Abstract
The number of reverse shoulder arthroplasties (RSAs) has increased tremendously over the last twenty years, with a substantial change from Grammont design to lateralized devices, having the goal of reducing the risk of complications and improve clinical outcomes.
Radiographic criteria have been established to evaluate lateralization in RSA and to speculate on their biomechanical and functional effects.
Some biomechanical changes produced by glenoid and/or humeral lateralization are not completely understood and require additional exploratory research, investigating issues that have not previously been studied in-depth.
This manuscript aims to outline the most common radiographic features evaluating lateralized RSA based on the evidence from literature.
This paper should help the surgeon in the postoperative evaluation of lateralized reverse prosthesis and improve the surgical technique for implant positioning and fixation
Keywords
Radiographic Features of Lateralized Reverse Shoulder Arthroplasty in Osteoarthritis
Background
RSA is a worldwide treatment to manage pain and improve shoulder function in patients with advanced gleno-humeral osteoarthritis. The main efforts of researchers were directed to address glenoid replacement, using devices that would preserve glenoid bone stock and ensure prosthesis stability [1]. The modified Walch classification described different glenoid deformities in primary osteoarthritis [2], while Favard and Coll reported variable grades of superior glenoid erosion in patients with cuff tear arthropathy (CTA) [3]. The modern software using 3D computed tomography (CT) scan for preoperative planning, demonstrated that glenoid deformity in osteoarthritis is multiplanar, thus changing our view and approach to shoulder replacement [4,5].
Good to excellent clinical outcomes have been reported with RSA in both, primary osteoarthritis and CTA [6,7]
The original design of Grammont RSA has a joint center of rotation (CoR) located medial to the glenoid-bone-prosthesis interface [8] and a neck-shaft angle (NSA) at 155° allowing the humeral tray to cover <50% of the glenosphere. These biomechanical features have the effects of lowering the humerus and increase deltoid tension [9]. Lacking appropriate tension of the deltoid may cause instability of the implant, which represents the most fearsome issue of RSA.
Lateralizing the glenoid improves deltoid wrapping and increases joint reaction forces, which may lead to greater stability [10]. Risk factors for RSA instability include shortening of the humerus, abnormal glenoid medialization and deficiency of the soft tissues [11-15]. Scapular notching, i.e erosion of the scapular neck as result of the abutment of the humeral cup on the glenoid during adduction, is the most common long-term radiographic complication of medialized reverse implant [16].
The main modification of the Grammont design directed to reduce the risk of scapular notching was the lateralization of the CoR. This change can be performed at the level of the baseplate, with bone or metallic increased offset implants (bony-increased offset RSA [BIO-RSA] and metallic-increased offset RSA [MIO-RSA]), or at the level of the glenosphere, changing its design. Lateralization of the CoR away from the glenosphere/glenoid interface increases the risk of mechanical loosening [17]. Changes of NSA, from 155° to 135°, and the new position of the reverse tray in onlay configuration, have produced a novel stem geometry that preserve the tuberosities, and the insertion of the rotator cuff (RC) (if present), with potential effects on external rotation recovery [18].
Radiographic Assessment of Lateralized RSA and Biomechanical Considerations
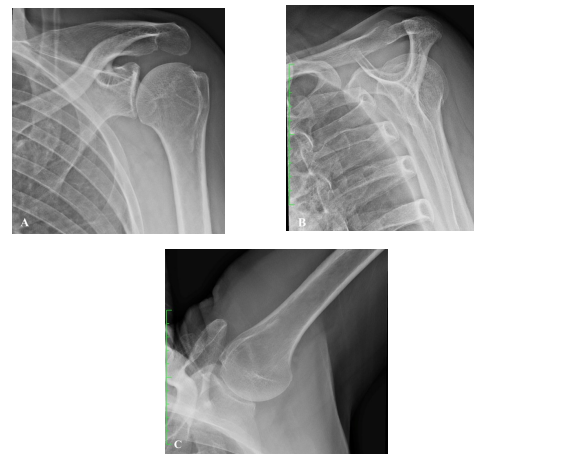
Radiographs to assess components position and lateralization of an RSA include true anterior-posterior (AP) Grashey, outlet and axillary views (Figures 1A-1C). Glenoid lateral offset (LO) is the sum of the radius of the glenosphere and of the CoR offset, and increases with increased glenosphere size. However, increasing the size of the glenosphere to the next available size, increases glenoid lateralization by a mean of only 1.14 mm. This is a very limited increase in lateral offset relative to CoR lateralization or humeral lateralization [19]. Therefore, glenoid lateralization is often referred to as CoR lateralization.
