Abstract
Ionizing radiation has been indispensable to medical diagnosis. In cancer, radiation therapy or radiotherapy (RT) offers patients a better chance of survival. It destroys cancer by depositing high-energy radiation on the cancer tissues, though it may directly damage a few normal cells. Therefore, the total radiation dose is administered in fractionated modalities over weeks or months. However, experimental evidence indicates that the irradiated cancer cells subsequently release cytokines in the blood that enter into nearby unirradiated nuclei/cells through several signaling pathways and cause radiation-induced bystander effects (RIBEs) such as DNA damage, chromosomal instability, mutation, and apoptosis in them as side effects of RT. Recently, many combined therapeutic protocols consisting of a few natural and synthetic products have been proposed to minimize RIBEs. This article reviews the present understanding of RIBEs and their possible countermeasures. Besides, a new protocol of combined therapy of nanoparticle-based ion treatment (NIT) and RT to minimize RIBEs has been proposed.
Keywords
Cancer, Radiation, RIBEs, Combined therapy, Nanoparticles, Ions
Introduction
Cancer continues to be a pervasive disease, and its management is a rising concern in an aging population [1,2]. Radiation therapy or radiotherapy (RT) is an effective treatment for cancer and management, conferring survival and palliative benefits [3-5]. For years, the belief was that the significant biological effects, or death, induced by ionizing radiation (IR) in mammalian cells were the direct consequence of radiation-induced unrepaired or misrepaired DNA damage in the irradiated cells [6], and no effect in cells that receive no direct radiation traversal. However, in 1992, an experiment revealed that 1% of cells irradiated with α-particles led to chromatid exchange in more than 30% of cells. It occurs due to the inter-relationship between irradiated and unirradiated cells and is called the radiation-induced bystander effect (RIBE) [7]. RIBE is the biological alterations manifested in unirradiated cells, called bystander cells, induced by signals from nearby irradiated cells within an irradiated volume [8]. Both bystander and irradiated cells exhibit genetic damage, chromosome aberrations, and possibly cancer formation [9].
The RIBE can be described in medium transfer experiments [10,11], in which a cell culture medium is harvested from irradiated cells to treat unirradiated cells. Unirradiated cells (either healthy or cancerous) that received the irradiated cell-conditioned medium (ICCM) show lethal mutations and marked cell death [12]. The mechanism of bystander signal transmission has become a fascinating topic of research. For example, Ariyoshi et al. reported that exosome-like vesicles (ELV) mediate the radiation-induced bystander signal from irradiated cells [13]. They observed DNA damage in normal human fibroblast cells cultured with ICCM ELV and mouse serum ELV irradiated with a 4 Gy of X-ray dose. Another report indicated that irradiated MCF-7 breast cancer cells could induce bystander death in unirradiated MCF-7 and hFOB 1.19 (human osteoblast) bystander cells where the bystander signal was mediated by the reactive oxygen species (ROS) generation during the irradiation with HDR brachytherapy [14]. Chen et al. reported that the up-regulation of ROS by mitochondria-dependent bystander signaling (γ-H2AX) contributes to the genotoxicity of bystander effects [15].
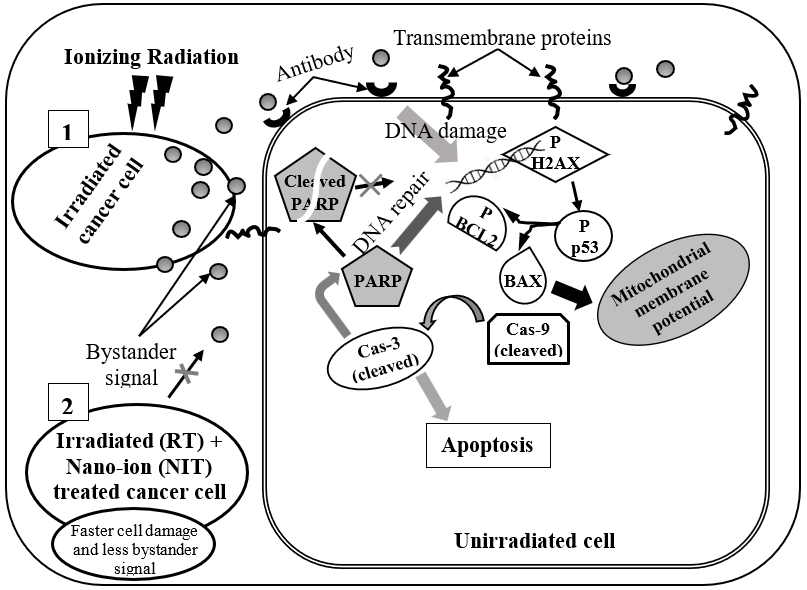
