Abstract
Human Papillomavirus (HPV) is a common virus transmitted primarily through sexual contact. However, it can also be spread through other ways, such as pool water, contaminated personal items, and even from mother to infant during childbirth or breastfeeding. It infects the basal cells of the epidermis and replicates in differentiated cells. HPV infection is a global health concern linked to various cancers. This short communication explores HPV infections, genetic characteristics, immune interactions, and vaccines, aiming to provide a brief understanding of HPV and its associated diseases.
Keywords
Cancer and the Immune System, Cervical Cancer, HPV infection, Human Papillomavirus, immune interactions
Introduction
Bot avatar
HPV, a prevalent sexually transmitted virus, is primarily transmitted through close skin-to-skin contact during sexual intercourse. However, it can also be spread through other means, such as swimming pool water, contaminated syringes, infected razors, contaminated laser devices, and even from mother to infant during childbirth or breastfeeding. This virus can cause cervical cancer and various other malignancies [1]. High-risk HPV infections can lead to precancerous lesions and progression to invasive cancer if untreated. Cervical cancer is closely linked to persistent HPV infections, with over 99% of cases associated with HPV-positive patients [2]. Additionally, HPV is implicated in other cancers such as head and neck cancers and penile cancer [3].
HPV genome
HPV is a DNA virus characterized by a circular, double-stranded genetic structure. Its genome includes coding and non-coding regions encoding essential viral proteins like E1, E2, L1, and L2, as well as accessory genes E4, E5, E6, and E7 [4]. There are over 200 types of HPVs classified into five categories. Some of its high-risk strains, particularly HPV types 16 and 18, are significantly linked to the development of cervical cancer. High-risk HPV E6 and E7 proteins play a crucial role in cell cycle regulation and carcinogenesis by targeting tumor suppressor proteins. It is known that the integration of HPV into the human genome plays a role in the cellular transformation and cancer development [4,5]. HPV integrations can cause unstable DNA regions with complex alterations affecting human and HPV genes [6].
Life cycle
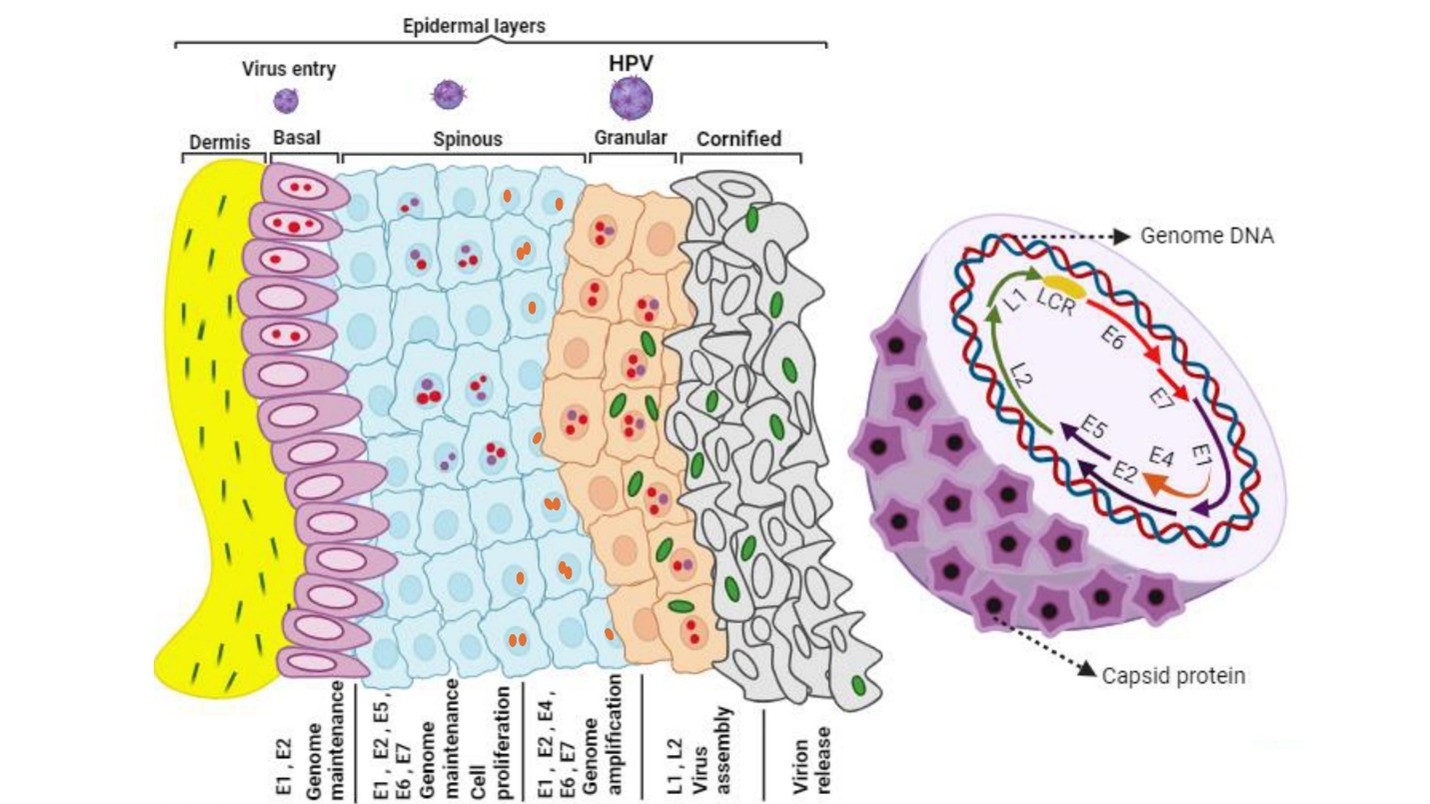
The life cycle of HPV involves three main stages: penetration into basal cells, minimal gene expression in the basal layer, and productive replication in differentiated cells [7]. HPV infects epithelial cells through cell surface receptors, specifically the α6β4 integrin complex, which is highly expressed in basal cells and epithelial stem cells. The virus remains latent in squamous cells and can become contagious in immunosuppressed individuals. Life Cycle of high-risk HPV is illustrated in Figure 1.
