Abstract
Background: Human immunodeficiency virus (HIV) is one of the world's most dangerous health issues. By the end of 2022, around 39 million HIV-positive people worldwide, more than half of the cases, were in Eastern and Southern Africa. The Hepatitis B Virus (HBV) is one of the most critical factors driving HIV drug resistance, resulting in treatment failure and poor adherence. A significant decrease in cluster of differentiation-4 (CD4) cell count disrupts the T cell immune system in the body. Therefore, this study aims to assess the effects of hepatitis-B virus co-infection on CD4 cell count recovery in HIV-infected patients on antiretroviral therapy (ART) in the Tigray region.
Methods: A retrospective cohort study design was conducted. The random sampling method was used in Mekelle health facilities from January 2009 to February 2018. The total sample size was 466, including 94 HIV-HBV co-infected and 372 HIV mono-infected. Data was entered, cleared, and coded into Epi-Info 7, and then analyzed using the Poisson regression model in STATA version 14.
Result: Of the total patients, 294 (64%) were females. The majority, 199(43%) of the participants were in the age group between 15 and 30 years old. Thirty-six (38.3%) participants in the HIV- HBV exposed group had CD4 cell counts greater than 200 cells/µl, while 58 (61.7%) clients in the HBV unexposed group had CD4 counts below 200 cells/µl. Compared to HIV mono-infected, the change of CD4 cell count over time was 0.25 units lower among the HIV-HBV co-infected individuals (adjusted coefficient -0.25; 95% CI: -0.26 to -0.23).
Conclusion: The HIV-HBV co-infected group showed a low rate of CD4 cell recovery. Then, HIV-infected people receiving (ART) should be tested for HBV co- infection. It is also suggested to do a prospective cohort study to investigate the impact of HIV-HBV co-infected patients on immune recovery.
Keywords
CD4 Recovery, HIV-HBV co-infected, HIV mono-infection, Mekelle city
Introduction
Human immunodeficiency virus (HIV), the cause of acquired immunodeficiency syndrome (AIDS), is one of the world's most significant health and development issues. By the end of 2022, over 39 million people worldwide would have HIV, including more than half of the 20.8 million people in Eastern and Southern Africa, including Ethiopia. Furthermore, since the beginning of the epidemic, 40.4 million people have perished from AIDS-related disorders [1,2].
HIV patients are vulnerable and have poor health outcomes since the condition necessitates rigorous adherence to medication, and delivering regular HIV care and securing a consistent supply of drugs in conflict zones can be difficult. Furthermore, stigma impedes HIV testing, ART administration, and client adherence [3]. Human Immunodeficiency Virus has remained a substantial obstacle for decades. Most HIV-positive people are unaware of their status, and many who seek testing and treatment do so too late to benefit completely from it. Lifelong treatment presents new issues for individuals, communities, and the health-care system [4]
Hepatitis B virus (HBV) is one of the most important factors influencing drug resistance to HIV/AIDS, resulting in treatment failure and poor adherence to ART. Following a mutation, the new HIV is not susceptible to the prior ART regimen, and the individual drug must be changed over time to another second-line regimen [5]. Several viral mutations associated with a high risk of HCC were commonly found in HIV-HBV co-infected patients, possibly explaining the high rates of carcinogenesis [6]. Recent studies have shown that these scores are accurate, noninvasive methods for distinguishing nonsignificant versus significant liver fibrosis in HIV-HBV co-infected patients [7,8]. It gives fast clinical information, with a sensitivity of about 78% and specificity of 81–82% for predicting severe fibrosis in HBV-infected people compared to liver biopsy [9,10].
Globally, about 254 million people are infected with the HBV, which is one of the infectious diseases with rising mortality rates. Sixty-three percentage of new hepatitis B infections arise in the WHO African region [11]. Chronic Hepatitis B (CHB) natural history is dynamic and complex. The sickness ranges from asymptomatic infection to severe chronic liver disease and HCC. Some persons do not suffer substantial liver damage with CHB, but others acquire liver fibrosis, potency, and a strong resistance barrier while having fewer renal and bone adverse effects [12,13]. Over 80% of patients with hepatitis cases in Sub-Saharan Africa, including Ethiopia, have HBV. Around 28% of HBV patients were co-infected with HIV, with over 40% in East Africa [14].
Patients on ART who are also infected with the HBV had poorer results than HIV-only patients [15]. Furthermore, HIV/HBV co-infected patients have greater levels of HBV viremia, a faster progression to chronic HBV infection, and an increased risk of hepatocellular carcinoma, as well as decreased cluster of differentiation-4 (CD4) cell recovery among antiretroviral treatment users [15–17]. Clients with HIV and HBV co-infection have lower CD4 cell counts than HIV mono-infected clients. However, most lower-income nations have suggested further exploration of the link between HBV and CD4+ T cell counts among co-infected clients, and the median CD4+ T cell count likewise did not vary by hepatitis B surface antigen status [18]
A significant decrease in CD4 cell count deconstructs the T cell immune system in the body, increasing opportunistic infections and cancer cells [19,20]. A CD4 cell count of less than 200 cells per microliter is too important in clinical practice. Patients with CD4 counts exceeding 200 cells/µL are at a lower risk of medical complications [21]. CD4 T cell counts are routinely used as important indicators of HIV/AIDS infection progression, as well as to initiate, prognosis, and monitor antiviral medication in the absence of HIV viral load quantitative monitoring tests. A steady increase in CD4 cells in response to highly active antiretroviral therapy (HAART) and HIV virological suppression was associated with higher CD4 T lymphocyte cell counts [22–24].
Approximately 15-20% of patients-initiated ART with very low CD4 counts (<200 cells/microliter) may persist to have usually low CD4 cell counts and are at the greatest risk of failing to achieve durable immunological recovery [25,26]. Patients enrolled on ART who experience low CD4 recovery at the early phase of treatment are at significant risk of acquiring advanced AIDS disease, AIDS related illnesses, and dying, despite viral load suppression. CD4 counts remain low among patients seeking care in many regions [27]. Nearly 17% of adults in Sub-Saharan Africa die within the first year of ART initiation [28].
The majority of CD4 irregularities that persist during drug therapy are similar to those reported in elderly people, confirming the theory that age-related immune system deterioration leads to adverse events and disease advancements [29]. Many nations have started life-saving therapy for co-infected patients; nevertheless, some countries like Ethiopia are still lagging in implementing HBV control strategies [29]. Therefore, there is potential for morbidity and mortality in HIV patients since the common way of transmission across co-infected patients leads to enhanced chronicity [30–33].
Hepatitis B is a dynamic infection, and patients with chronic infection require intensive follow-up and monitoring before, during, and after discontinuation of therapy to prevent disease progression and the development of HCC and liver toxicities [34].
The natural history and progression of HBV infection are complex, featuring developments in several identifiable cycles [30]. Therefore, the impact of ART on clinical and immunological outcomes and the associated factors with CD4 T cell count recovery among HIV and HBV co-infected patients is little investigated in Ethiopia. This helps to discover gaps and implement appropriate procedures to integrate HBV management with HIV care and treatment services packages. This study aimed to evaluate the effects of HBV co-infection on CD4 cell count recovery in HIV-infected patients on ART in the Tigray region.
Methods
Study area and period
This study was carried out in Mekelle, the capital of the Tigray regional state in northern Ethiopia, 783 kilometers from the capital city of Addis Ababa. The city is organized administratively into seven sub-cities. There was one teaching comprehensive referral hospital, two general hospitals, nine health centers, and 48 private clinics in the city [31]. Fourteen health facilities provided ART services by 2018 (Figure 1). And also, about 12,277 patients were receiving ART in these health facilities. According to Ethiopia’s predicted central statistical agency for 2018, the town’s population was 340,859. The study was carried out from March 1 to March 30, 2018.
Study design
A facility-based retrospective cohort study design was conducted.
The study population and participants
The source population consisted of HIV-infected clients undergoing ART registered in Mekelle health facilities, whereas the study population consisted of all registered adult clients over the age of 15 who had begun ART with positive HBV (exposed) and HBV negative (unexposed) results in six health facilities. Patients with known HBV status who received ART at six health facilities while utilizing their registered patient card were also included in the study subjects. As a result, the health research ethical review committee provided authorization from each study's health facilities, verbal consent from living participants, and written consent from each child’s parent or guardians aged 15 to 18 years. All the procedures used in this study were ethically evaluated in conformity with the Helsinki Declaration of human research.
Figure 1. Map of the study health facility in Mekelle city in Tigray region, 2018.
Eligibility criteria
The study included all registered patients over 15 years who started ART with known HBV status from January 2009 to February 2018. Patients with incomplete patient cards and those below the age of 15 were excluded from the study
Sample size determination
The sample size for the CD4 recovery was calculated using Epi-info statistical package 7.0.9.7, cohort (unexposed and exposed) with the assumption of two side confidence limits 95%, Power 80, the ratio of (unexposed: exposed group) 4:1, percent of outcome in the unexposed group (HBsAg negative) 52.4% [32], risk ratio 0.51 [32] and the percent outcome in the exposed group 26.7%. Using Fleiss w/CC, a total of 200 participants were recruited, with 40 exposed and 160 unexposed finally in a sample size of 233.
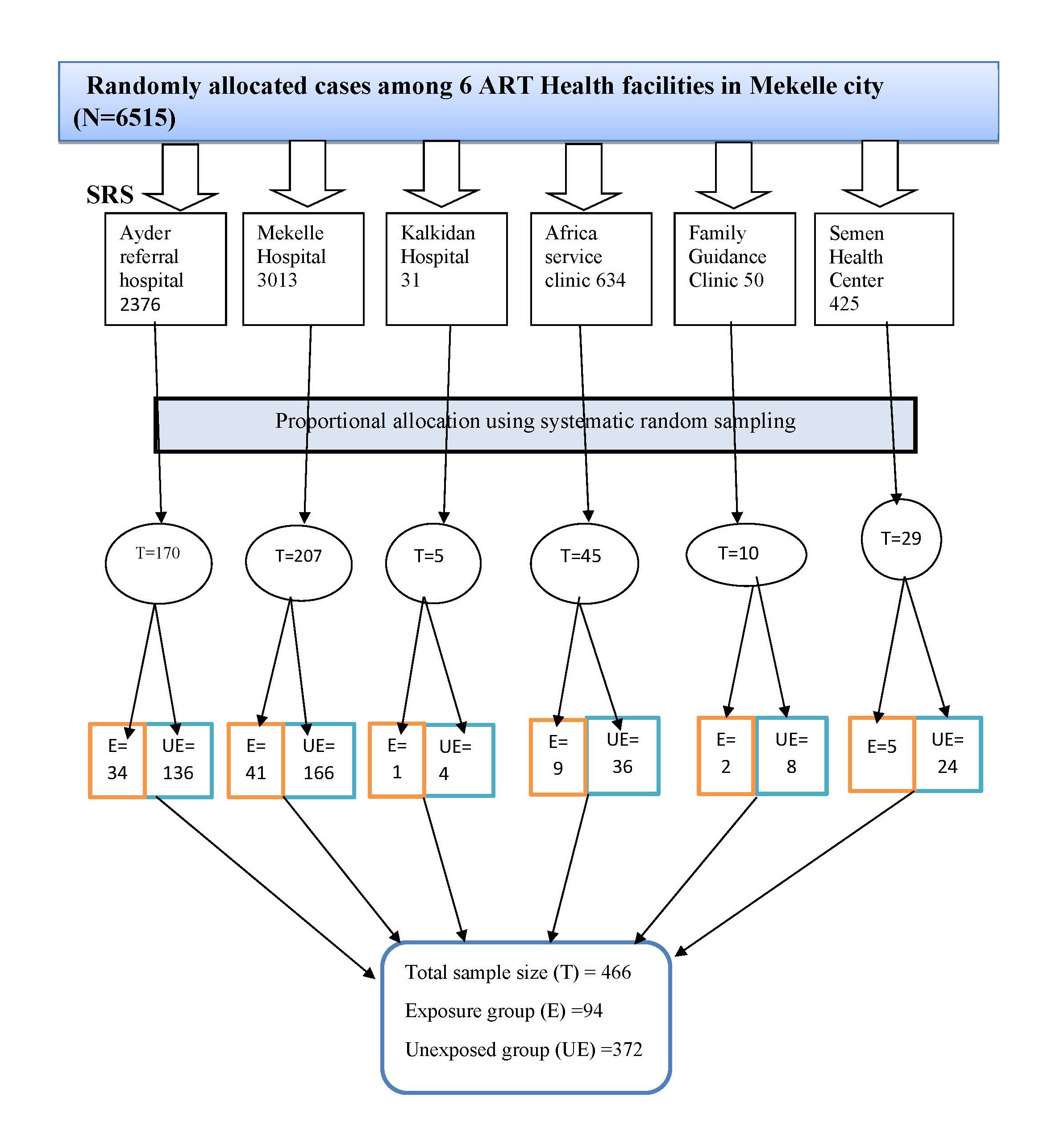
Sampling technique/ procedure: Six out of 14 ART health facilities in Mekelle were selected, using a simple random sampling method. In the study unit, 6,515 clients who started ART within the previous 8 years registered in the facility. Using this information as baseline, the total number of clients was assessed from each health facility using a systematic random sampling method, and the sample size was doubled using design effect (233*2=466, of which 94 were exposed and 372 were unexposed to HBV, 1:4). Finally, proportional allocation was employed for each health facility, additionally, and patient cards were assessed every 14 intervals (N/n=6515/466) (Figure 2).
Figure 2. Schematic presentation of the sampling procedure for the exposure and unexposed group of retrospective cohort design in Mekelle health facilities, 2018. Key: T: Total Allocated Sample; E: Exposed (HIV-HBV co-infected group); UE: Unexposed (HIV mono-infected group).
Study variables
Dependent variable: CD4 cell count recovery.
Independent variables: HBV status: Hepatitis B virus-positive results in ART patients and Hepatitis B Virus-negative results in ART patients; other variables such as, socio-demographic variables sex, age, marital status, occupation, educational status, and, the clinical and laboratory characteristics: Baseline CD4, baseline hemoglobin level, tuberculosis, baseline nutritional status (BMI), baseline ALT level, baseline AST Level, baseline creatinine, and baseline WHO clinical stage.
Operational definition
CD4 recovery: Progression (change) of CD4 cell count over time after initiation of ART at a first year and last examined result >24 months (>2 years).
Incomplete card: When two or more independent variables are not recorded in the patient registration book.
Data collection tools and procedures
Data were retrieved using a data extraction format (checklist) from different journals and medical records of HIV patients [33–35]. First unique medical record number of eligible groups was extracted from the ART registration log books, then based on the unique medical record number the chart of the patient was retrieved from the medical record room; we started data collection time from the date of ART initiated and serological tested for HBV, and patient cards were followed until study period. The status of the clients was reviewed by trained BSc nurses and laboratory technologists, who have experience with ART.
Data quality control and data management
To assure data quality, one supervisor and four data collectors received training. A Pretest of the checklist was carried out on 5% of the sample size in Mekelle health facilities. To reduce errors, double data entry was performed on selected medical charts of eligible patients mentioned in the ART registration logbook. In addition, the principal investigator and supervisor constantly monitored data collectors during the collection period.
Data analysis and interpretation
The data was coded, cleaned, validated, and entered into Epi Info version 7.0.9.7 and exported to STATA version 14 for analysis. In the descriptive analysis, the strategy for model building of the relationship between HIV-HBV co-infected and HIV mono-infected patients attending ART on socio demographic, clinical, and laboratory characteristics of variables was analyzed, and the median CD4 recovery at different time points was also calculated.
Interpreting coefficients
In Poisson regression, Y is often represented as a rate. Positive coefficients imply a higher rate, whereas negative coefficients suggest a lower rate. The Poisson distribution belongs to the exponential family.
Interprets the coefficients in terms of Poisson regression coefficients
In the Poisson model, our response variable is countable, and each subject received equal amounts of observation time. The regression coefficient predicts that the difference in logs of projected count will change for every unit change in the predictor variable.
Poisson model assumptions
When the mean and variance are the same, we get the Poisson mean-variance relationship. When the mean and variance are not equal (over-dispersion), the Poisson distribution is frequently replaced by the Negative Binomial Distribution. When the mean is greater than the variance, the dependent variable is counting data and positive integers.
Result
Socio-demographic characteristics
Between January 2009 and January 2018, a total of 6,515 patients enrolled in the ART Clinics in the selected six health facilities, and we analyzed them in March 2018. Of these, 94 HIV-HBV co-infected (exposed) and 372 HIV mono-infected (unexposed) groups were identified and evaluated in this study. Out of the total cases, 300 (64.3%) were females. The median age of 466 patients was 33 years, with a range of 15 to 68 years. The exposed group had a median age of 35 years, ranging from 15 to 65 years, whereas the unexposed group had a median age of 33, ranging from 15 to 68 years. Of the total patients, 216 (46.35%) were married, and 164 (35.19%) were employed (Table 1).
|
S. No |
Variables |
Category |
Exposed=94 (20%) |
Unexposed=372 (80%) |
|
No (%) |
No (%) |
|||
|
1 |
Sex |
Female |
44 (46.8) |
250 (67.2) |
|
Male |
50 (53.2) |
122 (32.8) |
||
|
2 |
Age group |
15–40 years old |
65 (69.1) |
302 (81.2) |
|
≥40 years old |
29 (30.9) |
70 (18.8) |
||
|
3 |
Marital status |
Married |
42 (44.7) |
174 (46.8) |
|
Never Married |
21 (22.3) |
87 (23.4) |
||
|
Divorced |
20 (21.3) |
79 (21.2) |
||
|
Widowed |
11 (11.7) |
32 (8.6) |
||
|
4 |
Occupation |
Daily laborers |
21 (22.34) |
59 (15.9) |
|
House wife |
12 (12.8) |
67 (18.0) |
||
|
Employed |
28 (29.8) |
136 (36.6) |
||
|
Unemployed |
33 (35.1) |
110 (29.6) |
||
|
5 |
Educational status |
No education |
19 (20.2) |
82 (22) |
|
Primary school |
32 (34) |
136 (36.6) |
||
|
Secondary |
33 (35) |
106 (28.5) |
||
|
Certificate and above |
10 (10.6) |
48 (12.9) |
Clinical and laboratory characteristics
In the HBV exposed group CD4 cell count was 36 (38.3%), with 58 (61.7%) in the CD4 >200 cell/µl group and 153 (41.1%) in the CD4 <200 cell/µl group. In the HBV-exposed group, 53 (56.4%) had normal (0–40 unit) ALT levels, and 41 (43.6%) had abnormal alanine aminotransferase (ALT) levels, where as in the HBV-unexposed group, 311 (83.5%) had normal ALT levels, and 61 (16.4%) had abnormal ALT levels [36] (Table 2).
|
S. No |
Variables |
Category |
Exposed=94(20%) |
Unexposed=372(80%) |
|
No (%) |
No (%) |
|||
|
6 |
HBV status |
Negative |
- |
372 (80) |
|
Positive |
94 (20) |
- |
||
|
7 |
Baseline CD4 |
>200 |
36 (38.3) |
219 (58.9) |
|
≤200 |
58 (61.7) |
153 (41.1) |
||
|
8 |
Baseline ALT |
Normal |
53 (56.4) |
311 (83.5) |
|
Abnormal |
41 (43.6) |
61 (16.4) |
||
|
9 |
Baseline AST |
Normal |
51 (54.3) |
322 (86.6) |
|
Abnormal |
43 (45.7) |
50 (13.4) |
||
|
10 |
Baseline creatinine |
Normal |
71 (75.5) |
337 (90.6) |
|
Abnormal |
23 (24.5) |
35 (9.4) |
||
|
11 |
Base line Hemoglobin |
Moderate or severe |
27 (28.7) |
41 (11) |
|
Non or mild Anemia |
67 (71.3) |
331 (89) |
||
|
12 |
WHO clinical stage |
Stage I&II |
29 (30.9) |
215 (57.8) |
|
Stage III&IV |
65 (69) |
157 (42.2) |
||
|
13 |
Tuberculosis |
No |
66 (70.2) |
315 (84.7) |
|
Yes |
28 (29.8) |
57 (15.3) |
||
|
14
|
Baseline BMI |
>25Kg/m2 |
2 (2.1) |
37 (9.95) |
|
<18.5kg/m2 |
40 (42.6) |
87 (23.4) |
||
|
18.5-25 |
52 (53.3) |
248 (66.7) |
||
|
15 |
ART TDF-based |
On TDF |
69 (73.4) |
258 (69.4) |
|
Not on TDF |
25 (26.6) |
114 (30.6) |
||
|
16 |
ART NVP-based |
Nevirapine based |
38 (40.4) |
143 (34.4) |
|
Efaveranze based |
56 (59.6) |
229 (61.6) |
||
|
Note: HIV: Human Immunodeficiency Virus; HBV: Hepatitis B Virus; ART: Antiretroviral Therapy; TDF: Tenofovir; NVP: Nevirapine; ALT: Alanine Transaminase; AST: Aspartate Transaminase; CD4: Cluster of Differentiation Receptor 4; WHO: World Health Organization |
||||
Evaluation of median CD4 progression
In the descriptive analysis, the median CD4 cell count progression was estimated at different time intervals, ranging from baseline to nine years. The median CD4 cell count progression was lower at baseline compared to time in the HIV mono-infected group, but greater at baseline in the HIV-HBV co-infected group during the follow-up period following ART commencement than at final follow-up (Table 3).
|
Duration of ART (months) |
HIV mono-infection |
Co-infected group |
||
|
CD4 cells/µl tested, in number |
Median (IQR) |
CD4 cells/µl tested, in number |
Median (IQR) |
|
|
At baseline CD4 |
372 |
255.5 (240) |
94 |
163.5 (203) |
|
At 6 months |
364 |
322.5 (234.5) |
91 |
237 (212) |
|
12 months |
358 |
406 (241) |
83 |
301 (245) |
|
18 months |
337 |
455 (237) |
81 |
314 (196) |
|
≥24 months |
302 |
543.5 (266) |
67 |
386 (245) |
|
Key: CD4: Cluster of Differentiation Receptor 4; ART: Antiretroviral Therapy; HBV: Hepatitis B Virus; IQR: Interquartile range |
||||
Effect of HBV co-infection on CD4 cell count recovery
In the Poisson regression analysis of the Cox proportional hazard model, the coefficient evaluates the effect of the covariate hepatitis B on the slope or change in CD4 count (unit cell/year).
In adjusted analysis on the final follow-up period (recent), CD4 status was evaluated. And the HIV-HBV co-infected group had a lower outcome compared to the HIV mono-infection group in terms of one-year duration and final investigated CD4 result (Table 4).
|
Random-effects Poisson regression (change in CD4 count) |
||||
|
Category |
Coefficient |
P-value |
Adjusted Coefficient |
P-value |
|
Change in CD4 in one year |
|
|
|
|
|
HIV-HBV co-infected group |
-.34 (-.36_-.33) |
<0.001 |
-.21 (-.22_-.20) |
<0.001 |
|
HIV mono-infected group |
Reference |
|
|
1 |
|
Change in (last) CD4≥2 years |
|
|
|
|
|
HIV-HBV co-infected group |
-.25 (-.26_-.23) |
<0.001 |
-0.25 (-.0.26--.23) |
<0.001 |
|
HIV mono-infected group |
Reference |
|
|
1 |
|
Key: CD4: Cluster of Differentiation Receptor 4; ART: Antiretroviral Therapy; HBV: Hepatitis B Virus |
||||
Discussion
This study found that HIV-HBV co-infected individuals had a lower median CD4 cell count than HIV mono-infected patients who attended ART. In the Poisson Cox regression model, CD4 cell recovery evaluation in one year and longer time at more than or equal to two years of follow-up was substantially higher in HIV-HBV co-infected individuals. According to the findings of this study, HIV patients with hepatitis B virus who were on ART experienced slower CD4 or immunological recovery.
In our study, HIV mono-infected patients had considerably higher CD4 counts than those infected with both HBV and HIV. This is consistent with the findings of a hospital-based descriptive cross-sectional study conducted in Nigeria [37] to look at the effect of hepatitis B virus co-infection on CD4 cell count and liver function in HIV-infected patients. According to [38], co-infected persons' CD4 levels were non-significantly lower [39]. Research on the effects of HIV and the hepatitis B virus yielded conflicting results. HIV/HBV co-infected subjects had considerably fewer CD4+ T cells than HIV mono-infected individuals. This suggests that HBV may hasten the progression of HIV.
According to the study's findings, individuals with HIV-HBV co-infection had a 0.25-unit reduction in CD4 cell count recovery over a long follow-up period compared to the HIV mono-infected group receiving ART in the city. Our findings are consistent with several African and Asian studies [17,40–43]. The majority of HIV patients enrolled and follow-up over one year, receiving antiretroviral medication, had a lower CD4 cell count in the HIV-HBV co-infected group by a factor of 0.25 than in the HIV mono-infected group; this study is similar to studies done in Cameroon and Switzerland [44,45].
However, differences in the USA [46] were highly significantly increased HIV-HBV than in the HIV mono-infected group, as well as some studies show no significant difference in CD4 recovery between HBV co-infected and HIV mono-infected patients, especially in contexts where Tenofovir-based ART regimens were used in Thailand, China, and Botswana [47–49]. The reason for the discrepancy with our study group may be due to the immunological differences in the HBV co-infected group and the awareness creation care given in different countries than in HIV mono-infected individuals attending ART.
The discrepancy across studies may relate to ART regimen composition, severity of liver fibrosis, HBV DNA levels, or additional co-infections [43,48,50–52]. Tenofovir, in particular, is known for its dual activity against HIV and HBV and may reduce HBV-associated immune suppression, thus mitigating the CD4 recovery gap [51,52]. Therefore, drug-specific effects and underlying liver disease status should be considered when interpreting immune recovery outcomes.
A study at Kisumu District Hospital [53] revealed that patients with co-infection had a lower mean CD4 cell count than those with HBV mono-infection, which supports our findings. A study at Mikelle Hospital in Tigray, Northern Ethiopia, reveals that HIV/AIDS-positive patients with a CD4 count of less than 200 cells/μl had a substantial link with hepatitis-positive patients [54]. Similar results were reported [53], where the authiors showed that the mean CD4 count of HIV mono-infected persons was much higher than that of co-infected patients, which is consistent with our current study.
Additionally, studies conducted in China [51,55], Vietnam [56], Cambodia [57], and South Africa [58] revealed that the CD4+ T cell count declined over a longer duration in people with HIV-HBV co-infection than in people with HIV mono-infection. The unfavorable effect of decreased CD4 cell count recovery on HIV-HBV co-infected patients may be explained by the possibility of developing several serious opportunistic infections, as well as the complex interaction between HIV-HBV co-infected individuals that results in immunological failure in ART users [59]. However, when HBV-co-infected and HIV-mono-infected patients started ART in South Africa and Cameroon, there was no difference in the change and progression of CD4 counts [45,60]; these discrepancies may be due to variations in adherence, genetics, and clinical settings.
Due to ART common hepatotoxicity, especially when co-infections are present, treating co-infections is frequently difficult. Delaying with a co-infected patient is more difficult [61]. Numerous studies have shown that co-infection with HBV has detrimental effects, such as a shorter life span, a correlation with an advanced stage of HIV clinical illness, a lower CD4 cell count, and more liver damage [62]. Studies examining the relationship between HIV and HBV infection have shown that people with HIV-HBV infection had a liver-related death rate that was ten times higher than that of patients with either infection alone [63].
Comparable studies conducted in Thailand and the United States of America, our study found that HIV-HBV groups significantly outnumbered those with no HBV in ART patients over time follow-up [64,65]. The different results may be due to the varied ways that healthcare practitioners in America and Thailand handle the treatment of patients who are co-infected with HIV and HBV.
According to a previous study conducted in Nigeria, patients who were co-infected with HIV-HBV had lower CD4 count values. While the mean CD4 cell count for solely HIV-infected patients was 478 cells/μl, patients who were co-infected with HBV-HIV had lower mean CD4 cell counts of 107 cells/μl. According to this, co-infections can happen at any stage of HIV infection, which hampered the trial's CD4 cell count response [66].
Age-related immune dysfunction in HIV patients may be mediated by regulatory genes such as Sirtuin 1 (SIRT1). This gene plays a critical role in T cell differentiation, immune modulation, and cellular senescence. Recent findings suggest that SIRT1 expression levels may influence the rate of CD4+ T-cell recovery, especially in aging or co-infected populations [67,68]. Although SIRT1 levels were not measured in our study, future prospective investigations might explore the potential correlation between plasma SIRT1 and CD4 recovery in HIV-HBV co-infected individuals. Furthermore, the role of SIRT1 activators or inhibitors may represent a promising therapeutic adjunct to enhance immune recovery [52,67,69].
Strengths and limitations of the study
A longitudinal cohort study gave more opportunities for patients to be screened for the HBV and begin antiretroviral medication. The drawback of our study was that hepatitis testing was not performed on the majority of patients in Mekelle health facilities as part of the usual test for patients starting ART. As a result, the quality of data derived from these findings was heavily reliant on the completeness of patient cards.
Conclusion and Recommendation
The study found that HIV mono-infected patients have higher CD4 counts than HBV-HIV co-infected patients. In addition, HIV mono-infected patients enrolled in the ART program showed significant CD4 T cell count recovery in response to ART. Patients on ART who have HIV and HBV co-infection have a worse rate of immunological recovery. These findings underline the significance of comprehensive HIV-HBV co-infection screening for ART patients. It is also important to do longitudinal prospective cohort studies for a better understanding of the association between HIV-HBV co-infection and immune system recovery.
Ethical Approval and Participant Consent
The approval of the Health Research Ethical Review Committee (HRERC) was made from Mekelle University College of Health Sciences (ERC-1350/2018), and a supportive letter was also attained hierarchically from the Tigray regional health Bureau to each health facility. As a retrospective study, this study was conducted on already available data collected during routine clinical practices. The dataset used for analysis was kept anonymous and used for the intended research. Hence, all procedures performed in studies involving human participants were conducted by the standard of the Health Research Ethical Review Committee of Mekelle University, College of Health Science.
Consent for Publication
Not applicable.
Availability of Data and Materials
The data set of this study is available from the corresponding author upon reasonable request.
Competing Interests
The authors declared that they have no conflict of interest.
Funding
This research has no external funds.
Author’s Contribution
HB has developed the proposal, written up research analysis, validation, and revising manuscript; AB and DM made revisions to the research proposal and revising the manuscript; and FG, GT, GH, YH, and MA contributed to revising the research manuscript and analysis of the study.
Acknowledgment
We'd like to thank Mekelle University, the College of Health Science, and the Department of Epidemiology for offering this precious opportunity, as well as the ART team members in the study health facilities.
My special thanks to those who have participated in the data collection and research process, especially Tesfaldet Weldegebrel, Dargie Tesfay, Abrar Mohamed, and Gebremedhin Gebreanenia.
References
2. PEPFAR. HIV and AIDS Epidemic Global Statistics. July 20, 2023. Available from https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics/.
3. MSF. HIV/AIDS: continuing against the deadly pandemic. MSF; 2022. Available from https://www.msf.org/hiv-depth.
4. World Health Organization. The global health sector strategy on HIV/AIDS 2011-2015: an interim review of progress: abridged report, May 2014. Geneva: World Health Organization; 2014. Available from https://iris.who.int/handle/10665/112790.
5. Gamell A, Muri L, Ntamatungiro A, Nyogea D, Luwanda LB, Hatz C, et al. A Case Series of Acquired Drug Resistance-Associated Mutations in Human Immunodeficiency Virus-Infected Children: An Emerging Public Health Concern in Rural Africa. Open Forum Infect Dis. 2015 Dec 17;3(1):ofv199.
6. Li KW, Kramvis A, Liang S, He X, Chen QY, Wang C, et al. Higher prevalence of cancer related mutations 1762T/1764A and PreS deletions in hepatitis B virus (HBV) isolated from HBV/HIV co-infected compared to HBV-mono-infected Chinese adults. Virus Res. 2017 Jan 2;227:88–95.
7. Iacob DG, Luminos M, Benea OE, Tudor AM, Olariu CM, Iacob SA, et al. Liver fibrosis progression in a cohort of young HIV and HIV/ HBV co-infected patients: A longitudinal study using non-invasive APRI and Fib-4 scores. Front Med (Lausanne). 2022 Jul 29;9:888050.
8. Yang R, Gui X, Ke H, Xiong Y, Gao S. Combination antiretroviral therapy is associated with reduction in liver fibrosis scores in patients with HIV and HBV co-infection. AIDS Res Ther. 2021 Dec 19;18(1):98.
9. Li Q, Chen L, Zhou Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels. Sci Rep. 2018 Mar 27;8(1):5224.
10. Qi X, An M, Wu T, Jiang D, Peng M, Wang W, et al. Transient Elastography for Significant Liver Fibrosis and Cirrhosis in Chronic Hepatitis B: A Meta-Analysis. Can J Gastroenterol Hepatol. 2018 May 24;2018:3406789.
11. World Health Organization. Global hepatitis report 2024: action for access in low-and middle-income countries. Geneva: World Health Organization; 2024 Apr 9. Available from https://www.who.int/publications/i/item/9789240091672.
12. Ryom L, De Miguel R, Cotter AG, Podlekareva D, Beguelin C, Waalewijn H, et al. Major revision version 11.0 of the European AIDS Clinical Society Guidelines 2021. HIV Med. 2022 Sep;23(8):849–58.
13. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017 Aug;67(2):370–98.
14. Okoth F, Mbuthia J, Gatheru Z, Murila F, Kanyingi F, Mugo F, et al. Seroprevalence of hepatitis B markers in pregnant women in Kenya. East Afr Med J. 2006 Sep;83(9):485-93
15. Deressa T, Damtie D, Fonseca K, Gao S, Abate E, Alemu S, et al. The burden of hepatitis B virus (HBV) infection, genotypes and drug resistance mutations in human immunodeficiency virus-positive patients in Northwest Ethiopia. PLoS One. 2017 Dec 27;12(12):e0190149.
16. Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection--a global challenge. N Engl J Med. 2012 May 10;366(19):1749–52.
17. Wandeler G, Gsponer T, Bihl F, Bernasconi E, Cavassini M, Kovari H, et al. Swiss HIV Cohort Study. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013 Nov 1;208(9):1454–8.
18. Singh KP, Avihingsanon A, Zerbato JM, Zhao W, Braat S, Tennakoon S, et al. Predictors of liver disease progression in people living with HIV-HBV co-infection on antiretroviral therapy. EBioMedicine. 2024 Apr;102:105054.
19. Chêne G, Binquet C, Moreau JF, Neau D, Pellegrin I, Malvy D, et al. Changes in CD4+ cell count and the risk of opportunistic infection or death after highly active antiretroviral treatment. Groupe d'Epidémiologie Clinique du SIDA en Aquitaine. AIDS. 1998 Dec 3;12(17):2313–20.
20. Weissberg D, Mubiru F, Kambugu A, Fehr J, Kiragga A, von Braun A, et al. Ten years of antiretroviral therapy: Incidences, patterns and risk factors of opportunistic infections in an urban Ugandan cohort. PLoS One. 2018 Nov 1;13(11):e0206796.
21. Opportunistic Infections Project Team of the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord; Young J, Psichogiou M, Meyer L, Ayayi S, Grabar S, et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9(3):e1001194.
22. Gottlieb GS, Sow PS, Hawes SE, Ndoye I, Redman M, Coll-Seck AM, et al. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J Infect Dis. 2002 Apr 1;185(7):905–14.
23. Cozzi Lepri A, Phillips AN, d'Arminio Monforte A, Castelli F, Antinori A, de Luca A, et al. When to start highly active antiretroviral therapy in chronically HIV-infected patients: evidence from the ICONA study. AIDS. 2001 May 25;15(8):983–90.
24. Smith CJ, Sabin CA, Youle MS, Kinloch-de Loes S, Lampe FC, Madge S, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004 Nov 15;190(10):1860–8.
25. Takuva S, Maskew M, Brennan AT, Long L, Sanne I, Fox MP. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J Int AIDS Soc. 2014 Mar 3;17(1):18651.
26. Engsig FN, Zangerle R, Katsarou O, Dabis F, Reiss P, Gill J, Porter K, e t al. Antiretroviral Therapy Cohort Collaboration (ART-CC) and the Collaboration of Observational HIV Epidemiological Research Europe (COHERE) in EuroCoord. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014 May;58(9):1312–21.
27. Deyno S, Toma A, Taddesse F. Pattern and predictors of cluster of differentiation 4 (CD4) cell count recovery among cohorts of human immunodeficiency virus (HIV)-infected patients on antiretroviral therapy in Hawassa University Referral Hospital. Journal of AIDS and HIV Research. 2018 Mar 31;10(3):40–8.
28. Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002-2013: a meta-analysis. Clin Infect Dis. 2015 Apr 1;60(7):1120–7.
29. Grover G, Vajala R, Swain PK. On the assessment of various factors effecting the improvement in CD4 count of aids patients undergoing antiretroviral therapy using generalized Poisson regression. Journal of Applied Statistics. 2015 Jun 3;42(6):1291–305.
30. Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell Mol Immunol. 2015 May;12(3):258–63.
31. Belay M. Determinants of demand for health care services in Mekelle City. Doctoral dissertation, Mekelle University; 2013.
32. Duong H, Nguyen S, Ray Shiraishi HT, Vo H, Tieu TV, Van H, et al. CD4 Recovery and Survival among Adults Co-infected with HIV and Hepatitis B or C Virus, Ho Chi Minh City, Vietnam. Universal Journal of Public Health. 2017;5(7):371–81
33. van Griensven J, Phirum L, Choun K, Thai S, De Weggheleire A, Lynen L. Hepatitis B and C co-infection among HIV-infected adults while on antiretroviral treatment: long-term survival, CD4 cell count recovery and antiretroviral toxicity in Cambodia. PLoS One. 2014 Feb 12;9(2):e88552.
34. Mbae M, Owen L, Elisha KK, Ndhere A, Mugambi NS, Yogev R, et al. Excess early mortality in HIV/hepatitis B virus co-infected patients initiating antiretroviral therapy in Kenya. AIDS. 2019 Jul 1;33(8):1404–6.
35. Sarkar J, Saha D, Bandyopadhyay B, Saha B, Kedia D, Guha Mazumder DN, et al. Baseline characteristics of HIV & hepatitis B virus (HIV/HBV) co-infected patients from Kolkata, India. Indian J Med Res. 2016 May;143(5):636–42.
36. World Health Organization. Training workshop on screening, diagnosis and treatment of hepatitis B and C. Geneva: World Health Organization; 2020. Available from https://cdn.who.int/media/docs/default-source/searo/hiv-hepatitis/training-modules/01b-overview-viral-hepatitis-southeast-asia-region-jan-2020.pdf?sfvrsn=b392d166_2.
37. Olawumi HO, Olanrewaju DO, Shittu AO, Durotoye IA, Akande AA, Nyamngee A. Effect of Hepatitis B Virus Co-Infection on CD4 Cell Count and Liver Function of HIV Infected Patients. Ghana Med J. 2015 Mar;49(1):2–6.
38. Sarkar J, Saha D, Bandyopadhyay B, Saha B, Kedia D, Guha Mazumder DN, et al. Baseline characteristics of HIV & hepatitis B virus (HIV/HBV) co-infected patients from Kolkata, India. Indian J Med Res. 2016 May;143(5):636–42.
39. Obeagu EF, Onyenweaku FC, Nwobodo HA, Ochei KC, Ochiabuto Ogochukwu MT, et al. Impact of HIV and hepatitis b virus coinfection on selected haematological markers of the patients in Umuahia, Abia State, Nigeria. Ann Clin Lab Res. 2017;5(2):175.
40. Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007 Jun;7(6):402–9.
41. Kwofie TB, Adigbli D, Osei-Yeboah J, Ativi E, Lokpo SY. Hepatitis B and C infections in HIV-1 patients on combination antiretroviral therapy (cART) in Ghana: implications for immunologic recovery, clinical response to treatment, and hepatotoxicity. Heliyon. 2021 May 28;7(6):e07172.
42. Sun HY, Sheng WH, Tsai MS, Lee KY, Chang SY, Hung CC. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: a review. World J Gastroenterol. 2014 Oct 28;20(40):14598–614.
43. Djuidje NM, Ambassa AC, Guiateu TM, Moundipa FP. Human immunodeficiency virus and hepatitis B virus (HIV/HBV) co-infection in people living with HIV/AIDs identified in Yaound Central Hospital, Cameroon: Seroprevalence and impact on the disease progression. Journal of AIDS. 2017;9(6):123–8.
44. Wandeler G, Gsponer T, Bihl F, Bernasconi E, Cavassini M, Kovari H, et al. Swiss HIV Cohort Study. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013 Nov 1;208(9):1454–8.
45. Marceline DN, Cyriaque AA, Marlene GT, Paul MF. Human immunodeficiency virus and hepatitis B virus (HIV/HBV) co-infection in people living with HIV/AIDs identified in Yaound Central Hospital, Cameroon: Seroprevalence and impact on the disease progression. Journal of AIDS and HIV Research. 2017 Jun 30;9(6):123–8.
46. Phusanti S, Manosudprasit K, Sungkanuparph S. Long-Term Liver Diseases after Initiation of Antiretroviral Therapy in HIV-Infected Patients with and without HBV or HCV Coinfection. J Int Assoc Provid AIDS Care. 2017 Mar/Apr;16(2):194–200.
47. Phusanti S, Manosudprasit K, Sungkanuparph S. Long-Term Liver Diseases after Initiation of Antiretroviral Therapy in HIV-Infected Patients with and without HBV or HCV Coinfection. J Int Assoc Provid AIDS Care. 2017 Mar/Apr;16(2):194–200.
48. Peierdun M, et al. Prevalence of HCV and HBV infection in HIV-infected patients receiving combined antiretroviral therapy and the impact of co-infections on mortality: a retrospective cohort. Int J Clin Exp Med. 2016;9(6).
49. Anderson M, Gaseitsiwe S, Moyo S, Thami KP, Mohammed T, Setlhare D et al. Slow CD4+ T-Cell Recovery in Human Immunodeficiency Virus/Hepatitis B Virus-Coinfected Patients Initiating Truvada-Based Combination Antiretroviral Therapy in Botswana. Open Forum Infect Dis. 2016 Aug 16;3(3):ofw140.
50. Marceline DN, Cyriaque AA, Marlene GT, Paul MF. Human immunodeficiency virus and hepatitis B virus (HIV/HBV) co-infection in people living with HIV/AIDs identified in Yaound Central Hospital, Cameroon: Seroprevalence and impact on the disease progression. Journal of AIDS and HIV Research. 2017 Jun 30;9(6):123–8.
51. Yang R, Gui X, Xiong Y, Gao SC, Yan Y. Impact of hepatitis B virus infection on HIV response to antiretroviral therapy in a Chinese antiretroviral therapy center. Int J Infect Dis. 2014 Nov;28:29–34.
52. Martins IJ. Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research. 2016;5(1):9–26.
53. Otedo AE. HBV, HIV co-infection at Kisumu District Hospital, Kenya. East Afr Med J. 2004 Dec;81(12):626–30.
54. Weldemhret L, Asmelash T, Belodu R, Gebreegziabiher D. Sero-prevalence of HBV and associated risk factors among HIV positive individuals attending ART clinic at Mekelle hospital, Tigray, Northern Ethiopia. AIDS Res Ther. 2016 Feb 4;13:6
55. Wang H, Li Y, Zhang C, Han Y, Zhang X, Zhu T, et al. Immunological and virological responses to cART in HIV/HBV co-infected patients from a multicenter cohort. AIDS. 2012 Sep 10;26(14):1755–63.
56. Tanuma J, Matsumoto S, Haneuse S, Cuong DD, Vu TV, Thuy PTT, et al. Long-term viral suppression and immune recovery during first-line antiretroviral therapy: a study of an HIV-infected adult cohort in Hanoi, Vietnam. J Int AIDS Soc. 2017 Dec;20(4):e25030.
57. van Griensven J, Phirum L, Choun K, Thai S, De Weggheleire A, Lynen L. Hepatitis B and C co-infection among HIV-infected adults while on antiretroviral treatment: long-term survival, CD4 cell count recovery and antiretroviral toxicity in Cambodia. PLoS One. 2014 Feb 12;9(2):e88552.
58. Martin NK, Devine A, Eaton JW, Miners A, Hallett TB, Foster GR, et al. Modeling the impact of early antiretroviral therapy for adults coinfected with HIV and hepatitis B or C in South Africa. AIDS. 2014 Jan;28 Suppl 1:S35–46.
59. Chung RT. Hepatitis C and B viruses: the new opportunists in HIV infection. Top HIV Med. 2006 Jun-Jul;14(2):78–83.
60. Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006 Dec 8;55(RR-16):1–33; quiz CE1-4. Erratum in: MMWR Morb Mortal Wkly Rep. 2007 Oct 26;56(42):1114. PMID: 17159833.
61. Maier I, Wu GY. Hepatitis C and HIV co-infection: a review. World J Gastroenterol. 2002 Aug;8(4):577–9.
62. Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009 May;49(5 Suppl):S138–45.
63. Treitinger A, Spada C, Ferreira LA, Neto MS, Reis M, Verdi JC, et al. Hepatitis B and hepatitis C prevalence among blood donors and HIV-1 infected patients in Florianópolis--Brazil. Braz J Infect Dis. 2000 Aug;4(4):192–6.
64. Tsuchiya N, Pathipvanich P, Rojanawiwat A, Wichukchinda N, Koga I, Koga M, et al. Chronic hepatitis B and C co-infection increased all-cause mortality in HAART-naive HIV patients in Northern Thailand. Epidemiol Infect. 2013 Sep;141(9):1840–8.
65. Chun HM, Mesner O, Thio CL, Bebu I, Macalino G, Agan BK, et al. Infectious Disease Clinical Research Program HIV Working Group. HIV outcomes in Hepatitis B virus coinfected individuals on HAART. J Acquir Immune Defic Syndr. 2014 Jun 1;66(2):197–205.
66. Forbi JC, Gabadi S, Alabi R, Iperepolu HO, Pam CR, Entonu PE, et al. The role of triple infection with hepatitis B virus, hepatitis C virus, and human immunodeficiency virus (HIV) type-1 on CD4+ lymphocyte levels in the highly HIV infected population of North-Central Nigeria. Mem Inst Oswaldo Cruz. 2007 Jun;102(4):535–7.
67. Hamaidi I, Kim S. Sirtuins are crucial regulators of T cell metabolism and functions. Exp Mol Med. 2022 Mar;54(3):207–15.
68. Li KW, Kramvis A, Liang S, He X, Chen QY, Wang C, et al. Higher prevalence of cancer related mutations 1762T/1764A and PreS deletions in hepatitis B virus (HBV) isolated from HBV/HIV co-infected compared to HBV-mono-infected Chinese adults. Virus Res. 2017 Jan 2;227:88–95.
69. Martins I. Sirtuin 1, a diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. EC Pharmacology and Toxicology. 2018;6(4):209–15.