of immune reaction in this process. The results obtained have been recently reviewed by Yoshinaga [91,92] and in a monograph [85].
These publications give us a colourful picture, including many immunological phenomena seen during embryo implantation, but no uniting idea can be detected. It is also clear at once that both authors are supporters of the same wrong conception on the necessity of embryo-damaging action neutralization and achieving tolerance to it. Of course, these authors do not mention the existence of the embryonic antigen of 37 kDa and its expression on embryonic cells. The authors insist preciously on the necessity of further study of implantation process details. Here is appropriate to mention once more a Ernst Mayr’s opinion: “no description of isolated parts cannot lead to the understanding of the system as a whole” [31].
We see the next scheme of the course of an implantation process.
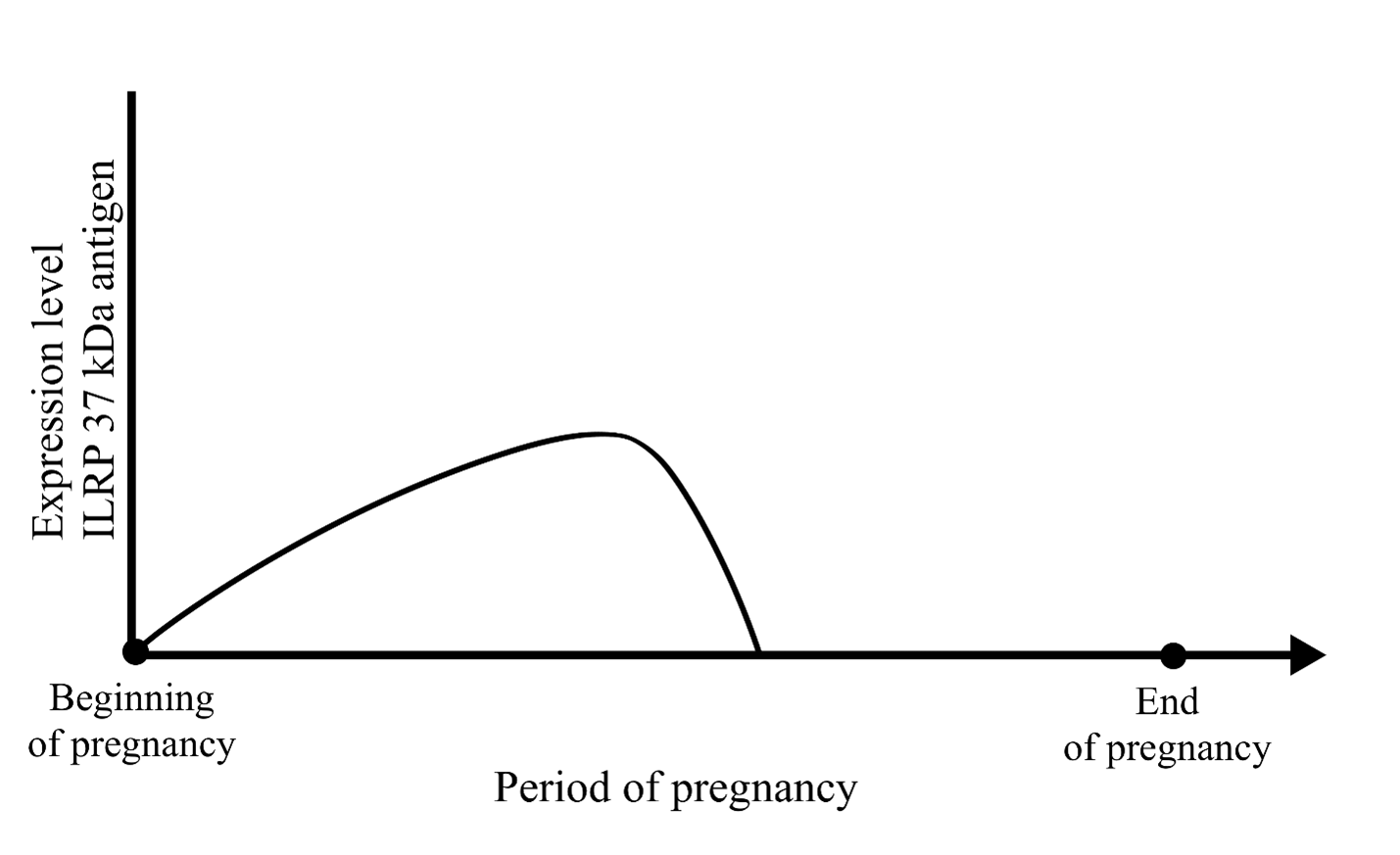
Embryonic cells have constitutive invasiveness at their disposal, which allows them to move inside the embryo itself, in its extracellular matrix. The density of uterine laminar structures, which the embryo must overcome to reach the uterine glands first and then the mother’s blood, exceeds incomparably the density of the intra-embryonic acellular matrix. The invasive force of embryonic cells should be increased to complete such a task. When the blastocyst comes into contact with the mother’s immune system, the cells of the blastocyst’s ectodermal layer being carriers of invasiveness and expressing embryonic antigens, stimulate an immune reaction. As a result of its stimulating action, the invasiveness level of embryo’s trophectodermal cells rises to the required level followed by the autocrine immunoregulation regime based on the activity of the ILRP 37 kDa, responsible for the invasion. The embryo implants, and a trophoblastic process begins. To perform this absolutely necessary action for the existence and development of the embryo, evolution provided the body's immune system with the ability to respond to the 37 kDa embryonic autoantigen. This ability is preserved throughout the life of the organism, regardless of its age and sex. Our awareness of such scheme is due to the results of Coggin’s investigations. First, we evaluated the data concerning autoantigens expression by embryo cells during a limited period – from the beginning of the pregnancy to the end of its half period (Figure 2).