In addition to the bystander signaling mechanisms, investigation of the dependence of signal strength on various irradiation parameters such as dose and post-irradiation aging of targeted cells is also necessary. Mukherjee et al. studied the effect of dose-varied ICCM, collected at different post-irradiation times, on DNA damage and apoptosis of bystander cells [16]. In their study, different sets of culture media of human hepatocellular carcinoma HepG2 cells had undergone irradiation by γ-rays with doses of 2, 5, and 8 Gy; the respective ICCM was collected in the early and late post-irradiated stages (1, 2, and 24 h) to transfer to unirradiated cells. This study showed that compared to control (the directly irradiated cells), bystander cells had an increased level of H2AX phosphorylation, mitochondrial membrane depolarization, and elevation of intrinsic apoptotic pathway mediators such as p53, Bax, cas9, cas3, and PARP cleavage. These intrinsic apoptotic pathways in a nearby unirradiated cell (bystander cell), mediated by the bystander signals released from irradiated cell, is shown by the sketch in the scenario (1) in Figure 1 following reference [16]. The highest expression of the apoptosis markers was observed in 8 Gy irradiation-induced bystander cells, and the ICCM collected at the early time (1 or 2 h), though the 24 h ICCM induced the highest increase of H2AX and p53 phosphorylation and Bax levels.
Possible routes of the bystander effect involve gap-junction intercellular communications, soluble signal molecules, oxidative metabolism, plasma membrane-bound lipid rafts, and calcium fluxes [17-20]. Exposure to radiation causes unrepairable DNA damages, especially double-stranded breaks (DSBs), leading to the accumulation of p53 [21], a protein that stimulates the expression of pro-apoptotic mediators, thereby disrupting the intricate balance between cellular anti-apoptotic and pro-apoptotic proteins [16].
The transmission of bystander effects among cancer cells involves the activation of inflammatory cytokines, death ligands, and reactive oxygen/nitrogen species (ROS/RNS). Besides bystander effects, two other non-target effects (NTEs) in radiotherapy are the cohort and abscopal effects. In cohort effects, irradiated cells can produce signals that reduce the survival of neighbouring cells within an irradiated volume suggesting the importance of cellular communication under irradiation with non-uniform dose distribution. In abscopal effects, the NTEs typically occur in distant non-irradiated cells from an irradiated target, mediated primarily by immune cells such as T cells [22].
The availability of micro-beams that can deposit energy precisely to a part of the cell (such as the cytoplasm or nucleus) makes it possible to investigate the damage caused by irradiation of the nuclei versus the cytoplasm in a different manner [23]. It has been generally accepted in radiation biology that genotoxic effects, such as mutations and carcinogenesis, attributed to ionizing radiation exposure mainly result from direct damage to the nuclei. The extra-nuclear, i.e., cytoplasmic, irradiation may result in mutations in the nuclei. It has shown that cytoplasmic irradiation of human–hamster-hybrid (AL) cells with eight alpha particles led to around a threefold increase in CD59 mutations while inflicting minimal cytotoxicity [24]. More recent studies demonstrated that cytoplasmic irradiation is associated with mitochondrial dysfunction [25] and autophagy [26]. Cytoplasmic irradiation can also induce a bystander effect, exhibiting genotoxic effects such as a mutation in directly and indirectly irradiated cells [27].
Cell Signaling Pathways of RIBE and Their Inhibition
Figure 1 shows the sketch of a few signaling pathways of RIBE in nearby bystander cells, as reported by Mukherjee et al. [16]. Although gap junction communication and the presence of soluble mediator(s) are both known to play crucial roles in the bystander response, the specific cell signaling molecules are yet to identify. Researchers at Columbia University showed that the cyclooxygenase-2 (COX-2, also known as prostaglandin endoperoxide synthase-2) signaling cascade plays an essential role in the bystander process using the charged particle beam [28]. Also, the Cox-2 activity gets suppressed when bystander cells are treated with NS-298, an anti-inflammatory agent [29]. It reduces the bystander effect drastically.
