Abstract
Objectives: We aim to identify thrombotic events and risk factors for thrombosis (RFT) comparing polycythemia vera (PV) and secondary polycythemia (SP) patients.
Methods: We carried out a retrospective study of a cohort of 59 patients with PV (n=34) and SP (n=25) followed for a period of 14 years. Variables studied were the frequency and type of thrombosis, sociodemographic, clinical, and biological RFT. Statistical analysis was performed using SPSS software version 18. Multivariate analysis was performed to identify RFT.
Results: Mean age in PV was 53 years (33 - 79) and 44.5 years (5 - 78) in SP (p=0.74). The sex ratio in PV was 1.12 and 2.57 in PS (p=0.52). Sixteen patients had thrombotic events (27.1%) including 12 PV (35.3%) versus 4 SP (16%) (p=0.09). Twenty-two thrombotic events were identified; 14 (63.7%) of arterial thrombosis and 8 (36.3%) of venous thrombosis. There were 18 thrombotic events (81.8%) in PV versus 4 (18.2%) in SP (p=0.02). Arterial thrombosis was more frequent in PV (55.6%). Thrombosis events were more frequent in female patients (59.1%). RFT in PV were age (40-59 years), hematocrit >45% and thrombocytosis ≥ 600 G/L and in SP, RFT were hematocrit >45% and hypercholesterolemia. After multivariate analysis, hematocrit >45% was the only RFT independently associated with thrombosis occurrence.
Conclusion: We show through this study, a frequent occurrence of arterial thrombosis in polycythemia. Main RFT is the high hematocrit level (>45%). We insist on the interest of therapeutic bloodletting, sometimes associated with cytoreductive treatment in order to maintain a hematocrit level <45%.
Keywords
Hematocrit, Polycythemia vera, Secondary polycythemia, Thrombosis
Introduction
Polycythemia refers to an excessive proliferation of the erythrocyte lineage with permanent elevation of total blood volume [1]. There are two major groups of polycythemia disorders, the PV, which is a myeloproliferative syndrome characterized by the presence of a clonal abnormality (JAK2 gene mutation: JAK2V617F or exon 12), and SP, which are often reactive to hypoxic circumstances or to tumors inappropriately secreted erythropoietin [2,3].
Difficulties of diagnosis encountered in primary polycythemia are at the origin of the establishment of diagnosis criteria regularly revised by the WHO [4,5].
Polycythemia is characterized by blood hyperviscosity leading to venous or arterial thrombosis, which is the main cause of patient mortality [6-8]. Nevertheless, it is sometimes difficult to link thrombosis events directly to polycythemia because of frequent co-morbidities in patients. The prevention of thrombosis is based on maintaining a hematocrit below 45% through therapeutic phlebotomy and sometimes cytoreductive treatment [9].
While polycythemia is widely studied in the West due to the availability of molecular analyses, in Africa, polycythemia is a rarely reported condition and studies often involve small cohorts of patients [10,11].
In our country, two studies have been conducted on polycythemia and have compared the epidemiological, diagnosis and therapeutic aspects of primary and secondary polycythemia [11,12]. These studies had found that thrombotic events were the major cause of morbidity and early mortality in patients. Because polycythemia often occurs in elderly subjects, it is sometimes difficult to link thrombosis directly to polycythemia because of the frequent comorbidities in these patients [13]. In view of this, we initiated this study with the objective to describe thrombotic events and RFT in patients followed for polycythemia.
Methods
We followed a cohort of 59 polycythemia consisting of 34 PV and 25 SP patients over a 17-year period (2004-2020). Patients followed for PV were diagnosed according to the WHO 2016 criteria. The diagnosis of PS patients was retained in front of clinical data (young age, absence of splenomegaly, history of chronic respiratory or cardiovascular diseases, prolonged stay in altitude) and blood count data (absence of hyperleukocytosis and/or thrombocytosis) and biological inflammatory signs (accelerated blood sedimentation rate, polyclonal hypergammaglobulinemia, hyperfibrinemia). In the absence of these clinical and biological data, PV diagnosis was retained even the absence of molecular biological analyses.