Figure 2. Dynamics of ILRP 37 kDa antigen expression during pregnancy (by J. Coggin).
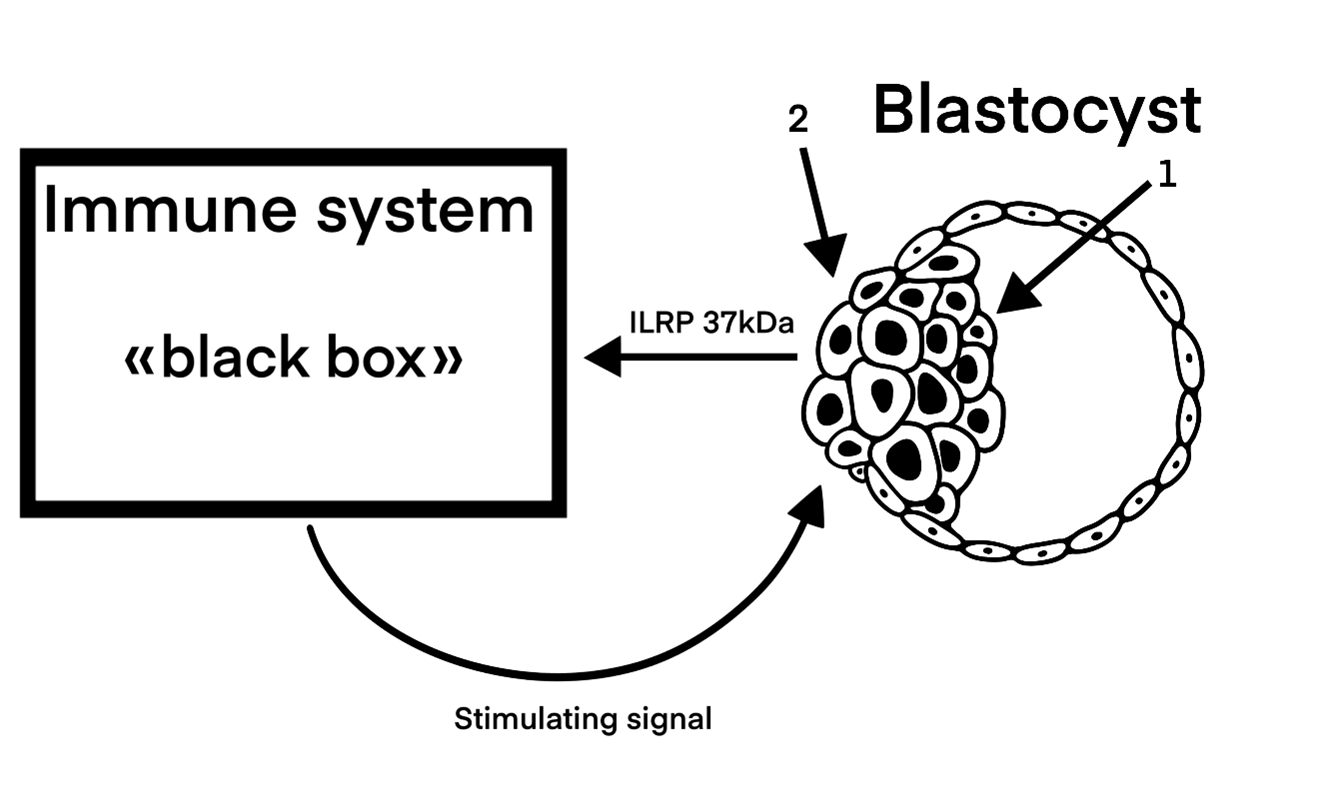
During this period, an extravillous trophoblast invades the decidual membrane's thickness and builds the placenta's main structures. According to the embryo development program, the invasive phase of trophoblast formation ends in the middle of pregnancy. At this point, the embryonic 37 kDa antigen is eliminated from the immune reaction having previously supported high invasiveness of trophoblast cells. The mechanism of its disappearance is not completely studied. Scientists are inclined to think immunogenic non-mature laminin-receptor 37 kDa to undergo acylation and to turn into a non-immunogenic mature laminin-receptor form with increased molecular mass [70]. It is possible that its synthesis stops. For clarity of our presentation we give now a scheme of the implantation process (Figure 3).
Figure 3. Process of blastocyst implantation. 1. Inner cell mass. 2. Beginning of the trophoblast invasion into the decidual uterus envelope.
The embryonic antigen being expressed by external cells of the blastocyst ectodermal layer are received by the immune system (let’s depict it as a “black box”). There its processing has place and a stimulating signal appears; coming out of “the box”, it increases the constitutive invasive function of blastocyst’s ectodermal cells to a high level required. The carriers of the stimulating signal are T-effector lymphocytes sensitized by embryonic antigen.
In such a way the bounding of the embryo to mother’s organism begins. We are convinced that researchers of embryogenesis immunology getting acquainted with our work will see without any difficulty how a lot of problems caused by a dogma of one-sided counter reaction will disappear.
Malignant Tumor Process and Ways of its Overcoming
According to the most developed mutagenic theory of carcinogenesis, the action of different carcinogens on organism’s cells leads to mutations of different nature; such mutations cause the restoration of stage-specific genes, i.e. oncogenes having been repressed in the course of the organism development. These genes are normal ones; they are fully functional at early embryogenesis stages and determine all the features of embryonic cells. Later, during the development of the embryo-fetus, they are repressed. If these genes' derepression has a place in any cell of the mature organism due to different carcinogenic factors, this cell becomes embryonized and acquires all the attributes of embryonicity – embryonic antigens expression, constitutive invasiveness, etc. Antigens stimulate immune reaction. Later, the events proceed according to the trophoblastic scenario described above: the immune reaction raises the constitutive invasiveness of embryonized cells to a high level. This is how the tumor process begins.