Multiple signal transduction pathways stimulated by IR are mediated by the mitogen-activated protein kinase (MAPK) superfamily, including extracellular signal-regulated kinase 1/2 (ERK 1/2), c-Jun NH2-terminal kinase (JNK) and p38 [20]. Various signals reaching the cell membrane activate MAPK pathways [30]. Radiation-induced oxidative stress induces multiple effects on cellular macromolecules and signaling cascades. Mukherjee et al. studied the consequences of ICCM, from γ-irradiated hepatocellular carcinoma (HepG2) cells, on the oxidative stress induction in bystander HepG2 and normal liver cells (BRL-3A) and their interactions with critical cell signaling mediators [31]. The experimental outcome revealed that the levels of pro-survival signaling factors (p-PI3K, p-Akt, p38-MAPK, p-JNK, and p-NF-κB) increase in bystander HepG2 cells but significantly decrease in bystander BRL-3A cells. Enhanced ROS levels down-regulate the activation of PI3K, Akt, JNK, and NF-κB in BRL-3A cells but add to the activation of DNA damage sensor ATM (ataxia telangiectasia mutated) kinase and cell cycle inhibitor p21.
Yin et al. reported that α-particle irradiation activates the TGF-β1-Smad2 (tumor growth factor β1-mothers against decapentaplegic homolog 2) pathway and consistently decreases the miR-21 level in HaCaT keratinocyte cell lines, which subsequently induces bystander micronucleus formation in unirradiated WS1 fibroblasts after co-culture. On the other hand, X-ray irradiation did not prompt a bystander effect in unirradiated WS1 cells and did not trigger the Smad2 pathway or decrease the miR-21 level in irradiated HaCaT cells [32]. IR activates TGF-β-Smad pathways [33]. Smad2 and Smad7 play a significant role in radiation-induced double-strand break (DSB) signaling [34].
Chen et al. investigated the bystander effect due to different types of irradiation, including gamma and lithium-ion, in the model human neuroblastoma cell line (SH-SY5Y) [35]; the gamma and lithium-ion irradiations cause several bystander effects in the unirradiated SH-SY5Y cell lines; for example, promotion of cell proliferation through activation of the ERK and AKT signaling pathways by the gamma irradiation, with minimal effect on the cell cycle of unirradiated SH-SY5Y cells. In contrast, lithium-ion irradiation inhibited cell proliferation, arrested the cell cycle, and activated the process of pro-apoptosis [35].
MAPK pathways link to the growth factor-mediated regulation of cellular events such as proliferation, senescence, differentiation, and apoptosis. Activation of multiple MAPK pathways, such as the ERK, JNK, and p38, has been shown to occur after exposure of cells to radiation and a variety of other toxic stresses. Lyng et al. investigated MAPK signaling pathways in bystander cells exposed to ICCM and the role of oxidative metabolism and calcium signaling in the induction of bystander responses [36]. These authors had irradiated HPV-G keratinocyte cell lines with varying doses, in the range of 0.005–5.0 Gy, using a cobalt-60 teletherapy unit, and then the media were harvested for 1-hour post-irradiation and, subsequently, transferred to the recipient HPV-G cells. It activated JNK and ERK proteins in bystander cells after exposure to ICCM. On the other hand, inhibition of the ERK pathway increases bystander-induced apoptosis, while inhibition of the JNK pathway decreases apoptosis.
Countermeasures for RIBE
Radiation-induced bystander signals cause various side effects (i.e., RIBEs), which commonly appear as changes in the skin (such as itching, peeling, and blistering) and fatigue; other side effects, such as diarrhea, nausea, hair loss, and vomiting depend on the exposed area of the body. Although most side effects tend to subside within a few weeks or months of completing radiation therapy, some may last for a longer time.
Many natural herbs help manage RIBEs to some extent. For example, curcumin (an antioxidant and anti-inflammatory compound found in turmeric) may help protect against radiation-induced skin damage, and Ginkgo biloba (a Chinese tree) may help shield against organ damage. Other natural antioxidants such as caffeine, melatonin, flavonoids, polyphenols, and phytochemicals (e.g., albana) help decrease radiation-induced damage in either plasmid or cellular DNA through scavenging of oxygen radicals and/or peroxides [37-42]. Several investigations revealed that those who take probiotics throughout their radiotherapy for various types of cancer are less likely to experience radiation-induced diarrhea [43-45].