The SP etiologies consisted of congenital cyanogenic heart disease (n=5), chronic lung disease (n=3), chronic renal failure (n=2), chronic hepatic disease (n=1), hypoxia without a specific cause identified (n=9) and 5 cases of unidentified etiologies without PV criteria.
Dependent variables were thrombotic events in polycythemia patients. The types of thrombosis were ischemic stroke confirmed by CT scan, myocardial infarction confirmed by ECG with persistent ST-segment elevation, deep venous thrombosis of the inferior members, of the superior members or superficial venous thrombosis confirmed by venous Doppler, pulmonary embolism confirmed on angioscan and portal thrombosis or Budd Thiari syndrome on abdominal CT scan or abdomino-pelvic ultrasound.
Independent variables consisted of sociodemographic data (age, sex, exposure to toxic substances, high altitude >2000 m), medical history such as chronic hepatic disease, chronic obstructive pulmonary disease, pulmonary neoplasia, heart disease, renal tumor), biological data (hematocrit, hyperleukocytosis, thrombocytosis, Jak2V617F mutation). The therapeutic data consisted of phlebotomy (8 ml/kg without exceeding 500 ml in emergency or basic treatment 1 to 3 sessions per month), low-dose aspirin (100 mg/d), hydroxyurea (10-20 mg/kg/d), anticoagulants (antivitamin K dose adjusted to INR and low-molecular-weight heparin at 100 IU/kg/12h)
Cardiovascular risk factors studied were age, sex, hypertension defined as blood pressure >140/90 mm Hg, diabetes, current smoking or smoking stopped for less than 3 years, hypercholesterolemia (LDL >1.6 g/L), obesity (BMI >30).
Statistical analysis was performed using SPSS software (version 18). A univariate and bivariate descriptive study summarized the quantitative variables as means with their standard deviations and the qualitative variables as numbers and percentages. Fisher test was used to measure the strength of the association as Relative Risk (RR), with the 95% confidence interval (CI). Multivariate analysis was performed by binary logistic regression to further investigate the RFT. A p value <0.05 was considered statistically significant
An informed consent form was signed by all patients, and anonymity and confidentiality were respected for all personal data collected.
Results
General baseline characteristics of patients
Mean age was 53 years (33-79) in PV and 44.5 years (5-78) in SP patients (p=0.74). The sex ratio M/F was l, 12 in PV and 2.57 in SP (p=0.52).
Only PV patients had presented thrombocytosis ≥ 600 G/L (p=0.002) and JAK2V617F mutation (p=0.000) (mutational load was >50% in 75% of PV patients.
Therapeutic bloodletting for background treatment was performed more in PS patients (p=0.001). Hydroxyurea was used only in PV patients (p=0.000).
No progression to primary myelofibrosis or acute leukemia was noted. Seven patients (11.8%) had died, 2 deaths in PV (5.8%) and 5 deaths in SP (20%) (Table 1). Causes of death in PV consisted of thrombosis, whereas in SP, deaths were due to complications of the baseline pathology.
|
Data |
PV (n=34) |
PS (n=25) |
p |
|
Mean age (years) |
53 |
44.5 |
0.74 |
|
Sex (M/F) |
18/16 |
18/7 |
0.52 |
|
High blood pressure |
41.10% |
44% |
1 |
|
History of diabetes |
8.80% |
4% |
0.63 |
|
LDL cholesterol |
8.80% |
4% |
0.63 |
|
Hematocrit > 45% |
50% |
32% |
0.46 |
|
Thrombocytosis ≥ 600 G/L |
38.20% |
0 |
0.002 |
|
JAK2V617F mutation |
47% |
0 |
0 |
|
Leucocytosis > 15 G/L |
32.30% |
0 |
0.054 |
|
Bloodletting (emergency) |
82.30% |
88% |
1 |
|
Bloodletting (basic treatment) |
14.70% |
84% |
0.001 |
|
Acetyl salicylic acid |
85.30% |
48% |
0.21 |
|
Hydroxyurea |
85.20% |
0 |
0 |
|
Number of deaths (n=7) |
5.80% |
20% |
0.23 |
Frequency and type of thrombosis in PV and SP patients
Sixteen patients had thrombotic events (27.1%) including 12 PV patients (35.3%) and 4 SP patients (16%) (p=0.09) (Figure 1).