Here there is no program being able to convert the embryonic antigen into a non-immunogenic form or to stop its synthesis during the embryo development; there is no such program being able to stop the immune reaction and, therefore, the invasive process. So this process continues indefinitely in time and space. A strong argument in favor of such occurrence of carcinogenesis is the phenomenon of malignancy concerning ectopic transplants of embryonic cells observed during the pre-implantation period [93-95].
Now we shall consider (from our previously justified position) the tumor interactions with body’s immune system and ways of our possible intervention into the tumor process.
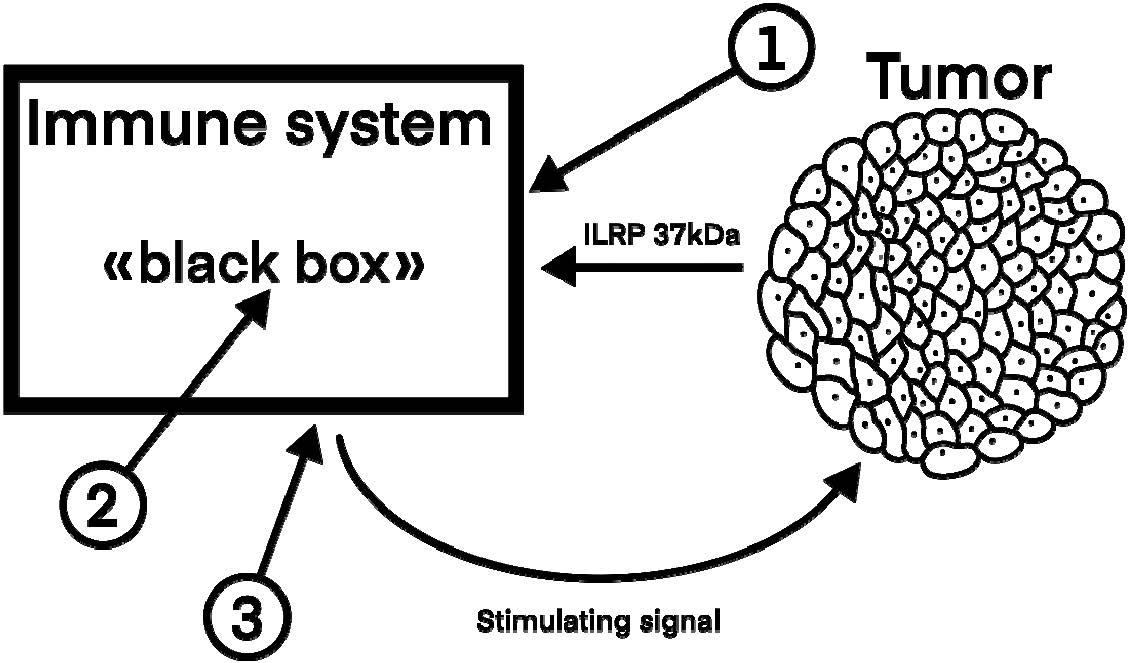
We shall use “the black box” model as in the case of analysis of the embryo implantation mechanism (Figure 4).
Figure 4. Interactions between the tumor and body’s immune system and approaches of possible intervention in the malignant tumor process.
There are three key links of initiation and development of the malignant tumor process including:
- The presence of an embryonic antigen (ILRP 37kDa) before the entrance into ‘the box”; this antigen comes firstly from “embryonized” cells and later from the tumor itself.
- This antigen reception on the entrance into “the box”, antigen processing, and formation of a tumor-stimulating signal.
- Signal output from “the box”. Its carriers are T-effector lymphocytes sensitized by the embryonic antigen.
To stop the tumor process, it is enough to remove from it only one of these key links. Let’s consider the possibilities to reach this aim.
The first link: The presentation of an embryonic antigen. As mentioned above, in order to stop the invasion of the trophoblast, the nature withdraws from the immune reaction the embryonic antigen ILRP 37kDa being responsible for the invasion. There is a real possibility to reproduce this process artificially. First of all, it can be realized using monoclonal antibodies – a fact confirmed by a lot of experimental researches. The vast majority of them were realized using in vitro models. It is clear the use of anti-ILRP 37 kDa antibodies decreases the invasiveness of cells taken from different tumors [96-101].
However, the in vitro investigations do not reflect completely the picture of a real malignant tumor process. They do not include such an important component of the process, as the body’s immune reaction against the tumor embryonic antigen.
Very important results were obtained using a combined in vitro + in vivo model. Following intravenous introduction of malignant tumor cells treated previously by a serum against laminin-receptor, their metastasis activity dropped almost by 20 times comparing to the control level (P<0.005) [102].
In 2015, a completely in vivo experiment was carried out [103]. Having used two tumor process models (leukemia and melanoma), the researchers studied engraftability and degree of tumor cells metastasis on the background of previously introduced anti-ILRP 37 kDa monoclonal antibodies activity. In two experiment variants using two tumor process models (leukemia and melanoma), the authors studied tumor cells engraftability and degree of metastases activity following the introduction of anti-ILRP 37 kDa monoclonal antibodies. Having received a positive result, the authors conclude that anti-ILRP 37 kDa monoclonal antibodies may be used as adjuvants for malignant tumor treatment in order to decrease their invasiveness and metastases formation. The authors did not understand the mechanisms having led to such results.