Figure 1. Standard radiograph series to assess the shoulder joint. A. True AP view, B. outlet view, C. axillary view. AP: Anterior-Posterior.
LO in RSA changed significantly, from the original medialized prosthesis of Grammont (mean 13.1 mm) to modern lateralized design (up to 35.8 mm).
Overall, the lateralization of the CoR can be achieved by either glenosphere shape modification or baseplate lateralization. When the RSA is medialized, compressive and shear components of the resultant force vector, act through a fixed CoR [8]. A CoR lateralization obtained through a specific glenosphere design, decreases compressive forces and increases destabilizing shear forces, creating a new moment (M) at the glenoid-implant interface [20] (Figures 2A-2B). When glenoid lateralization is achieved through the baseplate, the CoR falls at the interface baseplate-glenosphere. This design may theoretically reduce the micromotion at the glenoid bone-implant interface, and features that enhance fixation, such as larger screws, more screws, and porous coatings, can also contribute to minimize the risk of loosening. However, biomechanical studies supporting these speculations have not yet been published.
Figure 2. A. Reverse shoulder arthroplasty (RSA) acting with a fixed CoR with compressive (Fc) and shear (Fs) components of the resultant force vector (Fv). B. CoR lateralization increases the lever arm length which decreases compressive forces, increases destabilizing shear forces, and creates a new moment (M) at the glenoid-implant interface [34]. RSA: Reverse Shoulder Arthroplasty; CoR: Center of Rotation.
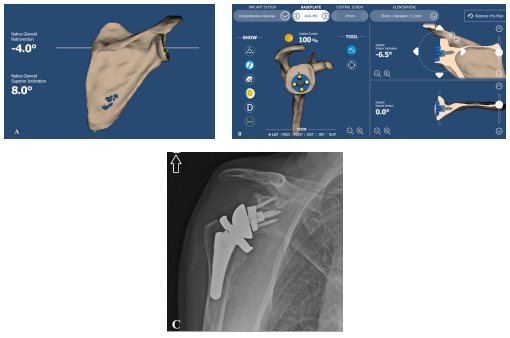
Preoperative CT planning guide the appropriate direction and orientation of glenoid baseplate and allows a perfect component seating (Figures 3A-3C).
Figure 3. Preoperative planning of primary shoulder osteoarthritis: A. computed tomography scan showing increased glenoid retroversion and superior inclination (posterior-superior glenoid bone loss). B. posterior-superior augmented baseplate (“bone preserving baseplate”) placed with a perfect seating (100% implant contact), - 6.5° inferior inclination and neutral glenoid version. C. Postoperative AP radiograph of the planned RSA with medium augmented baseplate and low GIO. GIO: Glenosphere Inferior Overhang; AP: Anterior-Posterior.
BIO-RSA is a type of “biologic lateralization” based on the concept that increasing the length of the scapular neck would lateralize both, CoR and glenoid-implant interface [21]. This would maintain the CoR at the glenoid-implant interface minimizing the torque on the glenoid component.
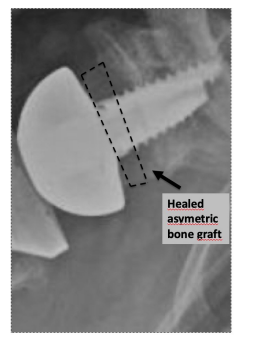
Radiographic evaluation of BIO-RSA includes assessment of bone graft radiolucency and thickness (mm) (Figure 4). Radiolucent lines >2 mm indicate that the bone graft is not healed and the baseplate is “at risk” of loosening. Glenoid radiolucent lines assessed at the interface “glenoid bone-metal” of MIO-RSA explain the baseplate seating (no radiolucent lines: perfect seating; radiolucent lines <2 mm: incomplete seating; radiolucent lines >2 mm: loosening).
Figure 4. BIO-RSA with a healed bone graft (dotted line). The CoR remains at the glenoid-implant interface. BIO-RSA: Bone Increased Offset-Reverse Shoulder Arthroplasty; CoR: Center of Rotation.
Additional radiographic features around the glenoid component include: radiolucent lines (RL) (5 zones), scapular notching, formation of bone scapular spurs and ossifications [22]. Multiple RL <2 mm or one RL ≥ 2 mm represent risk factors of glenoid loosening.
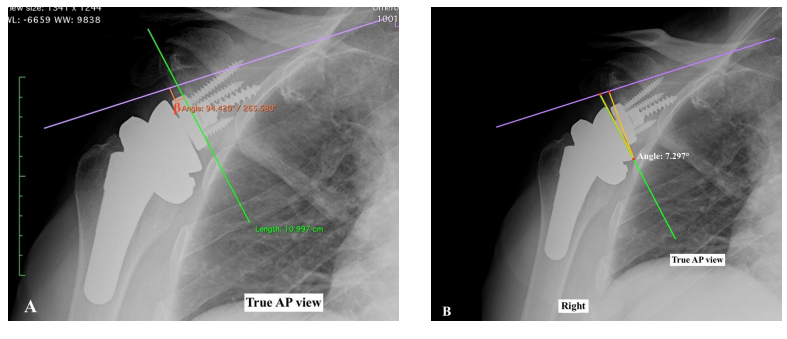
AP view radiographs are used to determine the vertical position of glenosphere according to the inferior glenoid rim (glenosphere inferior overhang [GIO]: high, flush, low and very low) [23] and glenosphere inclination, as measured by the β angle (global glenoid inclination) [24] and RSA angle [25] (Figures 5A and 5B). Insufficient GIO, following a high or flush placement of the glenospfere and a superior inclination of the baseplate (RSA >5°), are associated with higher risk of scapular notching. The best glenoid implant alignment should include a baseplate within 10° of neutral version in the axial plane and perpendicular to the floor of the supraspinatus fossa. The RSA angle and the β angle measure the focal inferior inclination and are helpful to determine the size of the bone or metallic augments needed to compensate for superior inclination in RSA [25].
Figure 5. Angle beta is the angle between the floor of the supraspinatus fossa and the glenoid fossa line (A). The RSA angle is the angle between the inferior part of the glenoid fossa (where the baseplate is implanted) and the perpendicular to the floor of the supraspinatus fossa (B).
Acromio-humeral distance and lateral humeral offset represent the amount of humeral distalization and lateralization and affect deltoid tension.
Additional radiographic methods described to measure the inferior and lateral position of the humerus, include the distalization shoulder angle (DSA) and lateralization shoulder angle (LSA) [26] (Figures 6A and 6B).
Figure 6. Distalization shoulder angle (DSA) (A) and lateralization shoulder angle (LSA) (B).
Active range of motion after RSA is correlated with specific ranges of LSA and DSA. LSA between 75° and 95° is correlated with an increased active external rotation and a DSA between 40° and 65° is correlated with increased active anterior elevation [26].
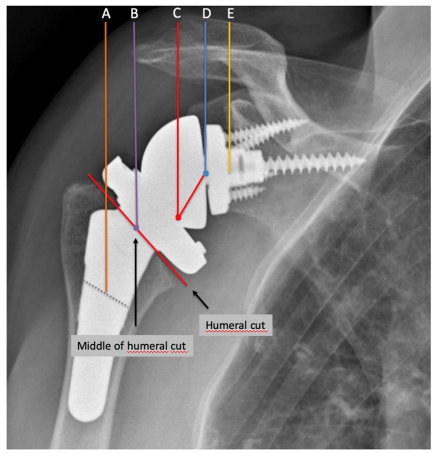
Lateralization of RSA can be represented schematically as described in the Figure 7 [19]. Overall, lateralization in RSA can be performed on the glenoid side, humeral side or both (global lateralization).