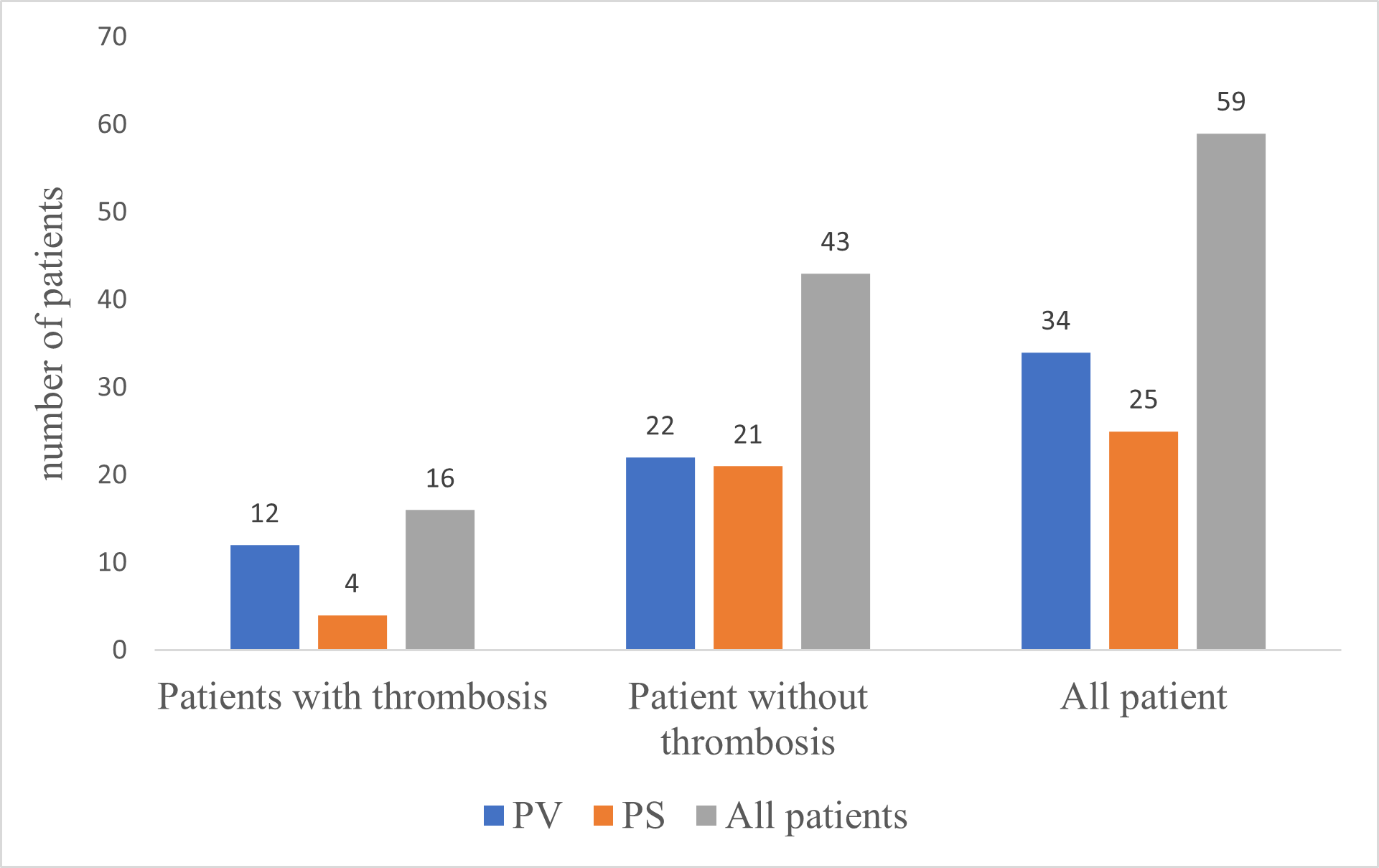
Figure 1. Frequency of thrombosis occurrence in polycythemia (PV and SP).
Twenty-two thrombotic events were identified including 18 thrombotic events (81.8%) in PV versus 4 cases (18.2%) in PS (p=0.02).
Arterial thrombosis was more frequent (63.7%) and represented 55.6% of thrombosis events for PV patients. IS type being more frequent (45.45%) but there was no significant difference in the type of thrombosis between PV and SP (p>0.05) (Table 2).
|
Thrombotic events (n=22) |
PV (n=18; 81.8%) |
PS (n=4; 18.2%) |
P |
|
IS (n=11) |
7 (63.6%) |
4 (36.4%) |
0.867 |
|
MI (n=3) |
3 |
0 |
0.22 |
|
DVT IM (n=3) |
3 |
0 |
0.127 |
|
SVT (n=2) |
2 |
0 |
0.387 |
|
DVT SM (n=2) |
2 |
0 |
0.22 |
|
PE (n=1) |
1 |
0 |
0.387 |
|
IS: Ischemic Stroke; MI: Myocardial Infarction; DVT IM: Deep Venous Thrombosis of the Inferior Members; DVT SM: Deep Venous Thrombosis of the Superior Members; SVT: Superficial Venous Thrombosis; PE: Pulmonary Embolism |
|||
Thrombosis events were more frequent in female patients (59.1%) but there was no difference for thrombosis type in terms of frequency between PV and PS according to sex (p>0.05) (Table 3).
|
|
PV (n=18) |
PS (n=4) |
|
||
|
|
M |
F |
M |
F |
P |
|
|
(n=6) |
(n=12) |
(n=3) |
(n=1) |
|
|
IS (n=11) |
27.3% |
36.4% |
27.3% |
9% |
0.867 |
|
MI (n=3) |
33.3% |
66.7% |
0 |
0 |
0.22 |
|
DVT IM (n=3) |
66.7% |
33.3% |
0 |
0 |
0.127 |
|
SVT (n=2) |
0 |
2 |
0 |
0 |
0.387 |
|
DVT SM (n=2) |
0 |
2 |
0 |
0 |
0.22 |
|
PE (n=1) |
0 |
1 |
0 |
0 |
0.387 |
RFT identified in PV and SP patients
The RFTs identified were age range between 40 and 59 years (RR=3.32), hematocrit >45% (RR=3.54), thrombocytosis ≥ 600G/L (RR=5.89) and hypercholesterolemia (RR=3.17) (Table 4).
|
Data |
Patients with thrombosis (PT+) (n=16) |
Patients without thrombosis (PT-) (n=43) |
P |
RR |
|
Age (years) |
|
|
|
|
|
≤40 |
18.7% |
30.2% |
0.38 |
0.62 |
|
40-59 |
68.7% |
37.2% |
0.00 |
3.32 |
|
≥60 |
12.5% |
32.5% |
0.12 |
0.38 |
|
Sex |
|
|
|
|
|
Male |
19.4% |
67.4% |
0.09 |
0.49 |
|
Female |
39.1% |
32.5% |
0.09 |
11.7 |
|
High blood pressure |
43.7% |
41.8% |
0.92 |
0.916 |
|
History of diabetes |
6.2% |
7% |
0.42 |
0.49 |
|
Smoking history |
6.2% |
14% |
0.38 |
0 |
|
Hematocrit > 45% |
81.2% |
27.9% |
0.00 |
3.54 |
|
Thrombocytosis ≥ 600G/L |
50% |
11.6% |
0.00 |
5.89 |
|
JAK2V617F |
37.5% |
23.2% |
0.27 |
1.61 |
|
Leukocytosis ≥15 G/L |
25% |
16.3% |
0.44 |
1.45 |
|
LDL cholesterol |
18.7% |
2.3% |
0.02 |
3.17 |
RFTs identified in PV were age (40-59 years) (p=0.02), hematocrit >45% (p=0.00), and thrombocytosis ≥ 600 G/L (p=0.00), whereas in SP, RFTs were hematocrit >45% (p=0.02) and hypercholesterolemia (p=0.01) (Table 5).
|
Data |
PV PT+ (n=12) |
PT- (n=22) |
P |
PS PT+ (n=4) |
PT- (n=21) |
P |
|
Age (years) |
|
|
|
|
|
|
|
≤40 |
8.3% |
18.2% |
0.43 |
50% |
42.8% |
0.79 |
|
40-59 |
75.0% |
45.5% |
0.02 |
50% |
28.6% |
0.40 |
|
≥60 |
16.7% |
36.4% |
0.23 |
0 |
28.6% |
0.22 |
|
Sex |
|
|
|
|
|
|
|
Male |
33.3% |
63.6% |
0.09 |
100% |
71.4% |
0.22 |
|
Female |
66.7% |
36.4% |
|
0 |
28.6% |
|
|
High blood pressure |
33.3% |
9.1% |
0.22 |
50% |
42.9% |
0.79 |
|
History of diabetes |
8.3% |
4.5% |
0.45 |
0 |
4.8% |
0.65 |
|
Smoking history |
0 |
4.5% |
0.45 |
25 |
33.3% |
0.74 |
|
Hematocrit > 45% |
66.7% |
22.7% |
0.00 |
75% |
42.9 |
0.02 |
|
Thrombocytosis |
66.6% |
22.7% |
0.00 |
0 |
0 |
NA |
|
≥ 600 G/L |
|
|
|
|
|
|
|
JAK2V617F |
50% |
45.5% |
0.80 |
0 |
0 |
NA |
|
Leukocytosis > 15 G/L |
33.3% |
4.5% |
0.65 |
0 |
0 |
NA |
|
LDL cholesterol |
8.3% |
4.5% |
0.65 |
25% |
0 |
0.01 |
After multivariate analysis, the only RFT independently associated with the occurrence of thrombosis in PV or SP was elevated hematocrit >45%.
Discussion
We observed a predominance of PV (34 PV versus 25 SP), which is not the case in several studies that had shown a predominance of SP compared to PV [14]. This difference can be explained on the one hand by the fact that our department focuses specifically the management of malignancy hemopathies, whereas SP have a multidisciplinary management including hematology and the specialty of the causative pathology.
It is noteworthy that patients with PV had a mean age of 53 years, which is lower than the mean age reported in the West, which is 60 years [15]. This finding has been found in other studies in sub-Saharan Africa where the mean age of patients was less than 60 years [6,16,17].
According to gender, approximately 53% of PV patients were male (sex ratio = 1.12). In SP, the sex ratio was 2.57. Male frequency in PV has also been described in other studies in the West and in Africa [4,16-18].