We have found no data concerning the use of monoclonal antibodies to terminate a malignant process having been already manifested. The reason is the lack of an idea about the malignant process's pathogenesis: the picture above (Figure 3) clearly shows that the binding of embryonic antigens should stop the malignant process. Now, there is an urgent need to close this gap and carry out wide investigations using different models of malignant tumor processes that have already manifested. We are completely sure that even the first studies carried out using shock doses of monoclonal antibodies should give impressive results. This opinion is supported by the fact that the introduction of monoclonal antibodies against the non-mature laminin-receptor into a pregnant murine uterus leads to the fetus's death and resorption [104]. The trophoblastic process is known to be a physiological homolog of the tumor one. The development of this approach is profitable also because here we have already tools of influence – monoclonal antibodies. So far, more than a dozen monoclonal anti-ILRP 37 kDa antibody variants have been proposed and patented [105]. We only have to use these tools correctly. Theoretically, the effectiveness of antibodies may depend on which epitope it binds to.
The idea of embryonic antigens blocking to overcome the tumor process was proposed for the first time nine years ago in our work “Specific immune response of the body as an initiating and promoter factor of carcinogenesis” [106]. At that time, there was still no complete understanding of the carcinogenesis process pathogenesis. We have only begun to consider it. This work was highly appreciated by R.T. Prehn, who considered it as “an excellent and extensive review” [107]. Prehn also gave justification for a similar idea in his publication “Cancer Immunotherapy by Immunosuppression” [108].
The second link is antigen reception at the entrance into “the box”. So far, there are no real possibilities for blocking it completely. Modern oncoimmunology is now involved in intervening in events taking place in “the black box,” hoping to achieve a breakthrough in cancer treatment. All efforts, according to the main slogan “let’s use the immune system to fight cancer”, are aimed at raising the power of the immune system’s effectory block to the tumor-destroying level.
Two approaches are used for this aim. The first one tries to increase the level of the effectory block power by its direct stimulation. The second approach aspires to reach the same aim due to the maximal weakening (ideally – due to the complete blocking) of the regulation block’s immunosuppressive ability. In the first case, different antitumor vaccines are mostly used for stimulation, the successes achieved being rather modest. They are measured only by the survival time of patients after treatment; it is not about a full recovery. Considering that this approach has been used for more than 50 years, we believe that all its possibilities have already been exploited. We are reminded of an Prehn article, in the title of which a question is included: “…why anticancer vaccines don’t work?” [109]. The author doubts that the situation will improve over the next half century.
The second approach is based on intervention in cellular and molecular regulatory mechanisms aiming to stop the regulatory block activity and to use its possibility to realize its potential cytotoxic tumor-destroying ability. In this case, different monoclonal antibodies are used directed against different links of the immunoregulation system. This approach's successes are more significant than those of the first one. Quite often, patients with generalized malignant processes recover completely. Unfortunately, only a third or even less of patients are sensitive to the drugs developed so far.
We think that the reason is the following. The immunity system is a super-complex set of different elements, so it can be assumed that its final task – a stimulating signal creation – is performed not by one, but by various combinations of elements. There is, for example, a combination of elements with crucial regulatory role of its immunoregulative sub-systems CTLA-4, or PD-L1, or both together. In this case the blocking of these sub-systems leads to the release of effectors’ cytotoxic activity and to the desired therapeutic effect. However, there are some other immunoregulation systems besides CTLA-4 and Pd-L1, such as Fas-Fas ligand, IDO, Tol-Tol tumor-necrotic factor etc. A message has recently appeared concerning one more mechanism allowing a cancer tumor to escape the immune response action; it was described at the Geneva branch of the Ludwig Institute. The third link is the presence of stimulating signal output from “the box”. Its carriers are lymphocytes activated by the tumor antigen. Such their function can be blocked using an immunosuppressive placenta factor found by Chaouat and colleagues in 1983 [110].
In the 1970s, a research group guided by the Komisarenko found water-salt extracts from human placentas taken during normal births to possess powerful antitumor properties.
This extract effect was studied using four models of tumor process:
- on rabbits (two protocol variants) using a model of generalized form of Brown-Pierce carcinoma;
- on mice with a solid form of Ehrlich carcinoma;
- on three horses being a model of spontaneous generalized melanoblastosis.
Effect of the treatment:
- full recovery in 80% of rabbits;
- full recovery in 50% of mice;
- all the three horses made a full recovery.
A number of biological phenomena having been not previously observed were discovered during this experiment:
- the presence of antitumor properties in the sera of rabbits having been cured from disseminated Brown-Pierce carcinoma;
- tumor regression against the background of its increased blood supply;
- expanding of the regeneration scale up to the organ regeneration (tail tips grew in cured mice);
- rejuvenation of the body (restoration of working capacity in healed horses, restoration of their sex activity and color of horse fur characteristic of Orlov trotters) (to be published).
A fair question arises: What has happened to tumors and their metastases after the blocking of the stimulating signal and the stopping of the tumor process?
Our results are the following:
- after the treatment, no metastatic nodes were palpated in the rabbit stomach; subcutaneous carcinoma nodes of mice disappeared; in horses, melanoblastic nodes disappeared almost completely, sometimes, small hardenings are seen on their place;
- a series of consecutive photos demonstrating the process of a cancerous node regression with a necrotic apex on a rabbit’s eye at the beginning of a necrotic scab resorption; then the tumor resolved within 120 days; all left is an eyesore.
- in a rabbit killed in the middle of the treatment process, there are:
- hematopoietic cells are visible in the centre of kidney metastatic nodes;
- a clear picture of tumor metastases degradation and fragmentation is seen on the liver slices; the normal hepatic tissue structure is found between tumor tissue fragments.
Next studies must help us understand the mechanism responsible for the resorption of tumors and their metastases after the tumor process terminates.
Evaluation of these methods leading to the blocking of nodal links of tumor process.
The first is undoubtedly the most perspective. It leads to the complete and quick termination of the tumor process in the whole organism. Besides, it is the most attractive one in terms of implementation. All the experimental research has already been realized, which indicates high prospects for the development of this approach and a real probability of achieving significant success in the near future. This is a universal approach.
