Abstract
Background: Antibiotic therapy remains the gold standard for the management of bacterial infections. However, the indiscriminate use of antibiotics has resulted in the emergence of resistant pathogens. Ethylene diamine tetra acetic acid (EDTA) is a well-known chelating agent that also acts as a potentiating and sensitizing agent when combined with antibiotics. The objective of the current study was to examine the antibacterial activity of commonly used beta-lactamases in Intensive Care Units (ICUs) with addition of EDTA to it against pathogens isolated from urine, blood, and sputum, and compare it with other antibiotics. The study also aimed to review antimicrobial resistance in India.
Methods: This retrospective study was conducted across various parts of India. We reviewed both published and unpublished data from different hospitals. Many Indian hospitals were approached to gather information on the sensitivity patterns of organisms prevalent in patients in their ICUs.
Results: In the present study, out of 2,977 isolates, 884 were ESBL, 1,772 were MBL, and 321 were ESBL+MBL. Among the MBL isolates, 29 were pan-drug resistant.
Conclusion: This novel antibiotic-adjuvant combination is a promising approach to tackling MDR bacterial infections. The combination of Ceftriaxone, Sulbactam, and Disodium EDTA was effective even against pathogens isolated from blood, urine, and sputum that were resistant to other antibiotics. The enhanced susceptibility may be due to the synergistic effect of all three molecules in the combination.
Keywords
Sepsis, MDR, ESBL, MBL, PDR, D-EDTA, Anti-microbial resistance
Introduction
Antimicrobial resistance is a significant global issue, posing a serious threat to the effective prevention and treatment of a growing range of resistant bacterial infections [1]. While resistance trends vary among populations, evidence shows that the prevalence of multi-drug resistance (MDR) is increasing. Infections caused by MDR organisms can lead to higher treatment costs, longer hospital stays, and increased mortality rates. Recently, there has been a rise in resistance among Gram-positive and Gram-negative pathogens causing both community and hospital-based infections [2].
The mechanisms of resistance among these infectious agents primarily include the production of extended-spectrum beta-lactamases (ESBLs) and carbapenemases in Gram-negative pathogens, as well as mutations in the transpeptidase gene in Gram-positive organisms, resulting in low affinity for beta-lactams. These studies highlight the urgent need for enhanced research on drug resistance and the development of new therapeutic strategies to expand the treatment options for effective infection control and antimicrobial use.
A novel Antibiotic Adjuvant Entity (AAE) comprising Ceftriaxone, Sulbactam, and EDTA (collectively known as CSE-1034), has been proposed as an alternative to address the MDR problem to some extent [3-5]. This drug leverages the synergistic action of ceftriaxone's inhibition of cell wall synthesis, sulbactam's inhibition of beta-lactamases, and EDTA's role as a non-antibiotic adjuvant that enhances the combination's efficacy. Reports indicate that CSE-1034 is effective against various types of MDR organisms [6,7].
EDTA (C10H16N2O8) is distributed in the extra cellular compartment. Within the plasma it enters and stays. Its ionic form prevents it from entering into cells. It is a well-known anionic permeation enhancer which appears to modulate the integrity of the tight junctions by chelation of extracellular Ca2+ and Mg2+ ions that require to maintain the integrity of the tight junctions [8,9]. EDTA can cause abdominal cramps, diarrhea, headache, low blood pressure, skin problems, and fever. It is unsafe to use more than 3 grams per day or to take longer than 5-7 days. Too much can cause kidney damage, dangerously low calcium levels and death. Special precautions are needed in Asthma, patients with abnormal heart rhythm, diabetes as well as in hypocalcemia cases.
In this study the researcher investigates into the antibacterial activity of commonly used beta- lactamases in Intensive Care Units (ICU) of India with or without EDTA. The researcher has taken patients data of Medical ICU’s. Pediatric population was excluded from this study. The data were taken from sepsis patients. The subgroups where dose modifications were not segregated from the population. The hospital that belongs to Private or Government could not be shown as the authorities of some hospitals objected to it.
Objectives
- To analyze the resistance pattern of microorganism in different hospitals of India
- To enquire into effectiveness of EDTA in overcoming resistance of MDR
- To compare effectiveness of Meropenem and Ceftriaxone with Sulbactum with or without EDTA
Significance
- This research is supposed to give an idea of antibiotic resistance profile
- This will help to select proper antibiotics in a timely manner to Sepsis patients which will save golden period of patients’ life
Methods
We conducted the multicentre retrospective study across many parts of India. We have gone through published as well as unpublished data from different hospitals of India. In this regard extensive literature review was done (N=25) from electronic databases of different journals like PubMed, Google Scholar, Springer, and DOI data bases, and relevant data has been searched. Many Indian hospitals are being approached for sensitivity pattern of their organisms prevalent in patients of their intensive care units. Some data were taken from data presented at various conferences conducted in India. All the compiled data has been analyzed statistically in a qualitative way and the significance of EDTA determined quantitatively. P value <0.05 is taken as significant at Confidence interval 95%. Antibiotic susceptibility testing was performed by Kirby diffusion method. ESBL (Extended spectrum beta lactamase) production was determined by double dick synergy test method.
To reach the objectives of the study the researcher proposed a hypothesis:
H1: The EDTA is effective in overcoming resistance of the organism.
H01: The EDTA is not effective in overcoming resistance of the organism.
Data of total of n= 2,977 non-duplicate isolates of E. coli, K. Pneumoniae, P aeruginosa, and A. baumannii collected from different hospitals of India (2013-2024) were tested for susceptibility against Meropenem, Colistin, Ceftriaxone, Sulbactam, Imipenem, and Ceftriaxone Sulbactam EDTA and Meropenem -EDTA combination. EDTA here is given in addition to the antibiotics to test the efficacy of the EDTA in overcoming resistance. Samples were taken by using Simple Random Sampling Method irrespective of private and government status.
Area of study
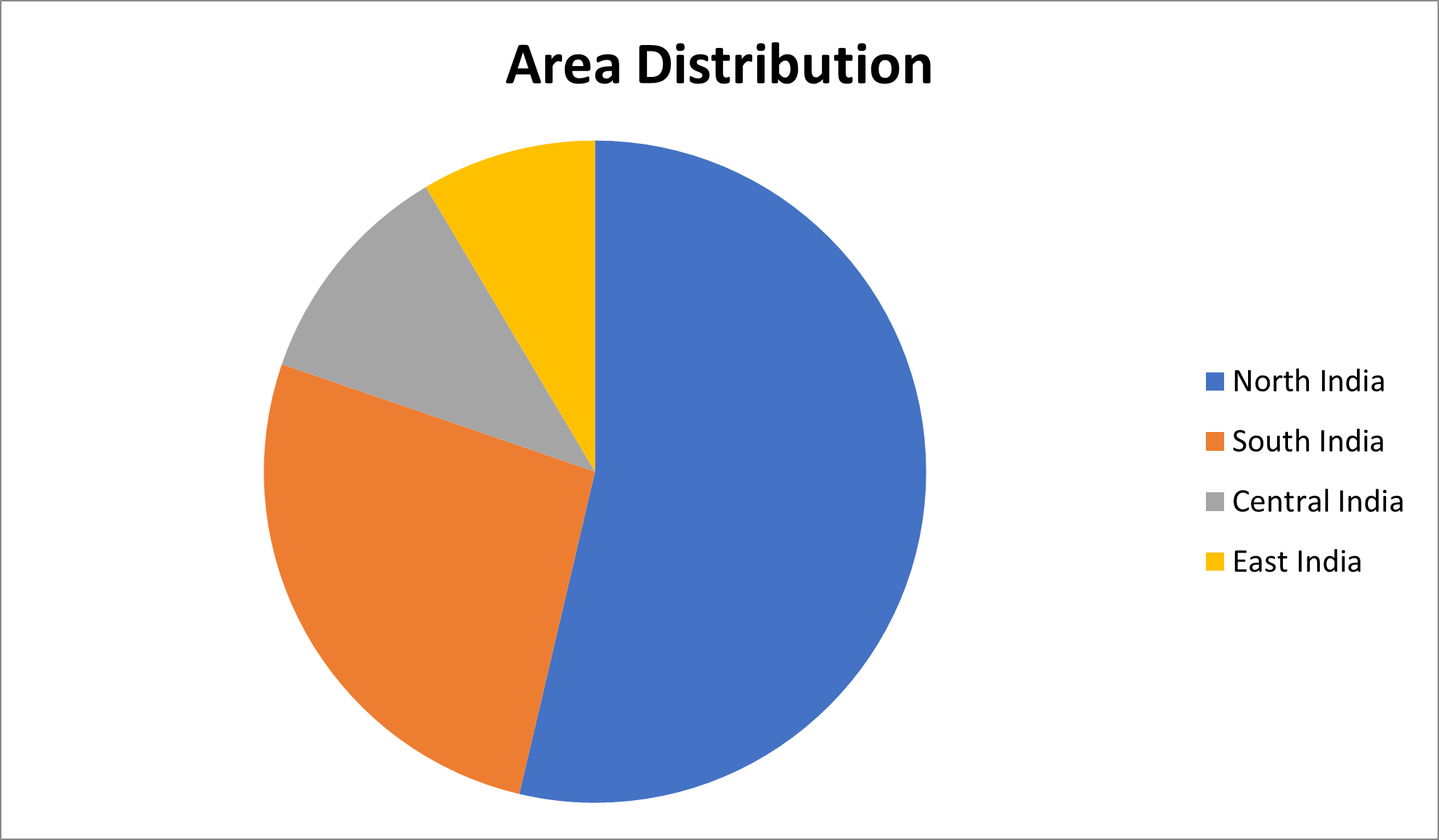
The samples were collected from different regions of India (n=2977) (Table 1 and Figure 1).
|
North India |
South India |
Central India |
East India |
West India |
|
1585 |
785 |
331 |
252 |
24 |
Figure 1. Area distribution of samples.
