Abbreviations
LC: Lung Cancer; TFAP2: Transcription Factor Activator Protein 2; ITPKA: Inositol-trisphosphate 3-kinase A; KRT16: Keratin 16, a type of I cytokeratin; TGFBR1: Transforming Growth Factor Receptor β1; LCIS: Lobular Carcinoma In situ; MMP9: Matrix Metallprotenaine 9; PTEN: Phosphatase and Tensin homology deleted on chromosome ten; ER: Estrogen Receptor; HER2: Human Epidermal Growth Factor Receptor 2; YY1: Yin-Yang1, a transcription factor
Commentary
Abnormality of transcription factors’ activity has been found in signal pathways of many cancers. The AP-2 family of transcription factors (TFAP2) is one of the most representative families with this characteristic. The family, consisting of five members (TFAP2A to TFAP2E), can activate or inhibit the target gene through signal transduction [1]. They have been found to play important roles in human embryonic development [2] and cell differentiation [3]. In addition to their influence on growth and development, TFAP2 family have also been reported to be involved in tumorigenesis and development in cancers of lung, nasopharynx [4], prostate [5], breast [6], glioma [7] and liver [8], etc.
Researches have been focused on the association between TFAP2 family and lung cancer (LC). For example, by inducing ITPKA [9] and KRT16 [10], TFAP2A acts as an oncogene in lung adenocarcinoma and lung tumorigenesis, in coordination with TFAP2C [11]. To further determine the role of TFAP2 in LC, the relationship between this family and the prognosis of LC was explored recently using bioinformatics in a recent study [12]. There are three significant findings in this study: (1) Compared with normal tissues, the expression of TFAP2A and TFAP2C increase in LC, while the expression of TFAP2B shows no significant difference. (2) Only TFAP2A is associated with the prognosis of LC, whose expression level is negatively correlated with the overall survival rate of LC. (3) The mutation rate of TFAP2A and TFAP2C in LC are higher than that of TFAP2B. Together, these findings suggest TFAP2A has prognostic value, which may improve survival and prognosis accuracy of LC patients.
However, some other researchers have different conclusions about the role of TFAP2B in LC. It is found in one research that the expression of TFAP2B is higher in lung cancer, which suggests poor prognosis [13]. But it is reported in another study that expression of TFAP2B in the nucleus is related to poor survival [14]. One possible reason of these different outcomes is the different sample sizes of the three studies. There were 147 patients contained in Fu’s study and totally 241 patients in Kim’s study, while Cheng et al. studied 1145 patients. Therefore, selection bias should be taken into consideration. To get further understanding about the role of TFAP2B in lung cancer, clinical studies with larger sample scale are still needed.
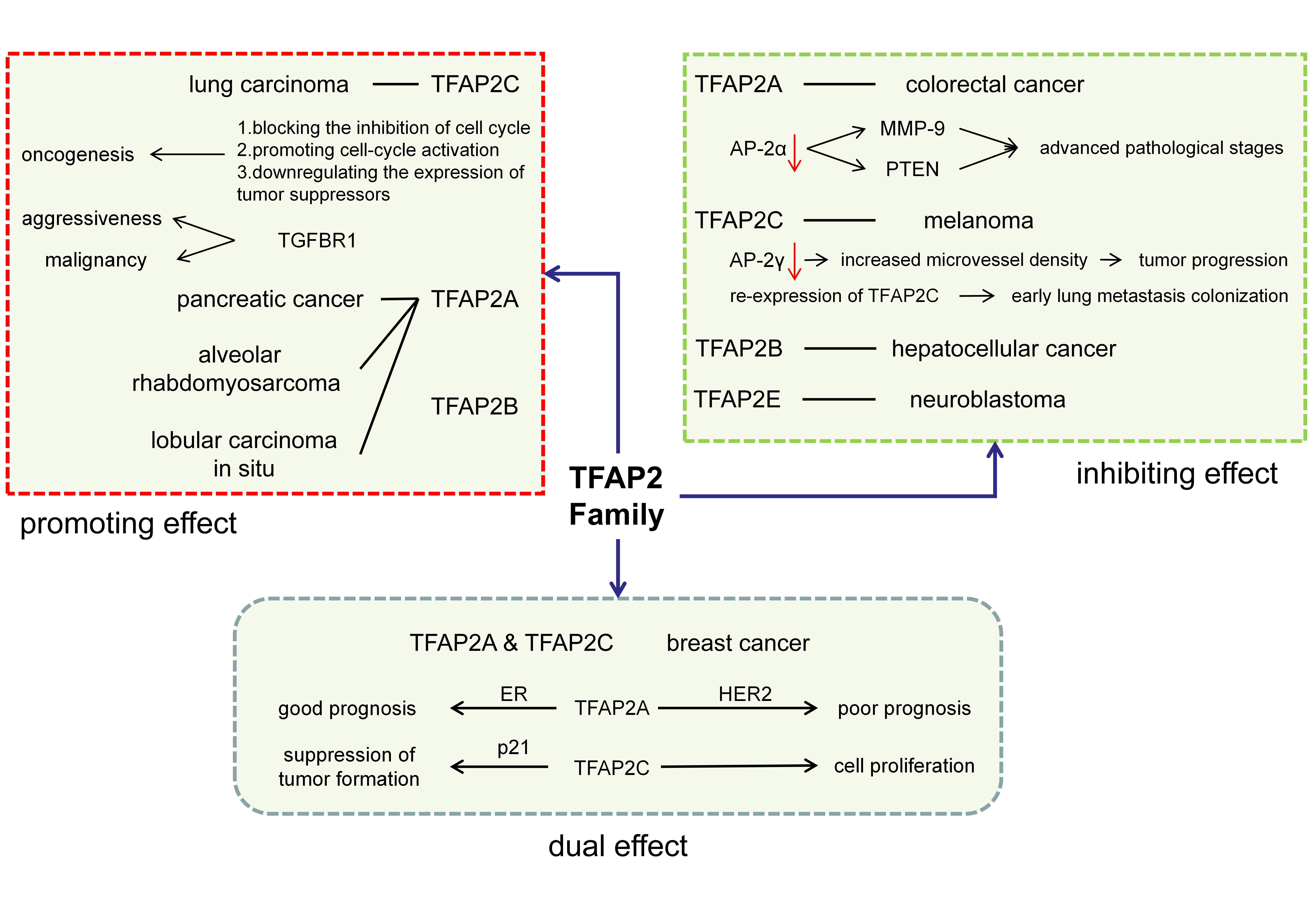
In summary, the dysregulation expression level of TFAP2 family and their prognostic value in LC are found in this study. However, except for what have been mentioned in this study, TFAP2 family also have tumor-promoting or inhibiting effect on different cancers.
The Tumor-promoting Effect of TFAP2
The TFAP2 family generally play a promoting role in the progression of cancers such as astroglioma [15], pancreatic cancer [16] and ovarian cancer [17].
In addition to their prognostic value, TFAP2 also show tumor-promoting effect on LC. The upregulation of TFAP2 expression in non-small cell lung cancer is reported in Zhou’s study, which is positively related to the pathological stage [18]. As a member of the TFAP2 family, TFAP2C shows similar effect on LC. By mediating the upregulation of TGFBR1 [11], the receptor of transforming growth factor, TFAP2C can enhance the aggressiveness and malignancy of LC. Besides, TFAP2C leads to the oncogenesis of LC through other pathways, including blocking the inhibition of cell cycle, promoting cell-cycle activation [19] and even downregulating the expression of tumor suppressors [20].
It should be noted that TFAP2B is also defined as a tumor promoter. Its overexpression has been associated with alveolar rhabdomyosarcoma [21] and lobular carcinoma in situ (LCIS) [22].
The Tumor-inhibiting Effect of TFAP2
The tumor-inhibiting effect of TFAP2 has been widely reported in colorectal cancer and melanoma.
AP-2α, encoded by TFAP2A, has been related to the suppression of colorectal cancer. It is indicated in Ropponen’s study that down-regulation of AP-2α expression occurs in advanced-grade pathological stages [23]. It is possible that the MMP-9 promoter can be directly bound by AP-2α [24], resulting in deduced promoter activity. Another explanation is that the expression of PTEN, a tumor suppressor gene, is positively correlated with AP-2α [25].
Another strong piece of evidence for the inhibitory effect of TFAP2 on cancer comes from studies of melanoma, in which silencing of TFAP2 is related to tumor invasion and metastasis [26]. The loss of TFAP2C expression, influenced by miR-214 [27], can increase microvessel density [26], downregulate the expression of AP-2α [28] and eventually contribute to the progression of melanoma [29]. Besides, the re-expression of TFAP2C reduces miR-214-induced cell motility and early lung metastasis colonization [28].
In addition, other members of TFAP2 family also have the inhibitory effect on tumor, such as TFAP2B on hepatocellular cancer [30] and TFAP2E on neuroblastoma [31].
The Bidirectional Effect of TFAP2
Interestingly, TFAP2 family sometimes seem to have bidirectional effect on cancers, among which TFAP2A and TFAP2C are the most representative.
Estrogen receptor(ER) is a useful predictor for breast cancer prognosis [32], which is the basis of endocrine therapy. In one clinical research, it has been found that nuclear AP-2α encoded by TFAP2A has positive association with ER expression [33], especially in breast cancer with ER-positive [34]. Another research on ER-positive breast tumor-derived cell line also confirms this result [35]. However, the overexpression of HER2, positively related to AP-2α [36], usually predicts a poor prognosis in breast cancer [37]. It is probably because that AP-2α can promote HER2 gene transcription [38] in combination with YY1 [39], a cofactor of AP-2α. And another possible explanation is that the inactivation of AP-2α protein reduces the activity of HER2 promoter [40].
Encoded by TFAP2C, AP-2γ seems to play a similar role in breast cancer. In breast tissue, over-expression of the AP-2γ stimulates cell proliferation and disrupts cell differentiation [41], suggesting that it may have carcinogenic effect. However, it is reported in another research [42] that AP-2γ can cause blocking of cell cycles by promoting p21 protein, contributing to the suppression of tumor formation. Therefore, TFAP2C may play a dual role [32] in tumor development, initially inhibiting tumor development [42] and then acting in the opposite direction when a certain balance is broken [43]. This conclusion is also confirmed by an in vivo experiment [44].
Different effects of TFAP2 family have been showed in Figure 1, which give them broad applications in clinic. For example, the expression of TFAP2A and TFAP2C are elevated in LC compared to normal tissue [12], which may be used as theoretically biomarkers for LC. Furthermore, because of the tumor-promoting effect of TFAP2, they may be considered as a target of anti-tumor drugs. However, there are still some issues to be studied. For instance, in order to detect their expression with the purpose of clinical diagnosis or prognosis, it is necessary to ensure the stable expression level of TFAP2A and TFAP2C in the peripheral circulation. Besides, standardized detection methods are also needed to guarantee sensitivity and specificity of detection [45]. What’s more, role of TFAP2B in lung cancer is still controversial. Therefore, we think TFAP2A and TFAP2C could be potential biomarkers and drug targets, but further researches are still needed to solve these problems.
In conclusion, the TFAP2 family play important and complex roles in human cancers. These studies may bring new ideas to the diagnosis, classification, treatment and prognosis of cancer, but there are still many mysteries to be explored. More in-depth studies are required for TFAP2 family to improve targeted therapy and acquire better prognosis.
Acknowledgment
This work was supported by the Science & Technology Department of Sichuan Province, No.2021YFS0111. The views expressed are those of the authors and not necessarily those of the Science & Technology Department of Sichuan Province. We apologize for not being able to cite all the publications related to this topic due to space constraints of the journal.
References
2. Nottoli T, Hagopian-Donaldson S, Zhang J, Perkins A, Williams T. AP-2-null cells disrupt morphogenesis of the eye, face, and limbs in chimeric mice. Proceedings of the National Academy of Sciences. 1998 Nov 10;95(23):13714- 9.
3. Schorle H, Meier P, Buchert M, Jaenisch R, Mitchell PJ. Transcription factor AP-2 essential for cranial closure and craniofacial development. Nature. 1996 May;381(6579):235-8.
4. Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu L, et al.TFAP2A regulates nasopharyngeal carcinoma growth and survival by targeting HIF-1α signaling pathway. Cancer Prevention Research. 2014 Feb 1;7(2):266-77.
5. Ruiz M, Pettaway C, Song R, Stoeltzing O, Ellis L, BarEli M. Activator protein 2α inhibits tumorigenicity and represses vascular endothelial growth factor transcription in prostate cancer cells. Cancer Research. 2004 Jan 15;64(2):631-8.
6. Williams CM, Scibetta AG, Friedrich JK, Canosa M, Berlato C, Moss CH, et al. AP-2γ promotes proliferation in breast tumour cells by direct repression of the CDKN1A gene. The EMBO Journal. 2009 Nov 18;28(22):3591-601.
7. Yuan J, Zhang N, Zheng Y, Chen YD, Liu J, Yang M. LncRNA GAS5 indel genetic polymorphism contributes to glioma risk through interfering binding of transcriptional factor TFAP2A. DNA and Cell Biology. 2018 Sep 1;37(9):750-7.
8. Liu Y, Yang Y, Wang T, Wang L, Wang X, Li T, et al. Long non-coding RNA CCAL promotes hepatocellular carcinoma progression by regulating AP-2α and Wnt/βcatenin pathway. International Journal of Biological Macromolecules. 2018 Apr 1;109:424-34.
9. Guoren Z, Zhaohui F, Wei Z, Mei W, Yuan W, Lin S,et al. TFAP2A induced ITPKA serves as an oncogene and interacts with DBN1 in lung adenocarcinoma. International Journal of Biological Sciences. 2020;16(3):504.
10. Yuanhua L, Pudong Q, Wei Z, Yuan W, Delin L, Yan Z, et al. TFAP2A induced KRT16 as an oncogene in lung adenocarcinoma via EMT. International Journal of Biological Sciences. 2019;15(7):1419.
11. Kim W, Kim E, Lee S, Kim D, Chun J, Park KH, et al. TFAP2C-mediated upregulation of TGFBR1 promotes lung tumorigenesis and epithelial–mesenchymal transition. Experimental & Molecular Medicine. 2016 Nov;48(11):e273-.
12. Cheng C, Ai Z, Zhao L. Comprehensive analysis of the expression and prognosis for TFAP2 in human lung carcinoma. Genes & Genomics. 2020 Jul;42:779-89.
13. Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. Journal of Biological Chemistry. 1999 Oct 8;274(41):29130- 7.
14. Carrière C, Mirocha S, Deharvengt S, Gunn JR, Korc M. Aberrant expressions of AP-2α splice variants in pancreatic cancer. Pancreas. 2011 Jul;40(5):695.
15. Ødegaard E, Staff AC, Kærn J, Flørenes VA, Kopolovic J, Tropé CG, et al. The AP-2γ transcription factor is upregulated in advanced-stage ovarian carcinoma. Gynecologic Oncology. 2006 Mar 1;100(3):462-8.
16. Zhou R, Chen P, Zhang GS. AP-2 expression and significance in the lung cancer tissues. Zhong nan da xue xue bao. Yi xue ban= Journal of Central South University. Medical Sciences. 2004 Apr 1;29(2):195-7.
17. Kang J, Kim W, Lee S, Kwon D, Chun J, Son B, et al. TFAP2C promotes lung tumorigenesis and aggressiveness through miR-183-and miR-33a-mediated cell cycle regulation. Oncogene. 2017 Mar;36(11):1585-96.
18. Do H, Kim D, Kang J, Son B, Seo D, Youn H, et al. TFAP2C increases cell proliferation by downregulating GADD45B and PMAIP1 in non-small cell lung cancer cells. Biological Research. 2019;52.
19. Wachtel M, Runge T, Leuschner I, Stegmaier S, Koscielniak E, Treuner J, et al. Subtype and prognostic classification of rhabdomyosarcoma by immunohistochemistry. Journal of Clinical Oncology. 2006 Feb 10;24(5):816-22.
20. Raap M, Gronewold M, Christgen H, Glage S, Bentires-Alj M, Koren S, et al. Lobular carcinoma in situ and invasive lobular breast cancer are characterized by enhanced expression of transcription factor AP-2 β. Laboratory Investigation. 2018 Jan;98(1):117-29.
21. Ropponen KM, Kellokoski JK, Pirinen RT, Moisio KI, Eskelinen MJ, Alhava EM, et al. Expression of transcription factor AP-2 in colorectal adenomas and adenocarcinomas; comparison of immunohistochemistry and in situ hybridisation. Journal of Clinical Pathology. 2001 Jul 1;54(7):533-8.
22. Anttila MA, Kellokoski JK, Moisio KI, Mitchell PJ, Saarikoski S, Syrjänen K, et al. Expression of transcription factor AP-2α predicts survival in epithelial ovarian cancer. British Journal of Cancer. 2000 Jun;82(12):1974-83.
23. Schwartz B, Melnikova VO, Tellez C, Mourad-Zeidan A, Blehm K, Zhao YJ, et al. Loss of AP-2 α results in deregulation of E-cadherin and MMP-9 and an increase in tumorigenicity of colon cancer cells in vivo. Oncogene. 2007 Jun;26(28):4049-58.
24. Choi HJ, Chung TW, Kim SJ, Cho SY, Lee YS, Lee YC, et al. The AP-2α transcription factor is required for the ganglioside GM3-stimulated transcriptional regulation of a PTEN gene. Glycobiology. 2008 May 1;18(5):395-407.
25. Gershenwald JE, Sumner W, Calderone T, Wang Z, Huang S, Bar-Eli M. Dominant-negative transcription factor AP-2 augments SB-2 melanoma tumor growth in vivo. Oncogene. 2001 Jun;20(26):3363-75.
26. Penna E, Orso F, Cimino D, Vercellino I, Grassi E, Quaglino E, et al. miR-214 coordinates melanoma progression by upregulating ALCAM through TFAP2 and miR-148b downmodulation. Cancer Research. 2013 Jul 1;73(13):4098-111.
27. Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E, et al. microRNA-214 contributes to melanoma tumour progression through suppression of TFAP2C. The EMBO journal. 2011 May 18;30(10):1990-2007.
28. Huang S, Jean D, Luca M, Tainsky MA, Bar-Eli M. Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. The EMBO Journal. 1998 Aug 3;17(15):4358-69.
29. Yang L, Qiu J, Xiao Y, Hu X, Liu Q, Chen L, et al. AP-2β inhibits hepatocellular carcinoma invasion and metastasis through Slug and Snail to suppress epithelialmesenchymal transition. Theranostics. 2018;8(13):3707.
30. Hoshi R, Watanabe Y, Ishizuka Y, Hirano T, Nagasaki-Maeoka E, Yoshizawa S,et al. Depletion of TFAP2E attenuates adriamycin-mediated apoptosis in human neuroblastoma cells. Oncology Reports. 2017 Apr 1;37(4):2459-64.
31. Pellikainen JM, Kosma VM. Activator protein-2 in carcinogenesis with a special reference to breast cancer—a mini review. International Journal of Cancer. 2007 May 15;120(10):2061-7.
32. Friedrichs N, Jäger R, Paggen E, Rudlowski C, Merkelbach-Bruse S, Schorle H, et al. Distinct spatial expression patterns of AP-2alpha and AP-2gamma in non-neoplastic human breast and breast cancer. Modern Pathology. 2005 Mar;18(3):431-8.
33. Gee JM, Robertson JF, Ellis IO, Nicholson RI, Hurst HC. Immunohistochemical analysis reveals a tumour suppressor-like role for the transcription factor AP-2 in invasive breast cancer. The Journal of Pathology. 1999 Dec;189(4):514-20.
34. Perissi V, Menini N, Cottone E, Capello D, Sacco M, Montaldo F, et al. AP-2 transcription factors in the regulation of ERBB2 gene transcription by oestrogen. Oncogene. 2000 Jan;19(2):280-8.
35. Delacroix L, Begon D, Chatel G, Jackers P, Winkler R. Distal ERBB2 promoter fragment displays specific transcriptional and nuclear binding activities in ERBB2 overexpressing breast cancer cells. DNA and Cell Biology. 2005 Sep 1;24(9):582-94.
36. Pegram MD. Treating the HER2 pathway in early and advanced breast cancer. Hematology/Oncology Clinics. 2013 Aug 1;27(4):751-65.
37. Li M, Wang Y, Hung MC, Kannan P. Inefficient proteasomal-degradation pathway stabilizes AP-2α and activates HER-2/neu gene in breast cancer. International Journal of Cancer. 2006 Feb 15;118(4):802-11.
38. Allouche A, Nolens G, Tancredi A, Delacroix L, Mardaga J, Fridman V, et al. The combined immunodetection of AP-2α and YY1 transcription factors is associated with ERBB2 gene overexpression in primary breast tumors. Breast Cancer Research. 2008 Feb;10(1):1-1.
39. Hoei-Hansen CE, Nielsen JE, Almstrup K, Sonne SB, Graem N, Skakkebaek NE,et al. Transcription factor AP-2γ is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clinical Cancer Research. 2004 Dec 15;10(24):8521-30.
40. Li H, Goswami PC, Domann FE. AP-2γ Induces p21 Expression, Arrests Cell Cycle, Inhibits the Tumor Growth of Human Carcinoma Cells. Neoplasia. 2006 Jul 1;8(7):568-77.
41. Park JM, Wu T, Cyr AR, Woodfield GW, De Andrade JP, Spanheimer PM, et al. The role of Tcfap2c in tumorigenesis and cancer growth in an activated Neu model of mammary carcinogenesis. Oncogene. 2015 Dec;34(50):6105-14.
42. Kołat D, Kałuzińska Ż, Bednarek AK, Płuciennik E. The biological characteristics of transcription factors AP2α and AP-2γ and their importance in various types of cancers. Bioscience Reports. 2019 Mar 29;39(3).
43. Jäger R, Friedrichs N, Heim I, Büttner R, Schorle H. Dual role of AP-2γ in ErbB-2-induced mammary tumorigenesis. Breast Cancer Research and Treatment. 2005 Apr;90(3):273-80.