Another approach is real but very complex, as described above. It is difficult to expect quick success in this approach, which takes a lot of time and research.
Cancer is a dynamic system in equilibrium. If a balanced system is acted upon from the outside, then processes aimed at counteracting these changes arise in this system (Le-Chatelier-Brown principle). This circumstance sheds light on the cause of relapses some time after treatment has previously led to a positive outcome.
The third approach is attractive because of its versatility and because it detects accompanying phenomena. But before starting to develop it, it is necessary to repeat the results of studies already conducted in reputable labs and verify the reproducibility of the results obtained.
Evaluating all the data considered above, it is clear there are absolutely real ways for the elaboration of new effective approaches to defeat malignant tumor disease.
Conclusions
The results of our research could be summarized as follows:
- A broad picture of modern ideas about the role of the immune system in the mechanisms of the tumor process was presented.
- The fundamental contradictions that arise within this picture are identified and described
- A new broad understanding of the malignant tumor process pathogenesis is formulated.
- It is shown that the emergence and development of a malignant tumor process is based on the physiological mechanism of embryo implantation in the thickness of the decidual membrane of the uterus and the formation of a trophoblast created by evolution.
- The decisive role of the body's immune response to the ILRP 37 kDa antigen in the initiation and promotion of the tumor process was revealed, and its key links were named.
- It is indicated that it is possible to overcome the malignant tumor process by blocking the key links of the immune response to the ILRP 37 kDa antigen.
Methods for blocking key links of the immune response to the ILRP 37 kDa antigen have been proposed.
Conflict of Interest
The authors declare no conflict of interest.
Funding Statement
There was no funding got this publication.
Acknowledgement
We bow our heads before the bright memory of the unforgettable Richmond Prehn. We are grateful to him for his support and high appreciation of our work.
We are also particularly grateful to Dr. Victor Shevchenko for his invaluable moral, scientific, and organisational support.
The authors also sincerely thank Prof. Leonid Gorb for his long-term support and help collecting literary sources.
References
2. Muenst S, Läubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016 Jun;279(6):541-62.
3. Guzman-Genuino RM, Diener KR. Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol. 2017 Feb 17;8:172.
4. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016 Feb;39(1):98-106.
5. Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007 Mar;56(3):271-85.
6. Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807-39.
7. Berezhnaya NM, Chekhun VF. Immunology of malignant growth. Kyiv: Nauk. Dumka; 2005. 791 p. (in Russian).
8. Davydovskiy IV. Problems of Causality in Medicine (Etiology). Moscow: Medgiz; 1962. 176 p. (in Russian).
9. Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009 Nov;84(11):985-1000.
10. Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007 Mar-Apr;13(2):121-41.
11. Soundararajan R, Rao AJ. Trophoblast 'pseudo-tumorigenesis': significance and contributory factors. Reprod Biol Endocrinol. 2004 Mar 25;2:15.
12. Nehar-Belaid D, Courau T, Dérian N, Florez L, Ruocco MG, Klatzmann D. Regulatory T cells orchestrate similar immune evasion of fetuses and tumors in mice. J Immunol. 2016 Jan 15;196(2):678-90.
13. Sukhotin AK. Paradoxes of Science. Moscow: Molodaya gvardiya; 1980. 238 p. (in Russian).
14. Kavetsky RE. Interaction between the body and the tumor. Kyiv: Nauk. Dumka; 1977. 235 p. (in Russian)
15. Serebrov AI. About one of the possible areas of research in oncology. Voprosy Oncologii 1975; 21(8):3-13.
16. Greek R, Hansen LA. Questions regarding the predictive value of one evolved complex adaptive system for a second: exemplified by the SOD1 mouse. Prog Biophys Mol Biol. 2013 Nov;113(2):231-53.
17. Prehn RT, Outzen HC. Immunostimulation of tumor growth. Prog Immunol. 1980;4:651-75.
18. Finn OJ. Human tumor antigens yesterday, today, and tomorrow. Cancer Immunol Res. 2017 May;5(5):347-54.
19. Prehn RT, Lappe MA. An immunostimulation theory of tumor development. Transplant Rev. 1971;7:26-54.
20. Prehn RT. The immune reaction as a stimulator of tumor growth. Science. 1972 Apr 14;176(4031):170-1.
21. Ji Y, Hao H, Reynolds K, McMahon M, Zhou CJ. Wnt signaling in neural crest ontogenesis and oncogenesis. Cells. 2019 Sep 29;8(10):1173.
22. Szabó A, Mayor R. Mechanisms of neural crest migration. Annu Rev Genet. 2018 Nov 23;52:43-63.
23. Maguire LH, Thomas AR, Goldstein AM. Tumors of the neural crest: Common themes in development and cancer. Dev Dyn. 2015 Mar;244(3):311-22.
24. Powell DR, Blasky AJ, Britt SG, Artinger KB. Riding the crest of the wave: parallels between the neural crest and cancer in epithelial-to-mesenchymal transition and migration. Wiley Interdiscip Rev Syst Biol Med. 2013 Jul-Aug;5(4):511-22.
25. Coggin JH, Ambrose KR, Anderson NG. Fetal antigen capable of inducing transplantation immunity against SV40 hamster tumor cells. J Immunol. 1970 Aug;105(2):524-6.
26. Coggin JH Jr, Ambrose KR, Bellomy BB, Anderson NG. Tumor immunity in hamsters immunized with fetal tissues. J Immunol. 1971 Aug;107(2):526-33.
27. Ambrose KR, Anderson NG, Coggin JH. Interruption of SV40 oncogenesis with human foetal antigen. Nature. 1971 Sep 17;233(5316):194-5.
28. Leffell MS, Coggin JH Jr. Common transplantation antigens on methylcholanthrene-induced murine sarcomas detected by three assays of tumor rejection. Cancer Res. 1977 Nov;37(11):4112-9.
29. Coggin JH Jr, Adkinson L, Anderson NG. Fetal antigens shared as transplantation rejection antigens on chemically induced mouse and hamster sarcomas. Cancer Res. 1980 May;40(5):1568-73.
30. Coggin JH Jr. Embryonic antigens in malignancy and pregnancy: common denominators in immune regulation. Ciba Found Symp. 1983;96:28-54.
31. Singh K, Lester J, Karlan B, Bresee C, Geva T, Gordon O. Impact of family history on choosing risk-reducing surgery among BRCA mutation carriers. Am J Obstet Gynecol. 2013 Apr;208(4):329.
32. Grant JP, Wells SA Jr. Tumor resistance in rats immunized to fetal tissues. J Surg Res. 1974 May;16(5):533-40.
33. Ting CC, Grant JP. Humoral antibody response and tumor transplantation resistance elicited by fetal tissues in mice. J Natl Cancer Inst. 1976 Feb;56(2):401-4.
34. Medawar P, Hunt R. The significance of embryonic reexpression in cancer. Cancer Res. 1976 Sep;36(9 PT 2):3453-4.
35. Bansal BR, Mark R, Rhoads JE Jr, Bansal SC. Effect of embryonic tissue immunization on chemically induced gastrointestinal tumors in rats. I. Can embryonic antigens act as rejection antigens? J Natl Cancer Inst. 1978 Jul;61(1):189-201.
36. Weppner WA, Coggin JH Jr. Antigenic similarity between plasma membrane proteins of fetal hamster cells and simian virus 40 tumor surface antigens. Cancer Res. 1980 May;40(5):1380-7.
37. Gautam S, Deodhar SD. T-cell-mediated antitumor immune response induced by oncofetal antigens. J Natl Cancer Inst. 1981 Oct;67(4):939-45.
38. Gussack GS, Rohrer SD, Hester RB, Liu PI, Coggin JH Jr. Human squamous cell carcinoma lines express oncofetal 44-kD polypeptide defined by monoclonal antibody to mouse fetus. Cancer. 1988 Jul 15;62(2):283-90.
39. Radiation-induced lymphoblastic lymphomas/leukemias and sarcomas of mice express conserved, immunogenic 44-kilodalton oncofetal antigen. Am J Pathol. 1988 Jan;130(1):136-46.
40. Coggin JH. Shared cross-protective OFAs on chemically induced rodent sarcomas. Immunol Today. 1989 Mar;10(3):76-8.
41. Barsoum AL, Coggin JH Jr. Immunogenicity of a soluble partially purified oncofetal antigen from murine fibrosarcoma in syngeneic mice. J Biol Response Mod. 1989 Dec;8(6):579-92.
42. Barsoum AL, Coggin JH Jr. Isolation and partial characterization of a soluble oncofetal antigen from murine and human amniotic fluids. Int J Cancer. 1991 May 10;48(2):248-52.
43. Rohrer SD, Sarli RN, Barsoum AL, Hester RB, Coggin JH Jr. Expression of 44-kilodalton oncofetal antigen as a premalignancy marker in X irradiation-induced murine T-cell lymphoma. J Natl Cancer Inst. 1992 Apr 15;84(8):602-9.
44. Coggin JH Jr, Rohrer SD, Hester RD, Barsoum AL, Rashid HU, Gussack GS. 44-kd oncofetal transplantation antigen in rodent and human fetal cells. Implications of recrudescence in human and rodent cancers. Arch Otolaryngol Head Neck Surg. 1993 Nov;119(11):1257-66.
45. Coggin JH Jr. Classification of tumor-associated antigens in rodents and humans. Immunol Today. 1994 May;15(5):246-7.
46. Rohrer JW, Rohrer SD, Barsoum A, Coggin JH Jr. Differential recognition of murine tumor-associated oncofetal transplantation antigen and individually specific tumor transplantation antigens by syngeneic cloned BALB/c and RFM mouse T cells. J Immunol. 1994 Jan 15;152(2):754-64.
47. Rohrer JW, Coggin JH Jr. CD8 T cell clones inhibit antitumor T cell function by secreting IL-10. J Immunol. 1995 Dec 15;155(12):5719-27.
48. Coggin JH Jr, Barsoum AL, Rohrer JW. Tumors express both unique TSTA and crossprotective 44 kDa oncofetal antigen. Immunol Today. 1998 Sep;19(9):405-8.
49. Rohrer JW, Barsoum AL, Dyess DL, Tucker JA, Coggin JH Jr. Human breast carcinoma patients develop clonable oncofetal antigen-specific effector and regulatory T lymphocytes. J Immunol. 1999 Jun 1;162(11):6880-92.
50. Terranova VP, Rao CN, Kalebic T, Margulies IM, Liotta LA. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444-8.
51. Rao NC, Barsky SH, Terranova VP, Liotta LA. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804-8.
52. Malinoff HL, Wicha MS. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475-9.
53. Mafune K, Ravikumar TS, Wong JM, Yow H, Chen LB, Steele GD Jr. Expression of a Mr 32,000 laminin-binding protein messenger RNA in human colon carcinoma correlates with disease progression. Cancer Res. 1990 Jul 1;50(13):3888-91.
54. Castronovo V. Laminin receptors and laminin-binding proteins during tumor invasion and metastasis. Invasion Metastasis. 1993;13(1):1-30.
55. Martignone S, Menard S, Bufalino R, Cascinelli N, Pellegrini R, Tagliabue E, et al. Prognostic significance of the 67-kilodalton laminin receptor expression in human breast carcinomas. J Natl Cancer Inst. 1993 Mar 3;85(5):398-402.
56. Landowski TH, Dratz EA, Starkey JR. Studies of the structure of the metastasis-associated 67 kDa laminin binding protein: fatty acid acylation and evidence supporting dimerization of the 32 kDa gene product to form the mature protein. Biochemistry. 1995 Sep 5;34(35):11276-87.
57. Menard S, Castronovo V, Tagliabue E, Sobel ME. New insights into the metastasis-associated 67 kD laminin receptor. J Cell Biochem. 1997 Nov 1;67(2):155-65.
58. Sobel ME. Differential expression of the 67 kDa laminin receptor in cancer. Semin Cancer Biol. 1993 Oct;4(5):311-7.
59. Menard S, Tagliabue E, Colnaghi MI. The 67 kDa laminin receptor as a prognostic factor in human cancer. Breast Cancer Res Treat. 1998;52(1-3):137-45.
60. Vana K, Zuber C, Pflanz H, Kolodziejczak D, Zemora G, Bergmann AK, et al. LRP/LR as an alternative promising target in therapy of prion diseases, Alzheimer's disease and cancer. Infect Disord Drug Targets. 2009 Feb;9(1):69-80.
61. Pelosi G, Pasini F, Bresaola E, Bogina G, Pederzoli P, Biolo S, et al. High-affinity monomeric 67-kD laminin receptors and prognosis in pancreatic endocrine tumours. J Pathol. 1997 Sep;183(1):62-9.
62. Pesapane A, Ragno P, Selleri C, Montuori N. Recent advances in the function of the 67 kDa laminin receptor and its targeting for personalized therapy in cancer. Curr Pharm Des. 2017;23(32):4745-57.
63. Bhattacharya A, Limone A, Napolitano F, Cerchia C, Parisi S, Minopoli G, et al. APP maturation and intracellular localization are controlled by a specific inhibitor of 37/67 kDa laminin-1 receptor in neuronal cells. Int J Mol Sci. 2020 Mar 4;21(5):1738.
64. Zuber C, Knackmuss S, Zemora G, Reusch U, Vlasova E, Diehl D, et al. Invasion of tumorigenic HT1080 cells is impeded by blocking or downregulating the 37-kDa/67-kDa laminin receptor. J Mol Biol. 2008 May 2;378(3):530-9.
65. Scheiman J, Tseng JC, Zheng Y, Meruelo D. Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol Ther. 2010 Jan;18(1):63-74.
66. Naidoo K, Malindisa ST, Otgaar TC, Bernert M, Da Costa Dias B, Ferreira E, et al, Knock-down of the 37kDa/67kDa laminin receptor LRP/LR impedes telomerase activity. PLoS One. 2015 Nov 6;10(11):e0141618.
67. Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004 Apr;11(4):380-1.
68. Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011 Dec 15;82(12):1807-21.
69. Shirakami Y, Shimizu M. Possible mechanisms of green tea and its constituents against cancer. Molecules. 2018 Sep 7;23(9):2284.
70. Coggin JH Jr, Barsoum AL, Rohrer JW. 37 kiloDalton oncofetal antigen protein and immature laminin receptor protein are identical, universal T-cell inducing immunogens on primary rodent and human cancers. Anticancer Res. 1999 Nov-Dec;19(6C):5535-42.
71. La Rocca C, Carbone F, Longobardi S, Matarese G. The immunology of pregnancy: regulatory T cells control maternal immune tolerance toward the fetus. Immunol Lett. 2014 Nov;162(1 Pt A):41-8.
72. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012 Jul 6;150(1):29-38.
73. Viazov OE, Verbitskiĭ MSh. Izuchenie voprosov tkanevoĭ nesovmestimosti na modeli mat'-plod [Tissue incompatibility on a mother-fetus model]. Usp Sovrem Biol. 1968;66(3):424-38.
74. Schumacher A, Zenclussen AC. The paternal contribution to fetal tolerance. Adv Exp Med Biol. 2015;868:211-25.
75. Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. 2014;58(2-4):189-98.
76. Schumacher A, Zenclussen AC. Regulatory T cells: regulators of life. Am J Reprod Immunol. 2014 Aug;72(2):158-70.
77. Prehn RT. Perspectives on oncogenesis: does immunity stimulate or inhibit neoplasia? J Reticuloendothel Soc. 1971 Jul;10(1):1-16.
78. Beaman KD, Jaiswal MK, Katara GK, Kulshreshta A, Pamarthy S, Ibrahim S, et al. Pregnancy is a model for tumors, not transplantation. Am J Reprod Immunol. 2016 Jul;76(1):3-7.
79. Beaman KD, Dambaeva S, Katara GK, Kulshrestha A, Gilman-Sachs A. The immune response in pregnancy and in cancer is active and supportive of placental and tumor cell growth not their destruction. Gynecol Oncol. 2017 Jun;145(3):476-80.
80. Chaouat G. Reconsidering the Medawar paradigm placental viviparity existed for eons, even in vertebrates; without a "problem": Why are Tregs important for preeclampsia in great apes? J Reprod Immunol. 2016 Apr;114:48-57.
81. Moffett A, Loke YW. The immunological paradox of pregnancy: a reappraisal. Placenta. 2004 Jan;25(1):1-8.
82. Land WG. How evolution tells us to induce allotolerance. Exp Clin Transplant. 2015 Apr;13 Suppl 1:46-54.
83. Maxwell AJ, You Y, Aldo PB, Zhang Y, Ding J, Mor G. The role of the immune system during pregnancy: General concepts. In: Mor GG, editor. Reproductive Immunology. Basic Concepts. Academic Press; 2021. p. 1-21.
84. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med. 2013 May;19(5):548-56.
85. Aagaard-Tillery KM, Silver R, Dalton J. Immunology of normal pregnancy. Semin Fetal Neonatal Med. 2006 Oct;11(5):279-95.
86. Nancy P, Erlebacher A. T cell behavior at the maternal-fetal interface. Int J Dev Biol. 2014;58(2-4):189-98.
87. Davies CJ. Why is the fetal allograft not rejected? J Anim Sci. 2007 Mar;85(13 Suppl):E32-5.
88. Van den Elsen PJ, Gobin SJ, Van der Stoep N, Datema G, Viëtor HE. Transcriptional control of MHC genes in fetal trophoblast cells. J Reprod Immunol. 2001 Oct-Nov;52(1-2):129-45.
89. Hager H, Aboagye-Mathiesen G, Petersen PM, Nørskov-Lauritsen N, Juhl CB, Villadsen JA, Zdravkovic M, Gildberg A, Dalsgaard AM, Ebbesen P. Human trophoblast interferons enhance major histocompatibility complex class I antigen expression on human term trophoblast cells in culture. Placenta. 1994 Oct;15(7):709-14.
90. Lui PP, Cho I, Ali N. Tissue regulatory T cells. Immunology. 2020 Sep;161(1):4-17.
91. Yoshinaga K. Two concepts on the immunological aspect of blastocyst implantation. J Reprod Dev. 2012;58(2):196-203.
92. Yoshinaga K. A historical review of blastocyst implantation research. Biol Reprod. 2018 Jul 1;99(1):175-95.
93. Andrews PW, Matin MM, Bahrami AR, Damjanov I, Gokhale P, Draper JS. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. 2005 Dec;33(Pt 6):1526-30.
94. Damjanov I, Solter D, Belicza M, Skreb N. Teratomas obtained through extrauterine growth of seven-day mouse embryos. J Natl Cancer Inst. 1971 Mar;46(3):471-5 passim.
95. Solter D, Skreb N, Damjanov I. Extrauterine growth of mouse egg-cylinders results in malignant teratoma. Nature. 1970 Aug 1;227(5257):503-4.
96. Munien C, Rebelo TM, Ferreira E, Weiss SF. IgG1-iS18 impedes the adhesive and invasive potential of early and late stage malignant melanoma cells. Exp Cell Res. 2017 Feb 15;351(2):135-41.
97. Rebelo TM, Chetty CJ, Ferreira E, Weiss SF. Anti-LRP/LR-specific antibody IgG1-iS18 impedes adhesion and invasion of pancreatic cancer and neuroblastoma cells. BMC Cancer. 2016 Nov 24;16(1):917.
98. Vania L, Chetty CJ, Ferreira E, Weiss SFT. Anti-LRP/LR specific antibody IgG1-iS18 significantly impedes adhesion and invasion in early and late stage colorectal carcinoma cells. Mol Med. 2016 Oct;22:664-73.
99. Chetty C, Khumalo T, Da Costa Dias B, Reusch U, Knackmuss S, Little M, et al. Anti-LRP/LR specific antibody IgG1-iS18 impedes adhesion and invasion of liver cancer cells. PLoS One. 2014 May 5;9(5):e96268.
100. Khumalo T, Reusch U, Knackmuss S, Little M, Veale RB, Weiss SF. Adhesion and invasion of breast and oesophageal cancer cells are impeded by Anti-LRP/LR-specific antibody IgG1-iS18. PLoS One. 2013 Jun 18;8(6):e66297.
101. Omar A, Reusch U, Knackmuss S, Little M, Weiss SF. Anti-LRP/LR-specific antibody IgG1-iS18 significantly reduces adhesion and invasion of metastatic lung, cervix, colon and prostate cancer cells. J Mol Biol. 2012 May 25;419(1-2):102-9.
102. Narumi K, Inoue A, Tanaka M, Isemura M, Shimo-Oka T, Abe T, et al. Inhibition of experimental metastasis of human fibrosarcoma cells by anti-recombinant 37-kDa laminin binding protein antibody. Jpn J Cancer Res. 1999 Apr;90(4):425-31.
103. McClintock SD, Warner RL, Ali S, Chekuri A, Dame MK, Attili D, et al. Monoclonal antibodies specific for oncofetal antigen--immature laminin receptor protein: Effects on tumor growth and spread in two murine models. Cancer Biol Ther. 2015;16(5):724-32.
104. Zhang C, Duan E, Cao Y, Jiang G, Zeng G. Effect of 32/67 kDa laminin-binding protein antibody on mouse embryo implantation. J Reprod Fertil. 2000 May;119(1):137-42.
105. Jovanovic K, Chetty CJ, Khumalo T, Da Costa Dias B, Ferreira E, Malindisa ST, et al. Novel patented therapeutic approaches targeting the 37/67 kDa laminin receptor for treatment of cancer and Alzheimer's disease. Expert Opin Ther Pat. 2015 May;25(5):567-82.
106. Shevchenko OV, Shevchenko VolO, Shevchenko VO. Specific immune response of the body as an initiating and promoter factor of carcinogenesis (hypothesis). Journ. Acad. Med. Sci. of Ukraine. 2004; 10(1):50-64.
107. Prehn RT, Prehn LM. Is an immune reaction required for malignant transformation and cancer growth? Cancer Immunol Immunother. 2012 Jul;61(7):963-6.
108. Prehn RT, Prehn LM. Cancer immunotherapy by immunosuppression. Theor Biol Med Model. 2010 Dec 15;7:45.
109. Prehn RT. On the nature of cancer and why anticancer vaccines don't work. Cancer Cell Int. 2005 Aug 1;5(1):25.
110. Chaouat G, Chaffaux S, Duchet-Suchaux M, Voisin GA. Immunoactive products of mouse placenta: I. immuno-suppressive effects of crude and water soluble extracts. J Reprod Immunol. 1980;2(3):127-39.