Figure 7. Radiographic references to measure humeral and glenoid offset in lateralized RSA (global lateral offset), as described by Werthel et al. [19]. Line A is the vertical line passing through the middle of the diaphysis of the humeral stem. Line B is the horizontal line passing through the middle of the surface of the humeral implant at the level of the humeral cut. Line C is the vertical line passing through the “pivot point” defined as the deepest point of the articular surface of the humeral insert measured perpendicular to the surface of the humeral insert. Line D is the vertical line passing through the centre of rotation of the joint. Line E is the vertical line passing through the bone-glenoid baseplate interface. Humeral lateral offset (distance AC) was defined as the sum of the humeral stem offset (distance AB) and of the humeral insert offset (distance BC). Glenoid lateral offset (distance CE) was defined as the sum of the “perceived radius of the glenosphere” (distance CD) and of the centre of rotation offset (distance DE).
Global lateralization is the sum of humeral LO and glenoid LO. The range of global lateralization that is possible to obtain with one given RSA implant varies from 3.3 to 20.9 mm [19]. Current RSA devices allow glenoid lateralization up to 7.7 with standard baseplate and up to 8.8 mm with bone or metallic augments.
Surgeon should be cautious with glenoid lateralization beyond +5–10 mm, depending on the amount of loss of medial bone stock, to avoid the risk of scapular facture or brachial plexus nerve palsy [10].
There is substantial evidence from biomechanical studies, that RSA design has effects on muscle moment arms [27]. The deltoid abductor moment arm increases with a medialized glenoid/lateralized humerus (MGLH) significantly more than lateralized glenoid/medialized humerus (LGMH) [27]. Improved deltoid efficiency has been documented by a reduction in force to achieve humeral elevation. When the CoR is distalized, the lines of action of the subscapularis and infraspinatus are affected in such a way that these muscles induce a much larger adduction. Offset of the humerus affects the efficiency of posterior deltoid, while a lateral CoR did not affect muscle moment arm of external rotators [27]. These biomechanical findings confirm the efficacy of reverse design to improve deltoid lever arm when the CoR is located at the glenoid bone interface. The role of augmented glenoid component with specific design of baseplate, remains crucial to preserve glenoid bone stock in arthritic shoulders with severe glenoid erosion [28]. By using CT-based computer modeling, Slowinski and Coll found that an augmented baseplate preserved, on average, 54% more native bone than a nonaugmented baseplate and results in 4.1 mm of lateralization[28]. Correction for glenoid bone defects without removing excess healthy bone, may may prevent possible complications of impingement and scapular notching.
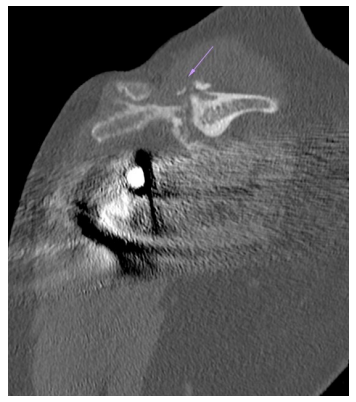
Despite the above reported biomechanical findings of Hamilton and co-workers [27] did not show significant effects of lateralized design on external rotators muscles, recent clinical studies demonstrated that RSA with 135° NSA and a lateralized glenosphere enables better preservation of external rotation and reduces the rate of scapular notching compared with the classic Grammont design in patients with cuff tear arthropathy [29]. Similar clinical findings were reported in other research studies describing reverse design with humeral and glenoid lateralization [18,30,31]. The use of reverse design with lateralized CoR or global lateralization, have reduced the risk of scapular notching, almost eliminating it [18,31], but have increased the risk of scapular spine and acromial fractures [18,32] (Figure 8). The effects of lateralized RSA on internal rotation mobility are unclear and results of recent clinical studies, assessing different reverse design, are elusive.
Figure 8. Scapular spine fracture (purple arrow) in lateralized RSA (“global lateralization”).
Recent research findings showed that a larger glenosphere size, a posterior offset humeral cup and an increased inferior glenosphere overhang were associated with excellent outcomes [33].
Analysis of other established radiographic parameters (radiolucency, condensation lines, cortical thinning, spot weld, subsidence and resorption of the tuberosities) complete the evaluation of the stemmed humeral component and give information about the stability of the humeral component and the risk of stress shielding and reduction of cortical thickness of the humerus [18].