HPV replication and maintenance rely on viral proteins E1, E2, E6, and E7, which play essential roles in establishing and sustaining viral DNA episomes within host cells [8]. E6 and E7 oncoproteins promote cell cycle advancement, inhibit tumor-suppressor proteins, and contribute to viral DNA amplification [9]. Persistent HPV infection, genetic and epigenetic changes are among the key risk factors for malignant progression [10].
Figure 1. High-Risk HPV Life Cycle: High-risk HPVs infect basal cells in the ectocervix through microwounds. These cells keep the viral genome. As they divide, new cells move to the surface. In high-risk HPV lesions, cells make E6 and E7 proteins that promote cell division, while mid-layer cells boost protein levels for genome copying. Upper layer cells stop dividing and produce proteins for viral genome packaging. The infection can spread to other cells, influencing disease advancement and leading to adenocarcinoma in the cervical transformation zone and endocervix [32].
Clinical presentation of HPV infection
HPV infections are often asymptomatic and clear on their own, but in the anogenital area, precancerous stages can lead to invasive cancer, whereas in the head and neck region, only invasive cancer stages have been observed. Early detection, especially in the uterine cervix, is aided by cytological and molecular tests, allowing treatment before the cancer becomes invasive. HPV infections can present in various forms, including warts or dysplasia, with genital warts displaying different shapes and colors, ranging from flat or raised to flesh-colored or cauliflower-like, and may appear alone or in groups [11,12].
HPV pathogenesis
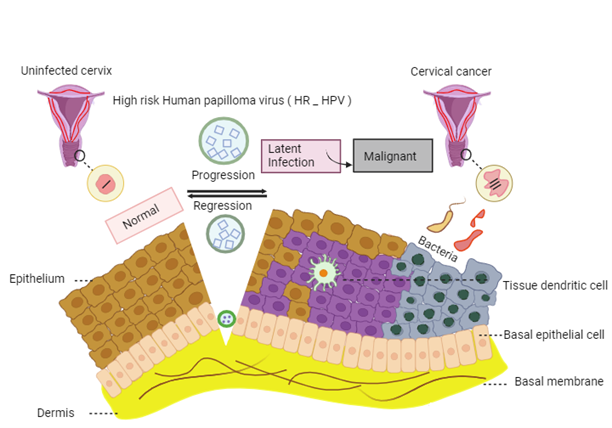
The pathogenesis of HPV involves integration of viral genetic material into the cellular genome, leading to increased expression of oncogenes E6 and E7 [13,14]. High-risk HPV strains can cause persistent infections and are associated with cervical neoplasia. Immune evasion and alterations in inflammatory cytokines play a role in HPV-associated cervical cancer [15]. Chronic inflammation, triggered by HPV infection, is linked to the development of cancer, involving complex interactions between viral, host, and environmental factors [16]. Moreover, interaction of chronic inflammation and oxidative stress contribute to HPV infection and development of HPV-induced cancer. [17]. The pathogenesis and natural progression of high-risk HPV infection illustrated in Figure 2.
Figure 2. The pathogenesis and natural progression of high-risk HPV infection begins when the virus enters the epithelium through a scratch or injury, leading to gradual growth. Most infections resolve on their own, but some can stay dormant. Eventually, the virus's genetic material integrates into the human genome, increasing replication and contributing to cervical cancer progression [32].
High-risk or oncogenic HPV types
Oncogenic or high risk HPV types, including HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59 have a significant association with the onset of cancer. HPV 16 and 18 are particularly common in cervical cancer. These high-risk HPV strains are associated with both benign and malignant cervical lesions [18]. HPV types are categorized into low-risk (LR) and high-risk (HR) based on their oncogenic potential and likelihood to induce cancer. These high-risk HPVs are commonly found in squamous epithelial lesions and are associated with various cancers, including those affecting the cervix, vagina, vulva, and anal squamous cell carcinomas [1,18]. Therefore, high-risk human papillomavirus (hrHPV) testing may serve as a key tool in cervical screening [19].
Low-risk HPV strains
Low-risk types of HPV, including HPV 6, 11, 42, 43, and 44 are linked to the development of non-cancerous genital warts and other seemingly benign epithelial lesions [18]. The immune system typically clears low-risk HPV infections more rapidly compared to high-risk types [20]. While low-risk HPV infections are generally not considered significant contributors to the malignancy within the general population. High-risk HPV strains exhibit distinct mechanisms involving E7 and E6 proteins that promote malignant transformation by disrupting cell cycle regulation and inhibiting tumor suppression pathways [21].