In a review article, Zhang et al. discussed the radioprotective properties of antioxidants and what those properties tell about the DNA and other cellular targets of radiation [46]. The radiation-induced DNA damage causes genotoxicity; preventing this damage requires an antioxidant during irradiation [47]. Two ways it may work, either (1) react with all the oxygen-related free radicals and detoxify them to radicals that are not themselves genotoxic, and/or (2) effectively compete with oxygen in chemically repairing the DNA damage through reactions with free radicals. For example, thiol-based compounds are especially capable of scavenging oxygen radicals and affecting the chemical restoration of some forms of DNA damage with the subsequent formation of sulphur-based radicals, which are non-reactive to DNA [48]. The incorporation of one or more positive charges on the thiol-based antioxidant has the effect of changing the proximity of the compound to the DNA [49,50]. The resulting counter-ion condensation between the positive charge of thiol and the negatively charged sugar-phosphate backbone of the DNA binds the thiol close to the DNA, facilitating the competition of the thiol with oxygen in reactions with DNA radicals, thereby reducing the DNA damage and increasing the cell survival [50,51].
A review article has discussed the advantages of using mesenchymal stem cells (MSCs) in combination with radiation therapy to treat rectal cancer [52]. This combined therapy on xenotumors, implanted in a murine model, showed the existence of a synergic mechanism that enhances the local and systemic actions of the radiation both on the treated tumor and its possible metastasis. These authors remarked that the physicochemical tropism of MSCs and the widespread functions of macromolecules, proteins, and exosomes released from activated MSCs have the effect of counteracting the pro-tumorigenic and pro-metastatic signals that contribute to the growth, spread, and resistance of the tumor cells in this combined therapy.
Application of Nanoparticles
Further research reveals that radiosensitizer agents made of high atomic number (Z) materials selectively increase the toxicity of radiation to the cancerous area and lessen the effect on healthy tissue [53-55]. Metallic/metal oxide nanoparticles such as gold nanoparticles (Au NPs), platinum nanoparticles (Pt NPs), iron oxide nanoparticles (Fe3O4 NPs), and bismuth oxide nanoparticles (Bi2O3 NPs) work as radiosensitizers and can increase the effect of radiation on cancerous areas by increasing the dose deposition in the target amount [56-59]. Recently, Rahman et al. explored the cellular outcomes of bismuth oxide nanoparticles (Bi2O3 NPs) on human breast cancer (MCF-7) and human fetal osteoblast (hFOB 1.19) cells due to the RIBE after irradiation with 6 MV clinical photon beam [60]. Bi2O3 NPs work as a radiosensitizer that increases the survival rate of the treated bystander cells (MCF-7 and hFOB 1.19) up to approximately 3–8% following a short and long-term incubation with ICCM treated with Bi2O3 NPs. Radiation causes the emission of more secondary electrons when metallic nanoparticles are present in the cells. The interaction of radiation with nanoparticles creates hydrolysis of water molecules within the cells and generates free radicals, which interact with DNA and cause excessive damage to them and cell death. Thus, it enhances the radiation treatment efficacy [61].
Functional nanomaterials have also been investigated for several other cancer therapeutic applications, such as photodynamic (PDT) and photothermal therapy (PTT) [62]. In PDT, the photosensitizer accumulates in cancerous sites; when irradiated with light, singlet oxygen and other cytotoxic reactive oxygen species (ROS) are generated that cause apoptosis and/or necrosis [63]. PTT uses materials with high photothermal conversion efficiency to elevate the temperature of targeted cancerous areas, leading to cancer cell death. Superparamagnetic iron oxide nanoparticles (SPION) have been investigated for cancer hyperthermia treatment due to their smaller size, higher targeting specificity, controllable releasing speed, and immune evasion capability [64].
Ion-conjugated Charged Nanoparticles (ICNPs): A New Proposal
Ion-conjugated charged nanoparticles (ICNPs) are surface-coated with counterion-conjugated charged molecules such as surfactants, citrates, or phosphates. The surface charge helps electrostatically binding ICNPs with oppositely charged biomolecules such as proteins and, subsequently, the release of counterions that intercalate into the bound molecules and irreversibly denature them [65-68]. This type of mechanism has the name ‘reverse charge parity counterion’ (RCPC) interaction [69], where the charge density (charge/volume) of counterions is an important parameter [70]. The denatured proteins lose their bio-functionalities [71]. Earlier, our in vitro experiment showed that the RCPC interaction causes cell membrane rupture and subsequent necrotic collapse of the cancer cell lines in the culture medium [72]. It gives hope that nanoparticle-based ion therapy (NIT) can be developed for cancer treatment to avoid using drugs, radiation, or surgery.