Cytoreductive therapy with hydroxyurea was used exclusively for the basic treatment of PV; of 85.3% of PV patients who received this treatment, 41.4% had at least one history of thrombosis. In patients without a history of thrombosis, this type of treatment was prescribed in 27.6% of patients over 60 years older, and in 31% of patients under 60 years who had hematocrit level >45%, leukocytosis >15 G/L, or thrombocytosis ≥ 600 G/L, resistant to therapeutic bloodletting. Due to the fact that we have a very young population at risk of thrombotic complications, the decision was made to use hydroxyurea in patients <60 years without a history of thrombosis, despite recommendations in the Western literature reserving Hydroxyurea for patients at high risk of thrombosis (age >60 years and/or a history of thrombosis). Therapeutic erythrocytapheresis is a more efficient strategy compared to therapeutic bloodletting to deplete levels of hematocrit in primary and secondary erythrocytosis [19-21]. The outcome of the treatment was favorable in most patients. It was marked by the control of hematocrit level at a value <45%. Contrary to the PVSG data, 1/3 of patients surviving the first thrombotic event will recur. In this study, 1/4 of patients with a history of thrombosis had at least one thrombosis recurrence. It was noted that thrombotic recurrence during follow-up occurred exclusively in patients with at least one history of venous thrombosis [22].
The frequency of JAK2V617F mutation was more than 60% in PV patients. Given the frequency of JAK2V617F mutation in PV (96%) [19], JAK2 mutation testing at exon 12 would have been requested if JAK2V617F-negative patients with strong suspicion of PV [20].
The therapeutic approach taken resulted in favorable outcomes, although two patients had recurrent thrombosis. This shows the difficulty of managing anticoagulant treatment in polycythemia. Anticoagulant treatment is often combined with therapeutic bloodletting to significantly reduce the blood hyperviscosity generated [21].
In this study, 27.1% of patients presented thrombotic events. We noted a predominance of arterial thrombosis as previously reported in several studies [4,23,24]. Female were more exposed to thrombosis but without any statistically significant difference compared to male patients. Nevertheless, female gender has been reported as a RFT in several studies [25].
Depending on the type of polycythemia, thrombotic events occurred more in PV patients. Laboratory studies show that coagulation activation markers are significantly higher in PV than in PS [24]. However, our results are better than other studies where the probability of thrombosis in PV was estimated to be more than 40% [26,27].
A total of 22 thrombotic events were identified with a predominance of ischemic stroke (more than 45%) followed by myocardial infarction, deep venous thrombosis of the members, superficial venous thrombosis and pulmonary embolism. All these types of thrombosis were observed in PV, but only ischemic stroke was noted in SP patients. This can be explained by the fact that the most frequent etiologies of SP in our study were pathologies at high risk of thrombosis such as heart disease and chronic broncho-pneumopathy.
Despite the higher frequency of thrombotic events and the presence of all types of thrombosis in PV [27], this study did not show any significant difference according to the type of thrombosis compared with SP.
This leads us to investigate, all factors that may influence the occurrence of thrombosis during primary or secondary polycythemia. Because polycythemia often occurs in elderly subjects, it is sometimes difficult to link thrombosis events directly to polycythemia because of the frequent comorbidities causing the occurrence of thrombosis in these patients [13].
The main sociodemographic or clinico-biological RFTs identified were the age range between 40 and 59 years, hematocrit level >45%, thrombocytosis ≥ 600 G/L and hypercholesterolemia. According to the type of polycythemia, RFTs identified in PV consisted of age, high hematocrit level and thrombocytosis, whereas in SP, RFTs identified were high hematocrit level and hypercholesterolemia. However, after multivariate analysis, the only RFT independently associated with the occurrence of thrombosis in PV or SP was hematocrit level >45%.
This study confirms the data in the literature that a hematocrit >45% is a cardiovascular risk factor in patients with PV or SP due to blood hyperviscosity [21,28,29].
Several studies have identified advanced age (>60 years) as a major RFT, due to vascular aging, and the tendency to hypercoagulability [30]. In our study, the predominance of young age would explain the fact that an age range between 40 and 59 years is considered an RFT.
Although somewhat unclear to some authors, thrombocytosis (>600 G/L) has been associated with a high risk of thrombosis in PV [31], as demonstrated in our study.
Hypercholesterolemia is a cardiovascular risk factor that favors the occurrence of thrombosis, as it would increase the risk of vascular injury through the action of arteriosclerosis. In addition, the concomitant presence of hypercholesterolemia with other factors such as hematocrit >45% would have an aditive pro-coagulant effect [25].
Some authors have suggested the presence of the JAK2V617F mutation, especially with a mutational load >50%, as a RFT in PV [32]; however, in this study, as in others [26,33], JAK2V617F mutation, even with a mutational load >50% was not identified among RTF.
The therapeutic approach chosen in our patients resulted in favorable outcomes, although some patients had recurrent thrombosis. This shows the difficulty of managing anticoagulant treatment in polycythemia. Anticoagulant treatment is often combined with therapeutic bloodletting to considerably reduce blood hyperviscosity generated in polycythemia.
However, our study has two limitations: the small size of the cohort and the fact that some RFTs such as the concomitant presence of thrombophilia factors, obesity, sedentary lifestyle or oral contraceptives could not be evaluated due to the retrospective study.
We show through this study, a frequency of arterial thrombosis in polycythemia. The main RFT is the high hematocrit level (>45%), even if, others RFTs were identified such as the age range (40-59 years), thrombocytosis ≥ 600 G/L and hypercholesterolemia. We insist on the interest of therapeutic bloodletting, sometimes associated with cytoreductive treatment in order to maintain a hematocrit level below 45% to reduce occurrence of thrombotic events.
Acknowledgement
The authors thank all the staff of the Hematology Department of Cheikh Anta Diop University and of the Clinical Hematology Department and the Laboratory of the National Blood Transfusion Center in Dakar, Senegal.
Funding
No funding received for this study
Conflict of Interest
None.
References
2. Randi ML, Bertozzi I, Cosi E, Santarossa C, Peroni E, Fabris F. Idiopathic erythrocytosis: a study of a large cohort with a long follow-up. Annals of Hematology. 2016 Jan;95:233-7.
3. Shizhong K, Shuzhen C, Zihui D, Christopher SH, Zhang Q, Liang T, et al. Erythrocytosis in Hepatocellular Carcinoma Portends Poor Prognosis by Respiratory Dysfunction Secondary to Mitochondrial DNA Mutations. Hepatology. 2017; 65(1):134-50.
4. Mesa RA. New guidelines from the NCCN for polycythemia Vera. Clin Adv Hematol Oncol. 2017;15(11):848-50.
5. Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018; Feb 9;8(2):15.
6. Michael HK, Laura CM, Srdan V. Mechanisms of Thrombogenesis in Polycythemia Vera. Blood Rev. 2015; 29(4):215-21.
7. Vijaya RB. Secondary Polycythemia and the Risk of Venous Thromboembolism. J Clin Med Res. 2014; 6(5):395-7.
8. Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Annals of Hematology. 2019; 98:1071-82.
9. Salinas IP, Flores VR, Montañés MÁ, García-Erce JA. Therapeutic erythroapheresis: Experience in patients with polycythemia vera and secondary erythrocytosis. Medicina Clínica (English Edition). 2020 Jan 10;154(1):16-9.
10. Padaro E, Agbetiafa K, Kueviakoe IM, Layibo Y, Amegbor K, Vovor A, et al. Syndromes myéloprolifératifs « Philadelphie négatifs » et mutation JAK2V617F: étude des 15 premiers cas ayant bénéficié de cette recherche au Togo. Ann Biol Clin. 2012; 70(5):591-4.
11. Seck M, Diop S, Diongue M, Ndiaye Dieye T, Touré Fall A O, Sall A et al. Les polyglobulies à Dakar : aspects épidémiologiques, diagnostiques et thérapeutiques. Dak Med. 2010; 55(1):31-5.
12. Sall A, Touré Fall AO, Seck M, Ndiaye FS, Faye BF, Gadji M, et al. Détection de la mutation de JAK2V617F dans les syndromes myéloprolifératifs « Philadelphie négatif ». Etude préliminaire à propos de 10 cas suivis à Dakar. Dak Med. 2012; 57(3):197-202.
13. Jey M, Josey P. Polycythemia and oxygen sensing. Pathol Biol. 2004; 52:280-4.
14. Barba T, Boileau JC, Pasquet F, Hot A, Pavic M. Polyglobulies héréditaires primitives et secondaires. Rev Med Interne. 2016; 37:460-5.
15. Barosi G, Mesa R, Finazzi G, Harrison C, Kiladjian J-J, Lengfelder E, et al. Revised response criteria for polycythemia vera and essential thrombocythemia: an ELN and IWG- MRT consensus project. Blood. 2013; 121(23):4778- 81.
16. Bolarinwa RA, Rurosinmi MA. Polycythemia Vera in Nigeria. Niger Post Grad Med J. 2009; 16: 68-72.
17. Azonbakin S, Awede B, Houssou B, Massi R, Adjagba M, Anani I, et al. La Mutation Jak2 V617F dans le diagnostic de la polyglobulie de Vaquez dans une cohorte de patients Beninois. Journal de la Société de Biologie Clinique du Bénin. 2018; 028: 75-79.
18. Bellanné-Chantelot C, Saint-Martin C, Malak S, Najman A. Les formes familiales des syndromes myéloprolifératifs Philadelphie négatifs : caractéristiques cliniques et moléculaires. Hematology. 2011; 17(5): 331-41.
19. Boussen I, Saillard C. Les syndromes myeloprolifératifs hors leucémie myéloïde chronique. Hematology. 2015; 21: 348-356.
20. Yoo JH, Park TS, Maeng HY, Sun YK, Kim YA, Kie H, et al. JAK2 V617F/C618R mutation in a patient with polycythemia vera a case study and review of the literature. Cancer Genet Cytogenet. 2009; 189(1): 43-47.
21. Tefferi A, Vannucchi AM, Barbui T. Polycythemia vera treatment algorithm 2018. Blood Cancer Journal. 2018; 8(1):3.
22. Barbui T, Carobbio A, Rumi E. In contemporary patients with Polycythemia Vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014; 124:3021-3.
23. Kralovics R. Genetic complexity of myeloproliferastive neoplasms. Leukemia. 2008; 22:1841-8.
24. Kornberg A, Rahimi-Levene N, Yona R, Mor A, Rachmilewitz EA. Enhanced generation of monocyte tissue factor and increased plasma prothrombin fragment1+2 levels in patients with polycythemia vera: mechanism of activation of blood coagulation. Am J Hematol. 1997; 56: 5-11.
25. Falanga A, Russo L, Milesi V, Vignoli A. Mechanisms and risk factors of thrombosis in cancer. Crit Rev Oncol Hematol. 2017 Oct;118:79-83
26. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol. 2017; 92: 95-108
27. Griesshammer M, Kiladjian JJ, Besses C. Thromboembolic events in polycythemia vera. Ann Hematol. 2019 May;98(5):1071-82.
28. Passamonti F, Rumi E, Pietra D. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24:1574- 1579
29. Di Nisio M, Barbui T, Di Gennaro L. European Collaboration on Low-dose Aspirin in Polycythemia Vera (ECLAP) Investigators. The haematocrit and platelet target in polycythemia vera. Br J Haematol. 2007;136(2):249-259.
30. Tefferi A, Jimma T, Sulai NH. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2012; 26(3):475-80.
31. V. Ugo. Stratégies diagnostiques dans les syndromes myéloprolifératifs en 2008. Correspondances in Onco-hematol. 2008; 3(3): 127-33.
32. Tefferi A, Barbui T. Polycythemia vera and essential thrombocythemia: 2017 update on diagnosis, risk-stratification and management. Am J Hematol. 2017; 92:95-108.
33. Barbui T, Carobbio A, Rambaldi A, Finazzi G. Perspectives onthrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood. 2009; 114:759-63.