The genomic similarities between HPV6/11 and HPV16, particularly in the E7 gene, suggest a limited capacity to inhibit cell differentiation. Also, the occurrence of Single or dual HPV6/11 infections in penile and laryngeal cancers highlight need for genotype-targeted vaccination to prevent warts and cancers [22]. Multiple factors play a defensive role in the HPV virus, as outlined below.
Immune Interactions
Immune interactions play an essential role in the development of HPV infections towards malignancy. The immune system of the host is capable of clearing most anogenital HPV infections, with only a small percentage leading to HPV-related tumors [23]. The latency phase of HPV infection, lasting approximately 10 years, is influenced by the host's immune response, which can either suppress or facilitate the advancement of high-risk HPV infections towards cervical neoplasia. Although high-risk HPV infections can enhance immune system resistance, they also create a microenvironment conducive to disease progression by interfering with IFN signaling pathways, promoting regulatory T cells (Tregs) infiltration, and compromising the function of cytotoxic T lymphocytes [24]. Combination of immunotherapies enhance immune system function and modulates HPV tumor microenvironment, improving treatment outcomes [25].
The Role of Innate Immunity and Its Evasion in HPV-infection
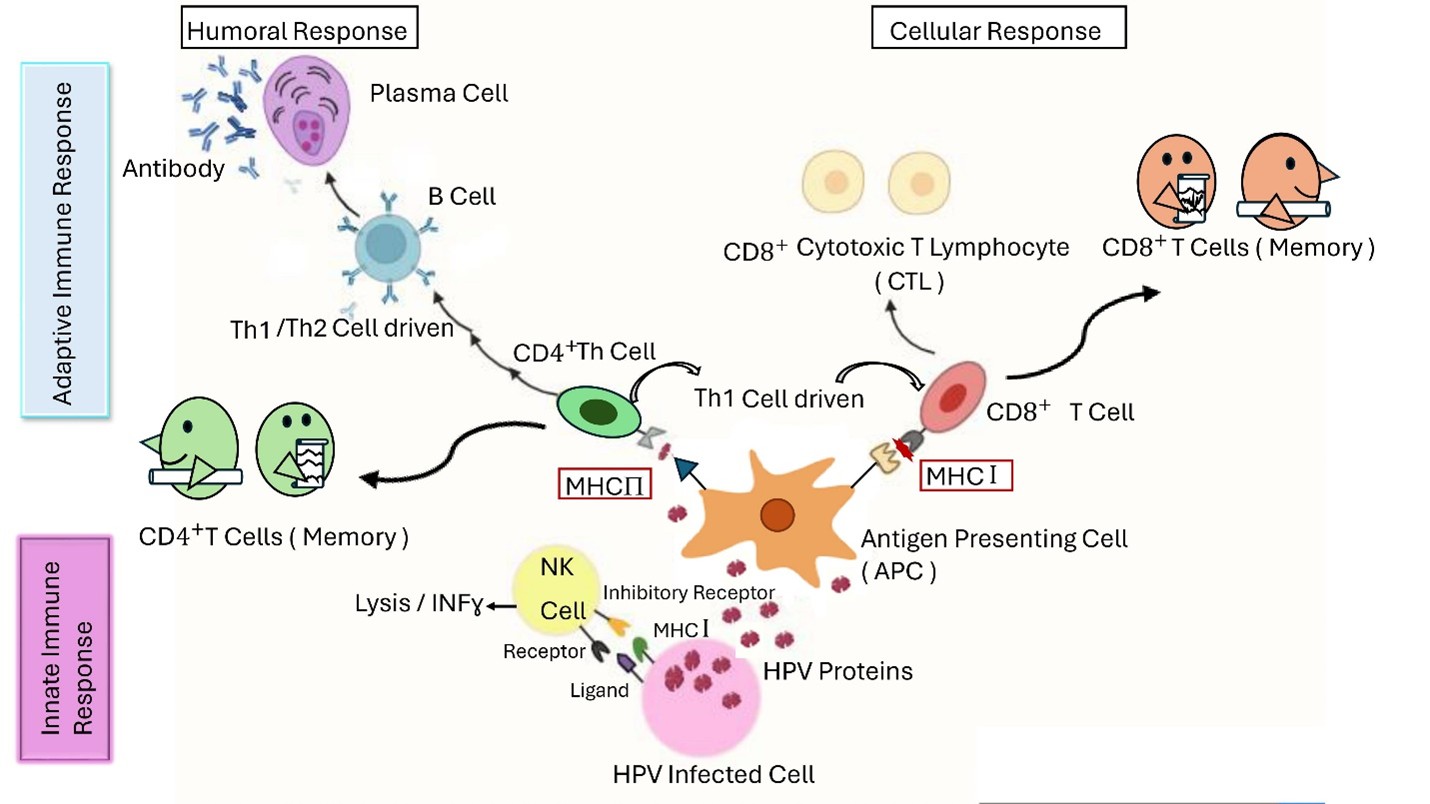
The innate immune response plays a crucial role in the initial defense against HPV infection, determining whether the virus will be cleared or persist. Pattern recognition receptors (PRRs), like Toll-like receptors (TLRs), are essential in the recognition of viral nucleic acids and contributed in HPV clearance. However, high-risk HPV types can undermine the innate immune response by downregulating TLR expression and interfering with critical pathways involving transcription factors [26]. HPV employs various strategies, such as altering cytokine expression and inhibiting interferon pathways, orchestrated by oncoproteins like E6 and E7, to evade the immune system and persist within the host. Additionally, HPV can impair the interferon-mediated response, disrupt immune cell movement and attachment, and influence cell phenotypes, contributing to immune evasion and tumor progression [27]. Various immune system components, including different cells, receptors, and biological factors, are targeted by viruses and play a role in cancer development. These targets involve natural killer cells, antigen-presenting cells, macrophages, as well as various cytokines and chemokines [28]. Variations in genes related to innate immunity, including ILs and sensors like IFI16, are linked to HPV infection and persistence (Figure 3).
Figure 3. In HPV infection, innate immune cells like NK cells, macrophages, and dendritic cells initiate the immune response by neutralizing the virus and releasing cytokines. Dendritic cells also act as Antigen-Presenting Cells, presenting viral peptides to MHC I and II molecules to trigger cellular and humoral immunity, lasting for a long time [32].
Role of Keratinocytes in HPV-infection
HPV primarily targets keratinocytes in the basal layer of stratified squamous epithelium, present in skin and mucosal sites like the anogenital and upper respiratory tracts [23]. Keratinocytes have pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) which recognize viral pathogens and start an innate immune response by releasing cytokines and chemokines. Despite the presence of PRRs, HPVs have developed strategies to create a cellular environment conducive to viral replication, persistence, and tumorigenesis [29].
Study findings show identifying HPV in keratinocyte skin cancers vary due to different detection methods used in research [30].
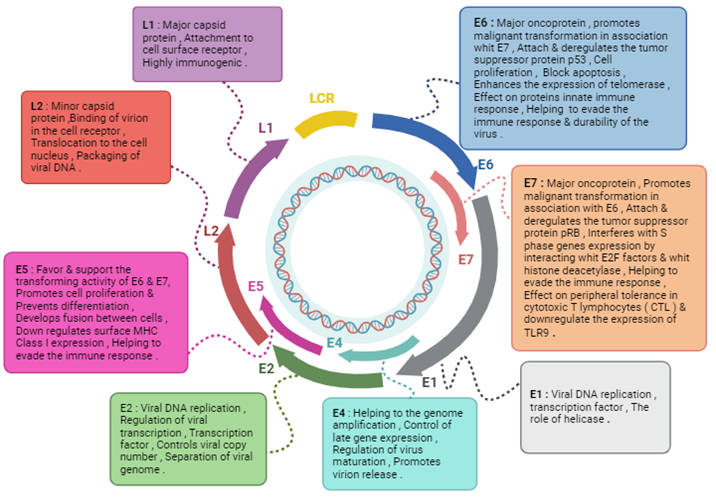
High-risk HPV genotypes can lead to persistent infection and cervical cancer through mechanisms involving the continuous overexpression of viral oncoproteins E6 and E7, often facilitated by the integration of viral DNA into the host's genetic material. This integration stabilizes E6 and E7 transcription, promoting cell growth and tumorigenesis [23]. Figure 4 illustrates functional properties of HPV proteins.
Figure 4. Schematic illustration of the functional properties of HPV proteins [32].
Role of Dendritic Cells (DCs) in HPV-infection
Dendritic cells (DCs) have an important function in starting and controlling immune reactions by recognizing foreign antigens and presenting them to T cells [31]. DCs play a crucial role in antigen presentation and are essential for activating naive T cells. They present antigens to both helper and cytotoxic T cells and are capable of cross-presentation, which involves displaying exogenous antigens on MHC class I molecules to activate cytotoxic T cells [32].
In cervical lesions, alterations in DC counts can impact immune evasion mechanisms and tumor progression [33]. Prostaglandin E2 (PGE2) is linked to the progression of malignant lesions and may influence DC migration within cervical lesions [34]. High-risk HPV infection can impair the activation of dendritic cells, hindering effective immune responses and promoting lesion growth. The activation of PD-1/PD-L1 pathway can weaken immune responses mediated by DCs against high-risk HPV infection [24]. Plasmacytoid dendritic cells (pDCs) are crucial in linking innate and adaptive immune responses, secreting interferon type I and potentially being stimulated by HPV VLPs (Virus-like particles). Toll-like receptors (TLRs) are essential in enhancing chemokine expression and migration of pDCs during their maturation [35].
HPV E7 proteins enhance plasmacytoid dendritic cells differentiation, maturation, and activate TLR and mitogen-activated protein kinase (MAPK) pathways for the host immune response [36] (Figure 1).
Role of Natural Killer Cells in HPV-infection
Natural Killer (NK) cells in the cervix play a crucial role in eliminating virus-infected and transformed cells through direct recognition of surface ligands, often down-regulated MHC class I molecules [37]. Diminished levels of activating receptors like NKp30, NKp44, NKp46, and NKG2D are associated with reduced NK cell cytotoxic activity in individuals with cervical cancer [38]. HPV can evade NK cell cytotoxicity by upregulating IDO expression, impairing NK cell function and facilitating immune evasion in cervical lesions. IDO expression in cancer has been reported in numerous cell types, both within the tumor microenvironment and in the peripheral blood [37,38].
The ex vivo experiments revealed reduced NK cell cytotoxicity in the cervix of HPV16+ patients compared to HPV18+ patients. HPV16 hinders NK cell increase in early lesions, suggesting hyporesponsiveness to HPV16 in cervix's immune system, potentially explaining its tendency for malignant transformation [39] (Figure 1).
Role of NKT Cells in HPV-infection
NKT cells, a subset of T lymphocytes, play an essential part in anti-tumor immunity by recognizing lipid antigens expressed by tumor cells. Invariant natural killer T (iNKT) cells activate different immune cells and rejuvenate exhausted cells in tumor microenvironment [40].
HPV 16-infected cells exhibit reduced CD1d levels, potentially aiding in evading NKT cell responses. In high-grade HPV-associated lesions, IFN-γ-producing NKT cells can have an immunosuppressive role in HPV-related carcinogenesis [41]. IDO1 expression in the HPV-associated tumor environment stimulated by NKT cells that produce IFN-γ and contribute to immunosuppression [37].
The Role of Adaptive Immunity and Its Evasion in HPV-infection
The HPV life cycle occurs within epithelial cells without inducing cytolysis or viremia, limiting systemic immune exposure [42]. T cell activation is crucial for clearing HPV-infected cells, with a correlation between regression of cervical precancerous lesions and the presence of cytotoxic T cells expressing granzyme B [43]. Host clearance of HPV infection relies significantly on T cell-mediated immune responses, supported by the immunogenicity and effectiveness of current HPV vaccines. HPV has evolved strategies to evade host antibody-mediated immune responses during natural infection [44].
HPV-associated oropharyngeal squamous cell carcinoma (OPSCC) tumors show high activity of immune response genes and inflammatory chemokines, promoting recruitment of cytotoxic T cells. This strong T-cell response is crucial in fighting the cancer [45].
Role of Humoral Response (B cells) in HPV-infection
B cells are essential in the humoral immune response because they generate antibodies that can deactivate and neutralize viral pathogens. Different subsets of B cells exist in the human immune system, including plasma cells, immature B cells, memory B cells, B-cell progenitors, and regulatory B cells [46]. Anti-HPV antibodies mainly target the L1 capsid protein, with predominant IgG1 class antibodies. Neutralizing L1 antibodies can prevent attachment to the cell surface or basement membrane and inhibit viral internalization [47]. After natural HPV infection, seroconversion and neutralizing antibodies can be detected about eight to nine months later, while vaccine-induced antibodies persisting at high levels compared to natural infection [48]. HPVs strategically evade the immune system by limiting the expression of capsid proteins in the skin's outer layers, reducing serum antibody responses during natural infections [49] (Figure 1).
Role of Cell Mediated Immunity (T cells) in HPV-infection
The innate immune response is triggered by pathogens, leading to the activation of adaptive immunity through the transformation of dendritic cells into antigen-presenting cells. Cytotoxic T cells (CD8+) and T helper cells (CD4+) play a crucial role in cellular and humoral immune responses [50]. HPVs possess characteristics that allow them to escape the immune response, hindering the cell-mediated immune response. Compromised adaptive immunity, particularly compromised CD4+ T cells, contributes to HPV-associated cancer development, characterized by an imbalance in Th1 and Th2 cytokines. HR-HPV modulates T cell activation through oncoproteins expression, disrupting the immune defense and promoting lesion progression [51].
Foxp3+ regulatory T cells (Tregs), a subset of CD4+ T cells, suppress anti-tumor immunity by limiting the activity of effector T cells. Tregs are associated with autoimmune diseases, viral infections, and are highly present in cervical cancer and may inhibit anti-HPV immunity. Tregs in cervical cancer are specific for HPV antigens [52] (Figure 1).
Vaccines
Most of females infected with HPV develop detectable antibody responses specific to the HPV L1 capsid protein, possibly providing natural immunity against subsequent infections [53]. HPV vaccines induce neutralizing antibody responses through virus-like particles (VLPs) and have shown cross-protection against multiple HPV types. Actual data indicates a significant reduction in HPV infections, genital warts, and cervical abnormalities among vaccinated individuals, highlighting the impact of HPV vaccination programs [54].
Researchers are developing therapeutic vaccines to treat infectious diseases and cancer by activating the immune system. Various types of vaccines, including bacterial and viral vectors, peptides, nucleic acids, and cells, are being studied along with combination therapies. Despite advances, no therapeutic vaccine has proven effective in curing HPV-associated cancers; combination therapy with PD-L1 antibodies and HPV vaccines shows promise in inhibiting tumor growth and enhancing immune response [55].
Common Treatments
Surgical methods like laser therapy, Loop electrosurgical excision procedure (LEEP), and cryosurgery are recommended for precancerous lesions induced by oncogenic HPVs. Chemotherapy, radiation therapy, and hysterectomy are common approaches for treating cervical cancer [56]. Checkpoint inhibitors like Pembrolizumab, Nivolumab, and Ipilimumab are used to target immune checkpoint pathways in cervical cancer treatment [57]. Additional clinical trials are required to enhance the effectiveness of therapeutic vaccines for HPV-related cervical cancer. The potential synergy between viral and neo-antigens and therapeutic vaccines should be explored. CRISPR/Cas genome-editing and advanced treatments like CAR-T therapy and radioimmunotherapy are also promising [58]. Suggested treatment strategies for low-risk papilloma infections, cervical precancerous lesions caused by high-risk HPVs and cervical cancer are summarized in Tables 1-3.
|
Treatment strategies to deal with low-risk papilloma infections |
||||
|
Type of treatment |
Trichloroacetic acid (TCA) |
Sinecatechins |
Podophyllotoxin |
Imiquimod |
|
Prescription |
Weekly examination by a specialist |
Every 8 hours |
In the time frame of four weeks, the medicine should be taken for 3 days and then not used for 4 days, during the time of taking the medicine 2 times a day. |
It should be used 3 times a week in a time efficiency of up to 16 weeks |
|
The possibility of treatment response |
56–94% |
47–59% |
36–83% |
28–100% |
|
The possibility of reactivating the infection |
36% |
4–8% |
4–100% |
6–26% |
|
Mechanism |
Protein inactivation by biochemical coagulation mechanism |
Inhibition of virus replication and control of immune response |
Germicidal properties |
Targeting the immune interaction |
|
Limited treatment strategies to deal with low-risk papilloma infections |
||||
|
Type of treatment |
Photodynamic therapy (PDT) |
Intralesional/topical interferon |
5-fluorouracil (5FU) |
|
|
Prescription |
Time frame of 15 days to 1 month, 2 to 5 times |
Time frame of 10 weeks |
Time frame of 14 weeks |
|
|
The possibility of treatment response |
96% |
17–90% |
10–50% |
|
|
The possibility of reactivating the infection |
9% |
9–69% |
50% |
|
|
Mechanism |
Disable with photo effects |
Antiviral inhibitors using proinflammatory chemokines |
Activation of anti-metabolic inhibitory genes |
|
|
Treatment strategies to deal with cervical precancerous lesions associated with high-risk HPVs |
||
|
Type of treatment |
Ablative treatment |
Excisional treatment |
|
Prescription |
Wart removal in one step |
Wart removal in one step |
|
The possibility of treatment response |
Almost 100% |
90–95% |
|
The possibility of reactivating the infection |
17–22% |
19–29% |
|
Mechanism |
Surgical operation |
Surgical operation |
|
Treatment strategies to deal with cervical cancer |
||||
|
Type of treatment |
Radiation |
Neoadjuvant chemotherapy |
Adjuvant chemotherapy |
Hysterectomy |
|
Prescription |
Depending on the progression of the disease and the HPV genotype |
According to the progress of the disease, different chemotherapies are used first, and surgery is recommended in appropriate conditions |
According to the progress of the disease, first surgery and in appropriate conditions the use of different chemotherapy |
Total/Radical surgical operation |
|
Mechanism |
Genome destruction in cells of malignant origin |
Suppressing the proliferation of cells of malignant origin, disrupting cell division |
Suppressing the proliferation of cells of malignant origin, disrupting cell division |
Removal of the uterus from the body by surgery |
Conclusion
HPV infection is a global health concern linked to various cancers. Prophylactic HPV vaccines have played a crucial role in reducing genital warts and cervical cancer prevalence. Moreover, Promoting vaccination, particularly in youth can decrease HPV infections and lower associated health risks globally.
References
2. Liu M, Yan X, Zhang M, Li X, Li S, Jing M. Influence of Human Papillomavirus Infection on the Natural History of Cervical Intraepithelial Neoplasia 1: A Meta-Analysis. Biomed Res Int. 2017;2017:8971059.
3. Tumban E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses. 2019 Oct 9;11(10):922.
4. Mirabello L, Clarke MA, Nelson CW, Dean M, Wentzensen N, Yeager M, et al. The Intersection of HPV Epidemiology, Genomics and Mechanistic Studies of HPV-Mediated Carcinogenesis. Viruses. 2018 Feb 13;10(2):80.
5. Pešut E, Đukić A, Lulić L, Skelin J, Šimić I, Milutin Gašperov N, Tomaić V, Sabol I, Grce M. Human Papillomaviruses-Associated Cancers: An Update of Current Knowledge. Viruses. 2021 Nov 6;13(11):2234.
6. Porter VL, Marra MA. The Drivers, Mechanisms, and Consequences of Genome Instability in HPV-Driven Cancers. Cancers (Basel). 2022 Sep 23;14(19):4623-30.
7. Kajitani N, Satsuka A, Kawate A, Sakai H. Productive Lifecycle of Human Papillomaviruses that Depends Upon Squamous Epithelial Differentiation. Front Microbiol. 2012 Apr 24;3:152.
8. Brianti P, De Flammineis E, Mercuri SR. Review of HPV-related diseases and cancers. New Microbiol. 2017 Apr;40(2):80-5.
9. Pinidis P, Tsikouras P, Iatrakis G, Zervoudis S, Koukouli Z, Bothou A, Galazios G, Vladareanu S. Human Papilloma Virus' Life Cycle and Carcinogenesis. Maedica (Bucur). 2016 Mar;11(1):48-54.
10. Mac M, Moody CA. Epigenetic Regulation of the Human Papillomavirus Life Cycle. Pathogens. 2020 Jun 18;9(6):483.
11. Prigge ES, von Knebel Doeberitz M, Reuschenbach M. Clinical relevance and implications of HPV-induced neoplasia in different anatomical locations. Mutat Res Rev Mutat Res. 2017 Apr-Jun;772:51-66.
12. Krzowska-Firych J, Lucas G, Lucas C, Lucas N, Pietrzyk Ł. An overview of Human Papillomavirus (HPV) as an etiological factor of the anal cancer. J Infect Public Health. 2019 Jan-Feb;12(1):1-6.
13. Hampras SS, Giuliano AR, Lin HY, Fisher KJ, Abrahamsen ME, Sirak BA, et al. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One. 2014 Sep 8;9(9):e104843.
14. Folliero V, Dell'Annunziata F, Chianese A, Morone MV, Mensitieri F, Di Spirito F, et al. Epigenetic and Genetic Keys to Fight HPV-Related Cancers. Cancers (Basel). 2023 Nov 25;15(23):5583.
15. Mac M, Moody CA. Epigenetic Regulation of the Human Papillomavirus Life Cycle. Pathogens. 2020 Jun 18;9(6):483.
16. Sadri Nahand J, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: a review. Int J Cancer. 2020 Jan 15;146(2):305-20.
17. Georgescu SR, Mitran CI, Mitran MI, Caruntu C, Sarbu MI, Matei C, et al. New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. J Immunol Res. 2018 Aug 27;2018:5315816.
18. Colpani V, Soares Falcetta F, Bacelo Bidinotto A, Kops NL, Falavigna M, Serpa Hammes L, et al. Prevalence of human papillomavirus (HPV) in Brazil: A systematic review and meta-analysis. PLoS One. 2020 Feb 21;15(2):e0229154.
19. Inturrisi F, Aitken CA, Melchers WJ, van den Brule AJ, Molijn A, Hinrichs JW, et al. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: an observational study. Lancet Reg Health Eur. 2021 Nov 9;11:100235.
20. Orlando PA, Gatenby RA, Giuliano AR, Brown JS. Evolutionary ecology of human papillomavirus: trade-offs, coexistence, and origins of high-risk and low-risk types. J Infect Dis. 2012 Jan 15;205(2):272-9.
21. Ivancic R, Iqbal H, Desilva B, Pan Q, Matrka L. Immunological tolerance of low-risk HPV in recurrent respiratory papillomatosis. Clin Exp Immunol. 2020 Feb;199(2):131-42.
22. Silva LLd, Teles AM, Santos JM, Souza de Andrade M, Medeiros R, Faustino-Rocha AI, et al. Malignancy Associated with low-risk HPV6 and HPV11: a systematic review and implications for Cancer Prevention. Cancers (Basel). 2023 Aug 11;15(16):4068.
23. Lo Cigno I, Calati F, Albertini S, Gariglio M. Subversion of host innate immunity by human papillomavirus oncoproteins. Pathogens. 2020 Apr 17;9(4):292.
24. Song D, Li H, Li H, Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol Lett. 2015 Aug;10(2):600-6.
25. Barros MR, de Melo CML, Barros MLCMGR, de Cássia Pereira de Lima R, de Freitas AC, Venuti A. Activities of stromal and immune cells in HPV-related cancers. J Exp Clin Cancer Res. 2018 Jul 5;37(1):137.
26. 26. Andrei EC, Munteanu MC, Busuioc CJ, Pisoschi CG, Mateescu GO, Drăcea SA, et al. Involvement of TLR9 in priming the immune response in oral papillomatosis induced by low-risk HPV. Rom J Morphol Embryol. 2023 Apr-Jun;64(2):181-8.
27. Yuan Y, Cai X, Shen F, Ma F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021 Jan 28;497:243-54.
28. Nunes RAL, Morale MG, Silva GÁF, Villa LL, Termini L. Innate immunity and HPV: friends or foes. Clinics (Sao Paulo). 2018 Oct 11;73(suppl 1):e549s.
29. Richards KH, Wasson CW, Watherston O, Doble R, Blair GE, Wittmann M, et al. The human papillomavirus (HPV) E7 protein antagonises an Imiquimod-induced inflammatory pathway in primary human keratinocytes. Sci Rep. 2015 Aug 13;5:12922.
30. Neagu N, Dianzani C, Venuti A, Bonin S, Voidăzan S, Zalaudek I, et al. The role of HPV in keratinocyte skin cancer development: A systematic review. J Eur Acad Dermatol Venereol. 2023 Jan;37(1):40-6.
31. Nikmanesh Y, Karimi MH, Yaghobi R, Marashi SM, Mahmoudi M, Moravej A, et al. Improved Function and Maturation of Dendritic Cells Stimulated by Recombinant pp65 Protein: In vitro Design. Iran J Immunol. 2019 Mar;16(1):11-25.
32. Nikmanesh N, Hosseini S, Mirbagheri F, Asadsangabi K, Fattahi MR, Safarpour AR, et al. Knowledge on Human Papillomavirus Infections, Cancer Biology, Immune Interactions, Vaccination Coverage and Common Treatments: A Comprehensive Review. Viral Immunol. 2024 Jun;37(5):221-39.
33. Kara PP, Ayhan A, Caner B, Gultekin M, Ugur O, Bozkurt MF, et al. Analysis of dendritic cells in sentinel lymph nodes of patients with endometrial and patients with cervical cancers. Int J Gynecol Cancer. 2009 Oct;19(7):1239-43.
34. Tsuge K, Inazumi T, Shimamoto A, Sugimoto Y. Molecular mechanisms underlying prostaglandin E2-exacerbated inflammation and immune diseases. Int Immunol. 2019 Aug 23;31(9):597-606.
35. Viscidi RP, Rowley T, Bossis I. Bioengineered Bovine Papillomavirus L1 Protein Virus-like Particle (VLP) Vaccines for Enhanced Induction of CD8 T Cell Responses through Cross-Priming. Int J Mol Sci. 2023 Jun 7;24(12):9851.
36. Han R, Song Y-J, Sun S-Y, Zhou Q, Chen X-Z, Zheng Q-L, et al. Influence of human papillomavirus E7 oncoprotein on maturation and function of plasmacytoid dendritic cells in vitro. Virol Sin. 2018 Dec;33(6):493-501.
37. Zhou C, Tuong ZK, Frazer IH. Papillomavirus immune evasion strategies target the infected cell and the local immune system. Front Oncol. 2019 Aug 2;9:682-90.
38. Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013 Oct 28;5(11):2624-42.
39. Zhang J, Jin S, Li X, Liu L, Xi L, Wang F, et al. Human papillomavirus type 16 disables the increased natural killer cells in early lesions of the cervix. J Immunol Res. 2019 Apr 28;2019:9182979.
40. Bae E-A, Seo H, Kim I-K, Jeon I, Kang C-Y. Roles of NKT cells in cancer immunotherapy. Arch Pharm Res. 2019 Jul;42(7):543-8.
41. Starska-Kowarska K. The Role of Different Immunocompetent Cell Populations in the Pathogenesis of Head and Neck Cancer—Regulatory Mechanisms of Pro-and Anti-Cancer Activity and Their Impact on Immunotherapy. Cancers (Basel). 2023 Mar 7;15(6):1642.
42. Hewavisenti RV, Arena J, Ahlenstiel CL, Sasson SC. Human papillomavirus in the setting of immunodeficiency: Pathogenesis and the emergence of next-generation therapies to reduce the high associated cancer risk. Front Immunol. 2023 Mar 7;14:1112513.
43. Borella F, Preti M, Bertero L, Collemi G, Castellano I, Cassoni P, et al. Is There a Place for Immune Checkpoint Inhibitors in Vulvar Neoplasms? A State of the Art Review. Int J Mol Sci. 2020 Dec 27;22(1):190.
44. Westrich JA, Warren CJ, Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017 Mar 2;231:21-33.
45. Subbarayan RS, Arnold L, Gomez JP, Thomas SM. The role of the innate and adaptive immune response in HPV-associated oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol. 2019 Aug 12;4(5):508-12.
46. Kim SS, Shen S, Miyauchi S, Sanders PD, Franiak-Pietryga I, Mell L, et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin Cancer Res. 2020 Jul 1;26(13):3345-59.
47. Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008 Apr;222:129-44.
48. Olczak P, Roden RB. Progress in L2-based prophylactic vaccine development for protection against diverse human papillomavirus genotypes and associated diseases. Vaccines (Basel). 2020 Oct 1;8(4):568.
49. Prabhu PR, Carter JJ, Galloway DA. B cell responses upon human papillomavirus (HPV) infection and vaccination. Vaccines (Basel). 2022 May 25;10(6):837-42.
50. Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. 2021 Jan;124(2):359-67.
51. Schindler S, Magalhaes RA, Costa ELF, Brites C. Genital Cytokines in HIV/Human Papillomavirus Co-Infection: A Systematic Review. AIDS Res Hum Retroviruses. 2022 Sep;38(9):683-91.
52. Shamseddine AA, Burman B, Lee NY, Zamarin D, Riaz N. Tumor immunity and immunotherapy for HPV-related cancers. Cancer Discov. 2021 Aug;11(8):1896-912.
53. Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis. 2016 May 1;213(9):1444-54.
54. Illah O, Olaitan A. Updates on HPV vaccination. Diagnostics (Basel). 2023 Jan 9;13(2):243-8.
55. Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines (Basel). 2020 Jul 16;8(3):391-7.
56. Miriyala R, Mahantshetty U, Maheshwari A, Gupta S. Neoadjuvant chemotherapy followed by surgery in cervical cancer: Past, present and future. Int J Gynecol Cancer. 2022 Mar;32(3):260-5.
57. Liu Y, Wu L, Tong R, Yang F, Yin L, Li M, et al. PD-1/PD-L1 inhibitors in cervical cancer. Front Pharmacol. 2019 Feb 1;10:65
58. Khairkhah N, Bolhassani A, Najafipour R. Current and future direction in treatment of HPV-related cervical disease. J Mol Med (Berl). 2022 Jun;100(6):829-45.