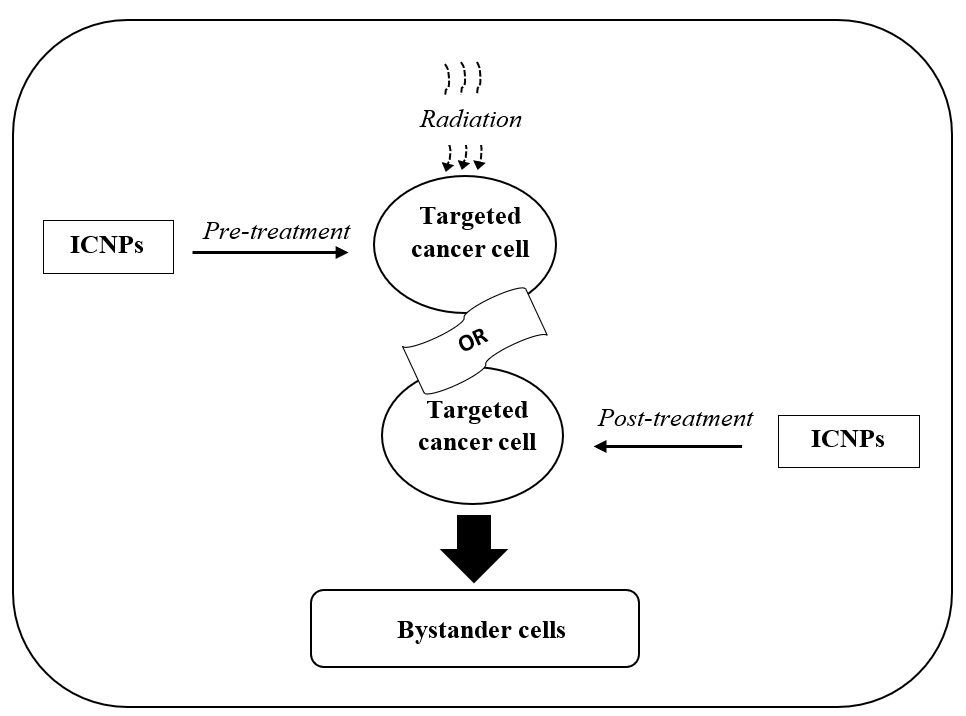
In this article, the author propose a new protocol in the following steps for a combined therapy using NIT and RT to minimize RIBE:
- Pre-treat the target cancer cells with ICNPs before radiation treatment (RT), or post-treat target cancer cells with ICNPs after RT, or simultaneous NIT and RT, and
- Incubation of NIT+RT treated cells with bystander cells.
NIT has its way of damaging target cells besides that due to RT. Consequently, target cells will die earlier than a pure RT; thus, the release of bystander signals will reduce. It is shown by the sketch in the scenario (2) in Figure 1.
A schematic presentation of the above protocol is shown in Figure 2. Work on this new protocol of combined therapy (NIT + RT) is undergoing, and the outcome will be published soon. On successful completion, a clinical test will follow. For clinical applications, a model ICNP has been proposed in our earlier review article [73].
Conclusion and Future Proposal
The author has reviewed research articles on the radiation-induced bystander effects (RIBEs) in unirradiated cells. Radiation causes several damages, such as unrepairable DNA damage in the nuclei of the targeted cells, which, in turn, induces apoptosis in the irradiated cells. However, nearby unirradiated cells (called bystander cells) get equally affected by the signals (called bystander signals) released from the irradiated cells; this is called RIBE. Recent reports suggest some combined therapies to countermeasure RIBE using herbal agents, antioxidant agents, mesenchymal stem cells (MSCs), or nanoparticles in addition to radiotherapy (RT). In this review article, the author has proposed a new therapeutic protocol to countermeasure RIBE using a nanoparticle-based ion treatment (NIT), with ion-conjugated charged nanoparticles (ICNPs), of the targeted cancer cells, either before or after RT. This protocol involves a nano-bio interaction model called ‘reverse charge parity counterion’ (RCPC) interaction, which causes necrotic death of the targeted cancer cells. In RT, a combination of NIT can inhibit bystander signal release in the medium.
References
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019 Jan;69(1):7-34.
3. Prise KM. New advances in radiation biology. Occupational Medicine. 2006 May 1;56(3):156-61.
4. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. Journal of Clinical Oncology. 2013 Jan 1;31(1):80.
5. Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Science Translational Medicine. 2013 Feb 20;5(173):173sr2-.
6. Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Frontiers in Molecular Biosciences. 2014 Nov 17;1:24.
7. Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of α-particles. Cancer Research. 1992 Nov 15;52(22):6394-6.
8. Marín A, Martín M, Liñán O, Alvarenga F, López M, Fernández L, et al. Bystander effects and radiotherapy. Reports of Practical Oncology and Radiotherapy. 2015;20(1):12-21.