Overall, lateralization in RSA can be performed on the glenoid side, humeral side or both (global lateralization).
Take Home Message
Radiographic features described in this study represent a pictorial essay to assess lateralized RSA.
RSA with CoR lateralization, both with bone or metal, represents a strategic choice for glenoid replacement in cases with severe bone loss. The role of augmented glenoid component with specific design of baseplate, remains crucial to preserve glenoid bone stock in arthritic shoulders with severe glenoid erosion. Glenoid lateralization should instead be cautiously considered in patient with shoulder arthritis without severe glenoid deformity.
Humeral lateralization improves posterior deltoid tension, while its action on the residual posterior rotator cuff muscles is still debated.
Funding
No funding was received for conducting this study.
Competing Interests
The author, his immediate family, and any research foundation with which they are affiliated did not receive any financial payments or other benefits from any commercial entity related to the subject of this article
References
2. Bercik M J, Kruse K, 2nd, Yalizis M, Gauci M O, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg. 2016;25:1601-6.
3. Favard L, Berhouet J, Walch G, Chaoui J and Levigne C. Superior glenoid inclination and glenoid bone loss : Definition, assessment, biomechanical consequences, and surgical options. Orthopade. 2017;46:1015-21.
4. Walch G, Vezeridis P S, Boileau P, Deransart P, Chaoui J. Three-dimensional planning and use of patient-specific guides improve glenoid component position: an in vitro study. J Shoulder Elbow Surg. 2015;24:302-9.
5. Jacquot A, Gauci MO, Chaoui J, Baba M, Deransart P, Boileau P, et al. Proper benefit of a three dimensional pre-operative planning software for glenoid component positioning in total shoulder arthroplasty. Int Orthop. 2018;42:2897-906.
6. Nunes B, Linhares D, Costa F, Neves N, Claro R, Silva MR. Lateralized versus nonlateralized glenospheres in reverse shoulder arthroplasty: a systematic review with meta-analysis J Shoulder Elbow Surg. 2021;30:1700-13.
7. Nunes J, Andrade R, Azevedo C, Ferreira NV, Oliveira N, Calvo E, et al. Improved Clinical Outcomes After Lateralized Reverse Shoulder Arthroplasty: A Systematic Review Clin Orthop Relat Res. 2022;480:949-57.
8. Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16:65-8.
9. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics J Shoulder Elbow Surg. 2005;14:147S-61S.
10. Bauer S, Corbaz J, Athwal GS, Walch G, Blakeney WG. Lateralization in Reverse Shoulder Arthroplasty. J Clin Med. 2021;10.
11. Chae J, Siljander M, Wiater JM. Instability in Reverse Total Shoulder Arthroplasty. J Am Acad Orthop Surg. 2018;26:587-96.
12. Padegimas EM, Zmistowski BM, Restrepo C, Abboud JA, Lazarus MD, Ramsey ML, et al. Instability After Reverse Total Shoulder Arthroplasty: Which Patients Dislocate? Am J Orthop (Belle Mead NJ). 2016;45:E444-E50.
13. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:737-44.
14. Boileau P, Melis B, Duperron D, Moineau G, Rumian AP, Han Y. Revision surgery of reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:1359-70.
15. Ladermann A, Walch G, Lubbeke A, Drake GN, Melis B, Bacle G, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21:336-41.
16. Bacle G, Nove-Josserand L, Garaud P, Walch G. Long-Term Outcomes of Reverse Total Shoulder Arthroplasty: A Follow-up of a Previous Study. J Bone Joint Surg Am. 2017;99:454-61.
17. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The Reverse Shoulder Prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87:1697-705.
18. Merolla G, Walch G, Ascione F, Paladini P, Fabbri E, Padolino A et al. Grammont humeral design versus onlay curved-stem reverse shoulder arthroplasty: comparison of clinical and radiographic outcomes with minimum 2-year follow-up. J Shoulder Elbow Surg. 2018;27:701-10.
19. Werthel J D, Walch G, Vegehan E, Deransart P, Sanchez-Sotelo J, Valenti P. Lateralization in reverse shoulder arthroplasty: a descriptive analysis of different implants in current practice. Int Orthop. 2019;43:2349-60.
20. Harman M, Frankle M, Vasey M and Banks S. Initial glenoid component fixation in "reverse" total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg. 2005;14:162S-7S.
21. Boileau P, Moineau G, Roussanne Y, O'Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res. 2011;469:2558-67.
22. Merolla G, De Cupis M, Walch G, De Cupis V, Fabbri E, Franceschi F, et al. Pre-operative factors affecting the indications for anatomical and reverse total shoulder arthroplasty in primary osteoarthritis and outcome comparison in patients aged seventy years and older. Int Orthop. 2020;44:1131-41
23. Boileau P, Morin-Salvo N, Bessiere C, Chelli M, Gauci MO, Lemmex DB. Bony increased-offset-reverse shoulder arthroplasty: 5 to 10 years' follow-up. J Shoulder Elbow Surg. 2020;29:2111-22.
24. Maurer A, Fucentese SF, Pfirrmann CW, Wirth SH, Djahangiri A, Jost B, et al. Assessment of glenoid inclination on routine clinical radiographs and computed tomography examinations of the shoulder. J Shoulder Elbow Surg. 2012;21:1096-103.
25. Boileau P, Morin-Salvo N, Gauci M O, Seeto B L, Chalmers P N, Holzer N, et al. Angled BIO-RSA (bony-increased offset-reverse shoulder arthroplasty): a solution for the management of glenoid bone loss and erosion. J Shoulder Elbow Surg. 2017;26:2133-42.
26. Boutsiadis A, Lenoir H, Denard PJ, Panisset JC, Brossard P, Delsol P, et al. The lateralization and distalization shoulder angles are important determinants of clinical outcomes in reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2018;27:1226-34.
27. Hamilton MA, Diep P, Roche C, Flurin PH, Wright TW, Zuckerman JD, et al. Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res. 2015;33:605-13.
28. Joshua J, Slowinski JAB, Feng L, Schoch N, Sperling JW, Duquin TR. CT-based 3D Modeling of Glenoid Bone Preservation with Augmented Baseplates. Seminars in Arthroplasty: JSES. 2022
29. Freislederer F, Toft F, Audige L, Marzel A, Endell D and Scheibel M. Lateralized vs. classic Grammont-style reverse shoulder arthroplasty for cuff deficiency Hamada stage 1-3: does the design make a difference? J Shoulder Elbow Surg. 2022;31:341-51.
30. Helmkamp JK, Bullock GS, Amilo NR, Guerrero EM, Ledbetter LS, Sell TC, et al. The clinical and radiographic impact of center of rotation lateralization in reverse shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2018;27:2099-107.
31. Lawrence C, Williams GR, Namdari S. Influence of Glenosphere Design on Outcomes and Complications of Reverse Arthroplasty: A Systematic Review. Clin Orthop Surg. 2016;8:288-97.
32. ASES Complications of RSA Research Group:, Mahendraraj KA, Abboud J, Armstrong A, Austin L, Brolin T, et al. Predictors of acromial and scapular stress fracture after reverse shoulder arthroplasty: a study by the ASES Complications of RSA Multicenter Research Group. J Shoulder Elbow Surg. 2021;30:2296-305.
33. Haidamous G, Ladermann A, Hartzler RU, Parsons BO, Lederman ES, Tokish JM, Denard PJ. Radiographic parameters associated with excellent versus poor range of motion outcomes following reverse shoulder arthroplasty. Shoulder Elbow. 2022;14:39-47.
34. Berliner JL, Regalado-Magdos A, Ma CB, Feeley BT. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:150-60.

![Figure 2. A. Reverse shoulder arthroplasty (RSA) acting with a fixed CoR with compressive (Fc) and shear (Fs) components of the resultant force vector (Fv). B. CoR lateralization increases the lever arm length which decreases compressive forces, increases destabilizing shear forces, and creates a new moment (M) at the glenoid-implant interface [34]. RSA: Reverse Shoulder Arthroplasty; CoR: Center of Rotation.](https://www.scientificarchives.com/public/assets/images/uploads/image-1670914705-1.png)