Results
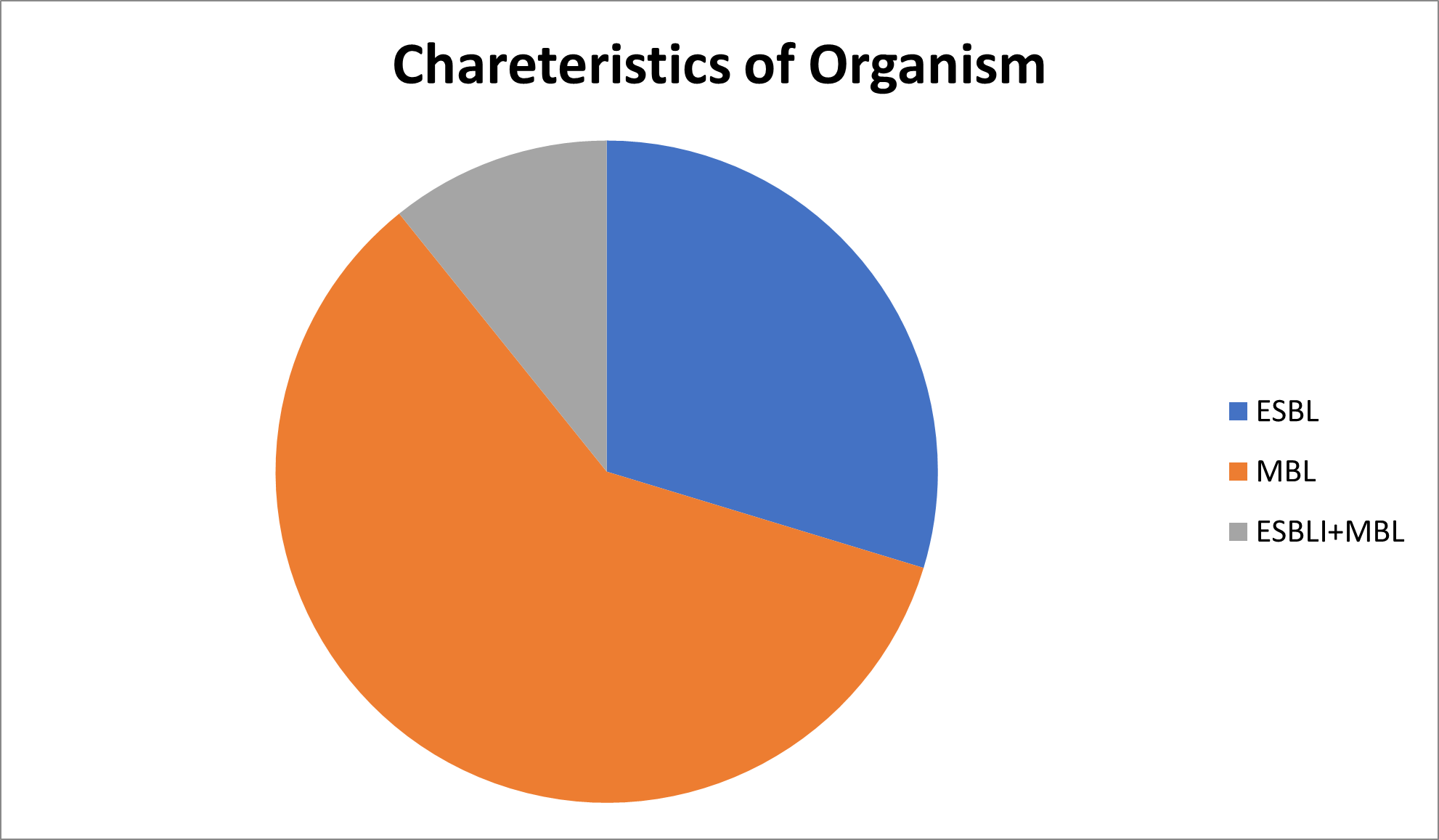
Out of n=2,977 isolates 884 were ESBL,1,772 were MBL and 321were ESBL+MBL. Amongst MBL isolates, 29 were Pan drug resistant (Table 2 and Figure 2).
Figure 2. Characteristics of organism.
|
ESBL |
MBL |
ESBL+MBL |
|
884 |
1772 |
321 |
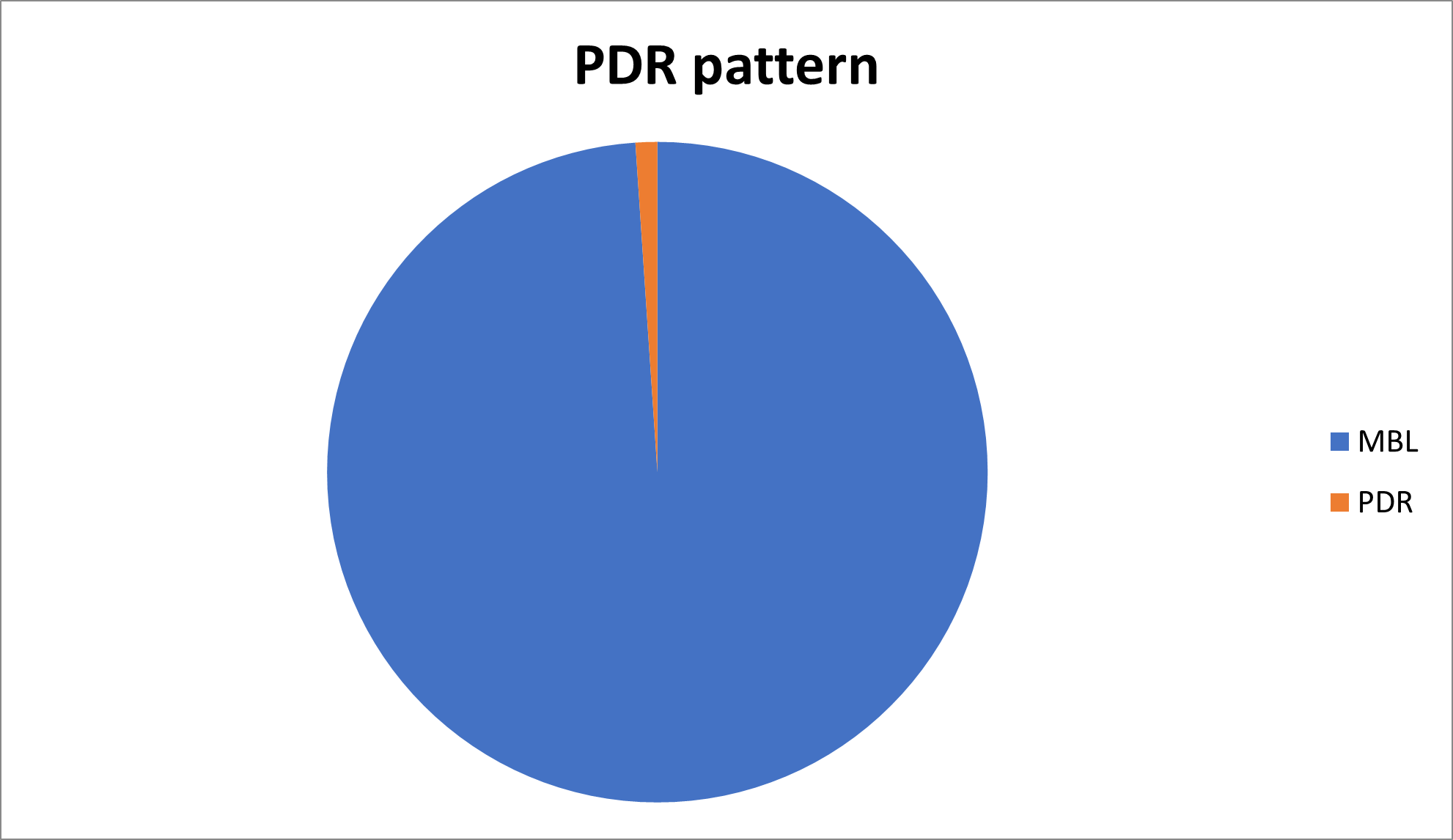
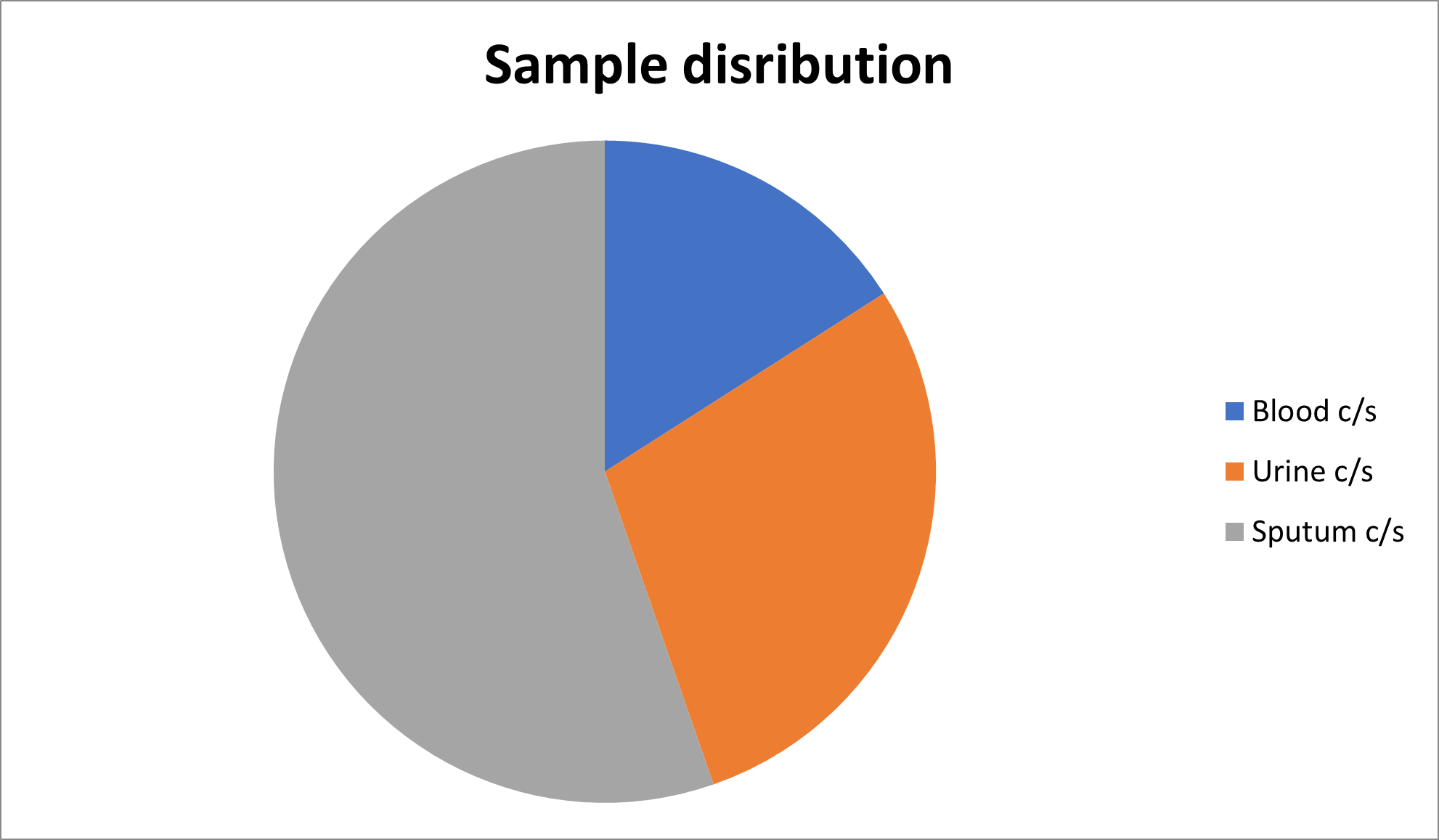
The sensitivity pattern according to different cultures can be summarized as presented in Tables 3 and 4, Figures 3 and 4.
|
MBL |
PDR (Pan drug resistance) |
|
1772 |
29 |
|
Blood C/S |
Urine C/S |
Sputum C/S |
|
475 |
855 |
1647 |
|
*C/S means culture sensitivity test. |
||
Figure 3. Sensitivity pattern of different cultures.
Figure 4. Sample distribution.
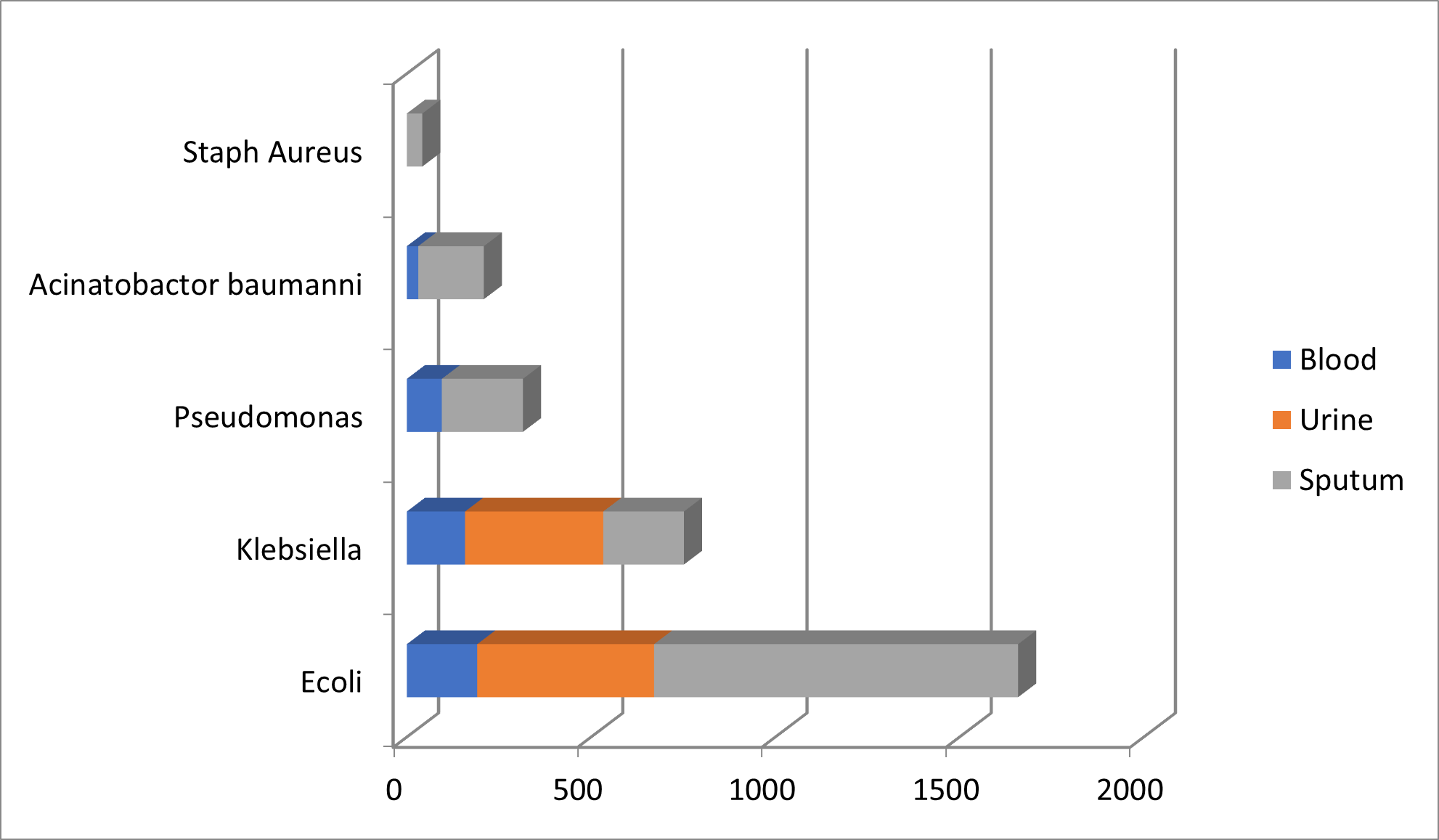
The organisms are isolated as per the pattern described in Table 5 and Figure 5.
|
Organism |
Blood |
Urine |
Sputum |
Total |
|
E. coli |
191 |
480 |
988 |
1659 |
|
Klebsiella |
158 |
375 |
219 |
752 |
|
Pseudomonas |
95 |
0 |
220 |
315 |
|
Acinetobacter baumannii |
31 |
0 |
178 |
209 |
|
Staphylococcus aureus |
0 |
0 |
42 |
42 |
|
Total |
475 |
855 |
1647 |
2977 |
Figure 5. Pattern of isolated organism.
The Antibiotic Meropenem and Ceftriaxone + Sulbactam and addition of EDTA is being observed to Meropenem and Ceftriaxone and Sulbactam. EDTA (D-EDTA) were used separately and also combined antibiotics with EDTA (Tables 6-18).
|
Organism |
Meropenem |
% |
Ceftriaxone + Sulbactam |
% |
Meropenem + EDTA |
% |
Ceftriaxone + Sulbactam + EDTA |
% |
|
E. coli |
110/475 |
23.1 |
85/475 |
17.83 |
118/475 |
24.84 |
237/475 |
50 |
|
Klebsiella |
112/475 |
23.57 |
118/475 |
24.8 |
114/475 |
24.0 |
152/475 |
32 |
|
Pseudomonas |
46/475 |
9.68 |
95/475 |
20 |
52/475 |
10.94 |
180/475 |
37.89 |
|
Acinetobacter |
23/475 |
4.8 |
114/475 |
24 |
26/475 |
5.47 |
323/475 |
68 |
|
Staphylococcus aureus |
0/475 |
0 |
0/475 |
0 |
0/475 |
0 |
0/475 |
0 |
|
Statistical values |
Meropenem |
Meropenem + EDTA |
|
Mean |
12.23 |
13.05 |
|
Median |
9.68 |
10.94 |
|
SD |
10.70 |
11.08 |
|
*P value 0.99 |
||
|
Statistical value |
Ceftriaxone + Sulbactam |
Ceftriaxone + Sulbactam + EDTA |
|
Mean |
21.65 |
46.97 |
|
Median |
22 |
43.94 |
|
SD |
3.30 |
15.89 |
|
*P value 0.035 |
||
|
Organism |
Meropenem |
% |
Ceftriaxone + Sulbactam |
% |
Meropenem + EDTA |
% |
Ceftriaxone + Sulbactam + EDTA |
% |
|
E. coli |
387/855 |
45.26 |
386/855 |
45.14 |
273/855 |
31.92 |
401/855 |
46.90 |
|
Klebsiella |
283/855 |
33.099 |
205/855 |
23.9 |
222/855 |
25.96 |
256/855 |
29.94 |
|
Pseudomonas |
0/855 |
0 |
0 |
0 |
0/855 |
0 |
0 |
0 |
|
Acinetobacter |
0/855 |
0 |
0 |
0 |
0/855 |
0 |
0 |
0 |
|
Staphylococcus aureus |
0/855 |
0 |
0 |
0 |
0/855 |
0 |
0 |
0 |
|
Statistical values |
Meropenem |
Meropenem + EDTA |
|
Mean |
39.175 |
28.94 |
|
Median |
39.175 |
28.94 |
|
SD |
8.599 |
4.214 |
|
*P value 1 |
||
|
Statistical value |
Ceftriaxone + Sulbactum |
Ceftriaxone + Sulbactum + EDTA |
|
Mean |
34.52 |
38.42 |
|
Median |
34.52 |
38.42 |
|
SD |
15.01 |
11.99 |
|
*P value 1 |
||
|
Organism |
Meropenem |
Ceftriaxone + Sulbactum |
Meropenem + EDTA |
Ceftriaxone + Sulbactum + EDTA |
||||
|
Total samples |
% Sensitivity |
Total Samples |
% Sensitivity |
Total Samples |
% Sensitivity |
Total Samples |
% Sensitivity |
|
|
E. coli |
767 /1659 |
46.23 |
315/1659 |
18.98 |
778/1659 |
46.89 |
893/1659 |
50.57 |
|
Klebsiella |
190/752 |
25.2 |
190/752 |
25,26 |
192/752 |
25.53 |
211/752 |
28.05 |
|
Pseudomonas |
106/315 |
33.60 |
66/315 |
20.9 |
107/315 |
33.96 |
108/315 |
34.28 |
|
Acinetobacter |
111/209 |
53.11 |
52/209 |
24.8 |
112/209 |
53.58 |
116/209 |
55.50 |
|
Staphylococcus aureus |
22/42 |
52.38 |
15/42 |
35.7 |
21/42 |
50 |
19/42 |
45.2 |
|
Code |
Organism |
Meropenem |
Meropenem + EDTA |
|
1 |
E. coli |
46.23 |
46.89 |
|
2 |
Klebsiella |
25.2 |
25.53 |
|
3 |
Pseudomonas |
33.60 |
33.96 |
|
4 |
Acinetobacter |
53.11 |
53.58 |
|
5 |
Staphylococcus aureus |
52.38 |
50 |
|
Statistical values |
Meropenem |
Meropenem + EDTA |
|
Mean |
42.104 |
41.992 |
|
Median |
46.23 |
46.89 |
|
SD |
12.265 |
11.81 |
|
* P value 0.995 |
||
|
Code No. |
Organism |
Ceftriaxone + Sulbactum |
Ceftriaxone + Sulbactum + EDTA |
|
1 |
E. coli |
18.98 |
50.57 |
|
2 |
Klebsiella |
25.26 |
28.05 |
|
3 |
Pseudomonas |
20.9 |
34.28 |
|
4 |
Acinetobacter |
24.8 |
55.50 |
|
5 |
Staphylococcus aureus |
35.7 |
45.2 |
|
Statistical value |
Ceftriaxone + Sulbactum |
Ceftriaxone + Sulbactum + EDTA |
|
Mean |
24.822 |
42.684 |
|
Median |
24.08 |
45.02 |
|
SD |
6.625 |
11.365 |
|
P value: 0.010589 |
||
|
Code No. |
Organism |
Colistin (I) |
Colistin + EDTA (I) |
|
1 |
E. coli |
15 |
21 |
|
2 |
Klebsiella |
26 |
28 |
|
3 |
Pseudomonas |
20 |
34 |
|
4 |
Acinetobacter |
22 |
44 |
|
5 |
Staphylococcus aureus |
19 |
65 |
|
I: Intermediate |
|||
|
Statistical value |
Colistin |
Colistin + EDTA |
|
Mean |
20.4 |
48.4 |
|
Median |
18 |
34 |
|
SD |
4.03 |
17.096 |
|
*P value0.03 (Significant) |
||
Discussion
MDR septicemia is often fatal and requires prompt diagnosis and treatment to prevent associated organ dysfunction or mortality [10]. Sepsis due to MDR microbes is one of the most pressing problems, necessitating newer strategies to safeguard the future of antibiotics.
The findings of clinical trials demonstrate that CSE is both microbiologically and clinically effective, with a safety and tolerability profile comparable to meropenem. Infection with MDR strains of Gram-negative bacterial pathogens can lead to severe morbidity and mortality [11]. The novel antibiotic adjuvant combination CSE comprises Ceftriaxone (a third-generation beta-lactam cephalosporin), Sulbactam (a beta-lactamase inhibitor), and EDTA (a class 1 antibiotic resistance breaker) [12,13]. This study suggests that this novel combination therapy (beta-lactam antibiotic + beta-lactamase inhibitor + EDTA) is highly effective against ESBL, MBL, and carbapenemase-producing MDR bacteria.
Indian hospitals are often filled with ESBL/MBL-producing Gram-negative microbes, and many clinicians recommend the use of beta-lactam/beta-lactamase inhibitor (BL/BLI) combinations to prevent the spread of these deadly infections. However, the likelihood of achieving targeted infection control through BL/BLI combinations is not optimized due to a lack of clinical data [14]. Microbiological data indicate that E. coli is the most prevalent microbe among septicemic patients, followed by A. baumannii, P. aeruginosa, and K. pneumoniae. Data of microbiological characterization suggests the infection of both ESBL and MBL-producing organisms in all patients. This MDR pattern of resistance has complicated the treatment scenario and rendered many antimicrobials ineffective.
ESBLs mediate resistance to extended-spectrum cephalosporins such as cefotaxime, ceftriaxone, and ceftazidime. Carbapenems and MBLs can hydrolyze all beta-lactam antibiotics and display resistance to these antimicrobials. Results of this study indicate that the combination therapy (BL/BLI + disodium edetate) is highly effective against ESBL/MBL-producing organisms. The enhanced susceptibility of ceftriaxone, sulbactam, and disodium edetate against all four microbes is likely associated with the synergistic activity of this combination. The presence of disodium edetate enhances the permeability of ceftriaxone and sulbactam, thereby enhancing activity against ESBL microbes synergistically [15]. Disodium edetate can also chelate the divalent ions required for the activity of MBLs, thus deactivating the MBLs and increasing the susceptibility of ESBL/MBL-producing microbes toward this combination. Hence, the frequent use of carbapenem class drugs can be avoided to treat infections by MDR bacteria in high-risk patients [16].
It is clearly observed in our study that for bacterial isolates, in Blood and Sputum Culture, sensitivity is increased with addition of EDTA as P value is significant for Ceftriaxone and Sulbactum but in case of Meropenem it is not significant. On the other hand, though sensitivity is increased in case of bacterial isolates in Urine Culture, P value is not found to be significant for both Meropenem and Ceftriaxone-Sulbactim combination. In case of Colistin with the addition of D-EDTA it is seen to be effective against bacterial isolates in Blood Culture. On the basis of these findings, we accept H1, but its effectiveness varies with antibiotic to which it is added.
However, a limitation of this trial is that it did not include patients from outside India. Nevertheless, the pharmacokinetics of Ceftriaxone and Sulbactam are not expected to vary by ethnic group or region. This study does not include pediatric population as well as cannot compare between Government as well as private hospitals. Though data of sputum analysis were found in adequate number, Tracheal secretion, BAL (Broncho alveolar lavage) samples were not found to be adequate retrospectively.
Conclusion
It is observed form this small study that the novel antibiotic-adjuvant combination is a promising approach to tackling MDR bacterial infections. The combination of Ceftriaxone, Sulbactam, and Disodium EDTA has an edge over Meropenem, was effective even against pathogens isolated from blood and sputum that were resistant to other antibiotics. Against urine though sensitivity is shown but not shown statistically significant. The enhanced susceptibility may be due to the synergistic effect of all three molecules in the combination. The author feels that it is needed to explore clinical correlation of effectiveness of EDTA in patients and also need to monitor side effects of EDTA.
Supplementary Data
Supplementary materials are available online, consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgements
We thank all investigators, study sites, and patients involved in this clinical trial program.
Financial Support
The study cost is being funded by DOTE Pharmaceuticals D-EDTA, Guwahati.
Conflict of Interest
None.
Disclaimer
All data are collected from retrospective sources where the author is not working. The culture, methodology and conditions may vary with lab.
References
2. WHO. Antimicrobial resistance: global report on surveillance 2014. WHO; 2016. Available from: http://www.who.int/drugresistance/documents/surveillancereport/en/ [cited 23.08.16].
3. Blot S, Vandijck D, Lizy C, Annemans L, Vogelaers D. Estimating the length of hospitalization attributable to multidrug antibiotic resistance. Antimicrob Agents Chemother. 2010 Sep;54(9):4046; author reply 4046-7.
4. Spellberg B, Guidos R, Gilbert D, Bradley J, Boucher HW, Scheld WM, et al. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin Infect Dis. 2008 Jan 15;46(2):155-64.
5. Kanj SS, Kanafani ZA. Current concepts in antimicrobial therapy against resistant gram-negative organisms: extended-spectrum beta-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin Proc. 2011 Mar;86(3):250-9.
6. Holmes NE, Howden BP. What's new in the treatment of serious MRSA infection? Curr Opin Infect Dis. 2014 Dec;27(6):471-8.
7. Chaudhary M, Payasi A. A randomized, open-label, prospective, multicenter phase-III clinical trial of Elores in lower respiratory tract and urinary tract infections. Journal of Pharmacy Research. 2013 Apr 1;6(4):409-14.
8. Barton CC, Harihara M, Mehedale. EDTA (Ethylenediaminetetraacetic Acid). http://doi.org/10.1016/80-12-369400-0/0.00363-X.
9. Kitchens KM, El-Sayed ME, Ghandehari H. Transepithelial and endothelial transport of poly (amidoamine) dendrimers. Adv Drug Deliv Rev. 2005 Dec 14;57(15):2163-76.
10. Gupta R. Antibiotic adjuvant therapy for multi-drug resistant carbapenemases producing Klebsiella pneumoniae associated sepsis: a case study. Journal of Clinical and Diagnostic Research: JCDR. 2016 Apr;10(4):DD08.
11. Singh S, Sahu C, Patel SS, Singh A, Yaduvanshi N. A Comparative In Vitro Sensitivity Study of "Ceftriaxone-Sulbactam-EDTA" and Various Antibiotics against Gram-negative Bacterial Isolates from Intensive Care Unit. Indian J Crit Care Med. 2020 Dec;24(12):1213-17.
12. Bernal P, Molina-Santiago C, Daddaoua A, Llamas MA. Antibiotic adjuvants: identification and clinical use. Microb Biotechnol. 2013 Sep;6(5):445-9.
13. Patil UN, Jambulingappa KL. A Combination Strategy of Ceftriaxone, Sulbactam and Disodium Edetate for the Treatment of Multi-Drug Resistant (MDR) Septicaemia: A Retrospective, Observational Study in Indian Tertiary Care Hospital. J Clin Diagn Res. 2015 Nov;9(11):FC29-32.
14. Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi Metallo-beta lactamase (NDM-1) in Enterobacteriaceae: treatment options with carbapenems compromised. J Assoc Physicians India. 2010 Mar; 58:147-9.
15. Ghafur A. Can India be the wing commander in the global fight against antimicrobial resistance? J Assoc Physicians India. 2012 May; 60:42-3.
16. Chaudhary M, Payasi A. Clinical, microbial efficacy and tolerability of Elores, a novel antibiotic adjuvant entity in ESBL producing pathogens: Prospective randomized controlled clinical trial. journal of pharmacy research. 2013 Apr 1;7(4):275-80.