9. Yakovlev VA. Role of nitric oxide in the radiation-induced bystander effect. Redox Biology. 2015 Dec 1;6:396-400.
10. Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiation Research. 1998 Mar 1;149(3):256-62.
11. Liu Z, Mothersill CE, McNeill FE, Lyng FM, Byun SH, Seymour CB, et al. A dose threshold for a medium transfer bystander effect for a human skin cell line. Radiation Research. 2006 Jul;166(1):19-23.
12. Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander‐killing environment. Radiation Oncology Investigations: Clinical and Basic Research. 1997;5(3):106-10.
13. Ariyoshi K, Miura T, Kasai K, Fujishima Y, Nakata A, Yoshida M. Radiation-induced bystander effect is mediated by mitochondrial DNA in exosome-like vesicles. Scientific Reports. 2019 Jun 24;9(1):1-4.
14. Nh MZ, Abdullah R, Rahman WN. Bystander effect induced in breast cancer (MCF-7) and human osteoblast cell lines (hFOB 1.19) with HDR-brachytherapy. Journal of Biomedical Physics & Engineering. 2020 Jun;10(3):319.
15. Chen S, Zhao Y, Zhao G, Han W, Bao L, Yu KN, et al. Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2009 Jun 18;666(1-2):68-73.
16. Mukherjee S, Dutta A, Chakraborty A. The cross-talk between Bax, Bcl2, caspases, and DNA damage in bystander HepG2 cells is regulated by γ-radiation dose and time of conditioned media transfer. Apoptosis. 2022 Apr;27(3):184-205.
17. Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced γH2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007 Feb;26(7):993-1002.
18. Fernandez-Palomo C, Seymour C, Mothersill C. Inter-relationship between low-dose hyper-radiosensitivity and radiation-induced bystander effects in the human T98G glioma and the epithelial HaCaT cell line. Radiation Research. 2016 Feb;185(2):124-33.
19. Shao C, Folkard M, Prise KM. Role of TGF-β1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008 Jan;27(4):434-40.
20. Hamada N, Matsumoto H, Hara T, Kobayashi Y. Intercellular and intracellular signaling pathways mediating ionizing radiation-induced bystander effects. Journal of Radiation Research. 2007:0702230007-.
21. Loewer A, Karanam K, Mock C, Lahav G. The p53 response in single cells is linearly correlated to the number of DNA breaks without a distinct threshold. BMC Biology. 2013 Dec;11(1):1-3.
22. Wang R, Zhou T, Liu W, Zuo L. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget. 2018 Apr 6;9(26):18637.
23. Zhou H, Hong M, Chai Y, Hei TK. Consequences of cytoplasmic irradiation: studies from microbeam. Journal of Radiation Research. 2009 Mar;50(Suppl_A):A59-65.
24. Wu LJ, Randers-Pehrson G, Xu A, Waldren CA, Geard CR, Yu Z, et al. Targeted cytoplasmic irradiation with alpha particles induces mutations in mammalian cells. Proceedings of the National Academy of Sciences. 1999 Apr 27;96(9):4959-64.
25. Zhang B, Davidson MM, Zhou H, Wang C, Walker WF, Hei TK. Cytoplasmic Irradiation Results in Mitochondrial Dysfunction and DRP1-Dependent Mitochondrial FissionCytoplasmic Irradiation and Mitochondrial Damage. Cancer Research. 2013 Nov 15;73(22):6700-10.
26. Wu J, Zhang B, Wuu YR, Davidson MM, Hei TK. Targeted cytoplasmic irradiation and autophagy. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2017 Dec 1;806:88-97.
27. Zhang Z, Li K, Hong M. Radiation-Induced Bystander Effect and Cytoplasmic Irradiation Studies with Microbeams. Biology. 2022 Jun 21;11(7):945.
28. Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proceedings of the National Academy of Sciences. 2005 Oct 11;102(41):14641-6.
29. Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994 Jan 1;47(1):55-9.
30. Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S. MAPK pathways in radiation responses. Oncogene. 2003 Sep;22(37):5885-96.
31. Mukherjee S, Dutta A, Chakraborty A. The interaction of oxidative stress with MAPK, PI3/AKT, NF-κB, and DNA damage kinases influences the fate of γ-radiation-induced bystander cells. Archives of Biochemistry and Biophysics. 2022 May 25:109302.
32. Yin X, Tian W, Wang L, Wang J, Zhang S, Cao J, et al. Radiation quality-dependence of bystander effect in unirradiated fibroblasts is associated with TGF-β1-Smad2 pathway and miR-21 in irradiated keratinocytes. Scientific Reports. 2015 Jun 16;5(1):1-2.
33. Dancea HC, Shareef MM, Ahmed MM. Role of radiation-induced TGF-beta signaling in cancer therapy. Molecular and Cellular Pharmacology. 2009 Jul 2;1(1):44-56.
34. Wang M, Saha J, Hada M, Anderson JA, Pluth JM, O'Neill P, et al. Novel Smad proteins localize to IR-induced double-strand breaks: interplay between TGFβ and ATM pathways. Nucleic Acids Research. 2013 Jan 1;41(2):933-42.
35. Chen B, Zhang P, Sun F, Li B, Chen Y, Pei S, et al. The mechanism of bystander effect induced by different irradiation in human neuroblastoma cells. Acta Astronautica. 2020 Jan 1;166:599-606.
36. Lyng FM, Maguire P, McClean B, Seymour C, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiation Research. 2006 Apr;165(4):400-9.
37. Kumar SS, Devasagayam TP, Jayashree B, Kesavan PC. Mechanism of protection against radiation-induced DNA damage in plasmid pBR322 by caffeine. International Journal of Radiation Biology. 2001 Jan 1;77(5):617-23.
38. Reiter RJ, Tan DX, Mayo JC, Sainz RM, Leon J, Czarnocki Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochimica Polonica. 2003 Dec 31;50(4):1129-46.
39. Maurya DK, Salvi VP, Nair CK. Radioprotection of normal tissues in tumor-bearing mice by troxerutin. Journal of Radiation Research. 2004;45(2):221-8.
40. Devi PU, Bisht KS, Vinitha M. A comparative study of radioprotection by Ocimum flavonoids and synthetic aminothiol protectors in the mouse. The British Journal of Radiology. 1998 Jul;71(847):782-4.
41. Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003 Jul 15;189(1-2):1-20.
42. Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. The Journal of Nutrition. 2003 Oct 1;133(10):3275S-84S.
43. Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, et al. Use of probiotics for prevention of radiation-induced diarrhea. World journal of gastroenterology: WJG. 2007 Feb 2;13(6):912.
44. Linn YH, Thu KK, Win NH. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study. Probiotics and Antimicrobial Proteins. 2019 Jun;11(2):638-47.
45. Giralt J, Regadera JP, Verges R, Romero J, de la Fuente I, Biete A, et al. Effects of probiotic Lactobacillus casei DN-114 001 in prevention of radiation-induced diarrhea: results from multicenter, randomized, placebo-controlled nutritional trial. International Journal of Radiation Oncology* Biology* Physics. 2008 Jul 15;71(4):1213-9.
46. Okunieff P, Swarts S, Keng P, Sun W, Wang W, Kim J, et al. Antioxidants reduce consequences of radiation exposure. Oxygen Transport to Tissue XXIX. 2008:165-78.
47. Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: current status and new directions. Oncology. 2002;63(Suppl. 2):2-10.
48. Held KD. Models for thiol protection of DNA in cells. Pharmacology & Therapeutics. 1988 Jan 1;39(1-3):123-31.
49. Smoluk GD, Fahey RC, Ward JF. Interaction of glutathione and other low-molecular-weight thiols with DNA: evidence for counterion condensation and coion depletion near DNA. Radiation Research. 1988 Apr 1;114(1):3-10.
50. Zheng S, Newton GL, Ward JF, Fahey RC. Aerobic radioprotection of pBR322 by thiols: effect of thiol net charge upon scavenging of hydroxyl radicals and repair of DNA radicals. Radiation Research. 1992 May 1;130(2):183-93.
51. Murray D, Prager A, Vanankeren SC, Altschuler EM, Kerr MS, Terry NH, Milas L. Comparative effect of the thiols dithiothreitol, cysteamine and WR-151326 on survival and on the induction of DNA damage in cultured Chinese hamster ovary cells exposed to γ-radiation. International Journal of Radiation Biology. 1990 Jan 1;58(1):71-91.
52. Farias VD, Tovar I, Del Moral R, O'Valle F, Expósito J, Oliver FJ, et al. Enhancing the bystander and abscopal effects to improve radiotherapy outcomes. Frontiers in Oncology. 2020 Jan 8;9:1381.
53. Mesbahi A. A review on gold nanoparticles radiosensitization effect in radiation therapy of cancer. Reports of Practical Oncology & Radiotherapy. 2010 Nov 1;15(6):176-80.
54. McMahon SJ, Paganetti H, Prise KM. Optimising element choice for nanoparticle radiosensitisers. Nanoscale. 2016;8(1):581-9.
55. Rosa S, Connolly C, Schettino G, Butterworth KT, Prise KM. Biological mechanisms of gold nanoparticle radiosensitization. Cancer Nanotechnology. 2017 Dec;8(1):1-25.
56. Rashid RA, Abidin SZ, Anuar MA, Tominaga T, Akasaka H, Sasaki R, et al. Radiosensitization effects and ROS generation by high Z metallic nanoparticles on human colon carcinoma cell (HCT116) irradiated under 150 MeV proton beam. OpenNano. 2019 Jan 1;4:100027.
57. KA MA, Ab Rashid R, Lazim RM, Dollah N, Razak KA, Rahman WN. Evaluation of radiosensitization effects by platinum nanodendrites for 6 MV photon beam radiotherapy. Radiation Physics and Chemistry. 2018 Sep 1;150:40-5.
58. Abidin SZ, Zulkifli ZA, Razak KA, Zin H, Yunus MA, Rahman WN. PEG coated bismuth oxide nanorods induced radiosensitization on MCF-7 breast cancer cells under irradiation of megavoltage radiotherapy beams. Materials Today: Proceedings. 2019 Jan 1;16:1640-5.
59. Sisin NN, Abdul Razak K, Zainal Abidin S, Che Mat NF, Abdullah R, Ab Rashid R, et al. Radiosensitization effects by bismuth oxide nanoparticles in combination with cisplatin for high dose rate brachytherapy. International Journal of Nanomedicine. 2019 Dec 18:9941-54.
60. Zainudin NH, Sisin NN, Ab Rashid R, Jamil A, Anuar MA, Razak KA, et al. Cellular analysis on the radiation induced bystander effects due to bismuth oxide nanoparticles with 6 MV photon beam radiotherapy. Journal of Radiation Research and Applied Sciences. 2022 Sep 1;15(3):318-25.
61. Paro AD, Shanmugam I, van de Ven AL. Nanoparticle-mediated X-ray radiation enhancement for cancer therapy. InCancer Nanotechnology. New York: Humana Press. 2017; pp. 391-401.
62. Gao W, Wang Z, Lv L, Yin D, Chen D, Han Z, et al. Photodynamic therapy induced enhancement of tumor vasculature permeability using an upconversion nanoconstruct for improved intratumoral nanoparticle delivery in deep tissues. Theranostics. 2016;6(8):1131.
63. Osaki T, Yokoe I, Sunden Y, Ota U, Ichikawa T, Imazato H, et al. Efficacy of 5-aminolevulinic acid in photodynamic detection and photodynamic therapy in veterinary medicine. Cancers. 2019 Apr 7;11(4):495.
64. Horst MF, Coral DF, van Raap MB, Alvarez M, Lassalle V. Hybrid nanomaterials based on gum Arabic and magnetite for hyperthermia treatments. Materials Science and Engineering: C. 2017 May 1;74:443-50.
65. Ghosh G, Panicker L, Ningthoujam RS, Barick KC, Tewari R. Counter ion induced irreversible denaturation of hen egg white lysozyme upon electrostatic interaction with iron oxide nanoparticles: a predicted model. Colloids and Surfaces B: Biointerfaces. 2013 Mar 1;103:267-74.
66. Ghosh G, Panicker L, Barick KC. Selective binding of proteins on functional nanoparticles via reverse charge parity model: an in vitro study. Materials Research Express. 2014 Jan 31;1(1):015017.
67. Ghosh G, Panicker L. Interactions of human hemoglobin with charged ligand-functionalized iron oxide nanoparticles and effect of counterions. Journal of Nanoparticle Research. 2014 Dec;16(12):1-0.
68. Dyawanapelly S, Jagtap DD, Dandekar P, Ghosh G, Jain R. Assessing safety and protein interactions of surface-modified iron oxide nanoparticles for potential use in biomedical areas. Colloids and Surfaces B: Biointerfaces. 2017 Jun 1;154:408-20.
69. Ghosh G. Counterion effects in protein nanoparticle electrostatic binding: a theoretical study. Colloids and Surfaces B: Biointerfaces. 2015 Apr 1;128:23-7.
70. Ghosh G, Panicker L, Barick KC. Protein nanoparticle electrostatic interaction: size dependent counterions induced conformational change of hen egg white lysozyme. Colloids and Surfaces B: Biointerfaces. 2014 Jun 1;118:1-6.
71. Ghosh G, Gaikwad PS, Panicker L, Nath BB, Mukhopadhyaya R. