Keywords
Persons living with HIV/AIDS (PLWHA) are at higher risk of developing adverse cutaneous reactions compared to the general population [1,2]. Numerous antiretrovirals approved for HIV treatment are associated with adverse dermatological reactions such as non-nucleoside reverse transcriptase inhibitors, protease inhibitors (specifically darunavir and amprenavir), and the CD4 T-lymphocyte attachment inhibitor ibalizumab [3]. Some antiretrovirals are associated with hypersensitivity reaction, one example is PLWHA who test positive for HLA-B*5701 allele and are prescribed the nucleoside reverse transcriptase inhibitor abacavir. This type of reaction is characterized by rash, fever, malaise, and flu-like illness and is the reason why all patients should receive HLA-B*5701 screening before initiating abacavir [4]. A few antiretrovirals (nevirapine and raltegravir) have been reported to cause drug rash with eosinophilia and systemic symptoms (DRESS), nevirapine has also been associated with Stevens-Johnson Syndrome and toxic epidermal necrolysis [5-7]. These reactions can be life-threatening, but are uncommon, and typically appear days to weeks after initiation of the triggering drug. Another potentially life-threatening adverse drug reaction is anaphylaxis, which is rarely associated with antiretrovirals, only a few cases have been reported in the literature [8,9].
HIV integrase strand transfer inhibitors (INSTI) are another class of antiretrovirals that dominate the HIV treatment landscape. They have become the preferred anchor for antiretroviral treatment naïve PLWHA and recommended by Department of Health and Human Services Adult HIV treatment guidelines [10]. Second generation INSTI (dolutegravir and bictegravir) have shown durable viral suppression, excellent tolerability, minimal toxicity, and high barrier to resistance. Bictegravir is only available co-formulated with tenofovir alafenamide (TAF) and emtricitabine and is not associated with adverse cutaneous reactions or anaphylaxis. We present a cutaneous drug reaction and anaphylaxis to the INSTI bictegravir.
Case Report
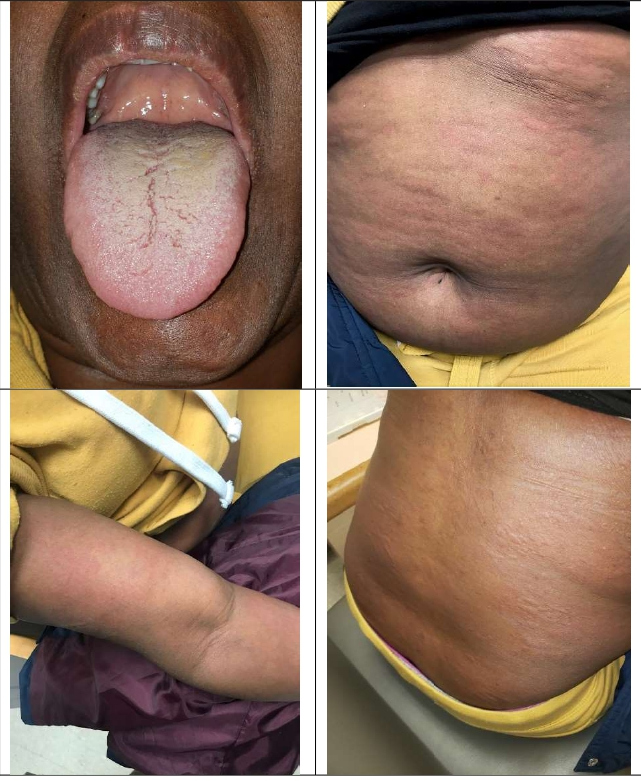
A 44-year old Ethiopian female with a history of HIV and latent tuberculosis treated with three months of rifampin presented to the Ruth M Rothstein CORE Center complaining of pruritic rash that started the previous night. HIV was diagnosed in 2004, however she did not start antiretroviral treatment until her arrival at our clinic in 2018. She had been living in Chicago for the previous five years. In the morning, the patient awoke with a mild sore throat and pruritic hives covering her torso and upper and lower extremities. She presented to our clinic complaining of rash and dysphagia, but denied cough, weight loss, fatigue, dizziness, headache, weakness, shortness of breath, wheezing, chest pain, diarrhea, nausea, vomiting, night sweats, and has no history of any prior allergic reactions. The patient states that her antiretroviral regimen was recently switched from tenofovir alafenamide/emtricitabine + dolutegravir to tenofovir alafenamide/emtricitabine/bictegravir (TAF/ FTC/BIC) two days ago. The patient reported the adverse cutaneous reaction began after the second dose of TAF/ FTC/BIC. She takes no other medications; however, she did receive influenza vaccine (Fluzone® Quadrivalent) eight days prior to change in antiretroviral therapy. No other changes to her diet or medications were identified. She did not report any allergies to food or latex and no recent insect bites or stings. Physical exam revealed a temperature of 99.9F, heart rate 97, respiratory rate 18, blood pressure 109/71 and oxygen saturation 100% on room air. She had oropharyngeal edema and diffuse urticaria (Figure 1), but no respiratory findings at this time. The patient was transferred to hospital emergency
Figure 1: Oropharyng eal edema and rash on abdomen , back, and right arm.
room for further evaluation of possible anaphylaxis reaction due to TAF/FTC/BIC. During emergency room evaluation her vital signs remained unchanged, however she developed bilateral wheeze on lung exam. Renal and liver function tests were within normal limits. She was given a clinical diagnosis of anaphylaxis. The patient received one dose of intravenous diphenhydramine, intravenous famotidine, intravenous methylprednisolone, albuterol, and ipratropium nebulizer treatment, and one dose of intramuscular epinephrine. Four hours later there was significant improvement in symptoms, chest X-ray showed atelectasis versus pneumonia, and patient was discharged to home with conservative management with a prescription for doxycycline 100mg twice daily for 10 days. The patient returned to our clinic six days later, her symptoms had resolved, and her antiretroviral regimen was changed to abacavir/lamivudine + doravirine.
When the patient arrived at our clinic (February 2018) her initial HIV viral load=585 copies/ml, CD4+ T-lymphocyte=471 and she was started on tenofovir/ emtricitabine + dolutegravir. She remained on this regimen without incidence until July 2018 when she was switched to tenofovir alafenamide/emtricitabine + dolutegravir. She remained on this regimen until December 2019 (total of 17 months) when she was switched to TAF/FTC/BIC. After just two doses of TAF/FTC/BIC our patient developed a cutaneous reaction with oropharyngeal edema, pulmonary wheezing, and was given clinical diagnosis of anaphylaxis.
Discussion
Results from clinical trials of TAF/FTC/BIC have reported excellent tolerability with few patients having to discontinue therapy due to adverse effects. Adverse cutaneous reaction of any type associated with TAF/FTC/ BIC are uncommon and not reported in the literature to our knowledge. Study 1878 was a large, phase three, randomized study in PLWHA who were taking a protease inhibitor and had an undetectable viral load were switched to TAF/FTC/BIC. This study reported one patient who discontinued treatment with TAF/FTC/BIC due to
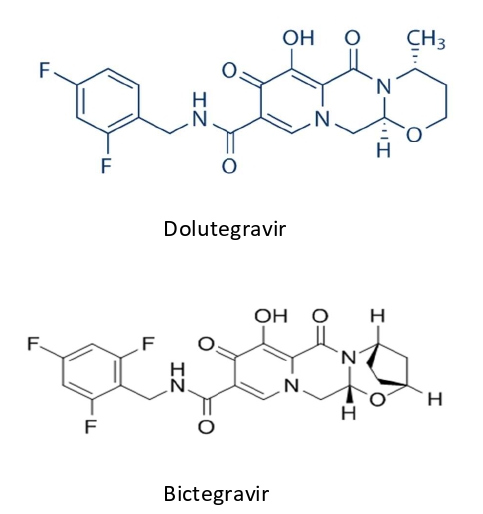
Figure 2: Chemical Structure of Dolutegravir (a) and Bictegravir (b).
rash while two other pivotal studies did not report any occurrence of rash [11-13]. Drug information for TAF/ FTC/BIC from the manufacturer report rash (all grades) occurring in less than 2% of patients administered TAF/ FTC/BIC in clinical trials and no reports of anaphylaxistype drug reaction [14].
Our patient already had significant exposure to tenofovir disoproxil (TDF), tenofovir alafenamide (TAF), emtricitabine, and dolutegravir without incident. Not until exposure to bictegravir did our patient develop and adverse cutaneous reaction with systemic symptoms consistent with anaphylaxis.
Cutaneous reactions are uncommon for nucleoside reverse transcriptase inhibitors; but have been reported with TDF in 5-7% from clinical trials. A published case series reported nine patients who developed skin abnormalities attributed to TDF with a range of 24 hours to six weeks after the initiation of TDF and seven out of nine patients the adverse skin reaction recurred after re-challenge with TDF [15]. Rash, either maculopapular or vesicular, was the most common manifestation with a mean onset of 15 days. Our patient had been exposed to TDF for approximately five months and TAF for 15 months before onset of her adverse cutaneous reaction and therefore highly unlikely that TAF was the cause of her adverse reaction.
Early clinical trials of emtricitabine reported skin discoloration manifested by hyperpigmentation on the palms and or soles that was generally mild and asymptomatic [16]. This adverse event was more common in children than adults and in clinical practice is rarely observed. Our patient had been exposed to emtricitabine for 22 months and therefore it is highly unlikely that emtricitabine caused this reaction.
Adverse cutaneous reactions are rarely reported in dolutegravir clinical trials (<1%). However, drug information provided by the manufacturer includes a warning of potential hypersensitivity reactions that have been reported and were characterized by rash, constitutional findings, and sometimes organ dysfunction [17]. This type of reaction can be life-threatening and requires immediate drug discontinuation. Raltegravir, another INSTI, also has the same warning, however the two other INSTI (elvitegravir and bictegravir) do not. Our patient had been prescribed dolutegravir for 22 months without incidence at the time of the switch in therapy. Not only is the chemical structure of dolutegravir and bictegravir almost identical (Figure 2), a large randomized clinical trial switching PLWHA from dolutegravir-based regimen to TAF/FTC/BIC was safe, well tolerated, few discontinuations, and no reported adverse cutaneous reactions [18].
The only other confounder for the cutaneous reaction and anaphylaxis observed was the administration of influenza vaccine, which occurred eight days before she began TAF/ FTC/BIC. Serious anaphylactic or cutaneous adverse reactions can occur with vaccines; but are uncommon [19]. For the Fluzone® Quadrivalent influenza vaccine cutaneous reactions or anaphylaxis were not reported in the manufacturer drug information insert [20]. After review of the patient’s medication list, prior medical history, timing, and severity of her symptoms we believe the only explanation for the drug induced anaphylaxis was bictegravir. To our knowledge, this is the first reported case of anaphylaxis attributed to bictegravir. An important consideration is that we have only described the case of one patient. Further investigations involving multiple patients would be helpful to begin to develop the incidence and risk factors for anaphylaxis due to this commonly prescribed antiretroviral medication.
References
2. Dover JS, Johnson RA. Cutaneous manifestations of human immunodeficiency virus infection: Part I & II. Archives of Dermatology. 1991 Sep 1;127(9):1383-91, 1549- 1558.
3. Borras-Blasco J, Navarro-Ruiz A, Borras C, Castera E. Adverse cutaneous reactions associated with the newest antiretroviral drugs in patients with human immunodeficiency virus infection. Journal of Antimicrobial Chemotherapy. 2008 Nov 1;62(5):879-88.
4. Rauch A, Nolan D, Martin A, McKinnon E, Almeida C, Mallal S. Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV Cohort Study. Clin Inf Dis. 2006;43:99-102.
5. O’Perry ME, Almaani N, Desai N, Larbalestier N, Fox J, Chilton D. Raltegravir-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome- Implications for clinical practice and patient safety. Int J STD AIDS. 2013;24(8):639-642.
6. Pollard RB, Robinson P, Dransfield K. Safety profile of nevirapine, a nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus infection. Clin Therapeutics. 1998;20(6):1071-1092.
7. Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC, EuroSCAR study group. Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS 2001; 15(14):1843-1848.
8. Wassef M, Keiser P. Hypersensitivity to zidovudine: Report of a case of anaphylaxis and review of the literature. Clin Inf Dis. 1995;20(5):1387-1389.
9. Kainer MA, Mijch A. Anaphylactoid reaction, angioedema, and urticaria associated with lamivudine. Lancet. 1996;348(9040):1519.
10. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://www. aidsinfo.nih.gov/ContentFiles/ AdultandAdolescentGL. pdf. Accessed [6/23/20] [pg 64, table 6a].
11. Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicenter, phase 3, randomized controlled non-inferiority trial. Lancet. 2017; 390:2063- 2072.
12. Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink HJ, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, noninferiority trial. Lancet. 2017;390:2073-2082.
13. Daar ES, DeJesus E, Ruane P, Crofoot G, Oguchi G, Creticos C, et al. Efficacy and safety of switching to fixeddose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2018;5:e347-e356.
14. Gilead Sciences. Biktarvy (bictegravir, emtricitabine, and tenofovir alafenamide). US Prescribing Information 2018. (Accessed 6/23/20) https://www.accessdata.fda. gov/drugsatfda_docs/label/2018/210251s000lbl.pdf
15. Lockhart SM, Rathbun RC, Stephens JR, Baker DL, Drevets DA, Greenfield RA, et al. Cutaneous reactions with tenofovir disoproxil fumarate: a report of nine cases. AIDS. 2007;21(10):1370-1372.
16. Saag MS, Cahn P, Raffi F, Wolff M, Pearce D, Molina JM, et al. Efficacy and safety of emtricitabine vs stavudine in combination therapy in antiretroviral-naïve patients: A randomized trial. JAMA. 2004;292(2):180-189.
17. ViiV Healthcare. Tivicay (dolutegravir). US Prescribing Information June 2020 (Accessed 6/25/20)
18. Molina JM, Ward D, Brar I, Mills A, Stellbrink HJ, Cortes LL, et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, noninferiority trial. Lancet HIV. 2005;5:e357-e365.
19. McNeil MM and DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463- 472.
20. Sanofi-Pasteur. Quadrivalent influenza vaccine (Fluzone®), [package insert], Swiftwater, PA, Revised July 2019.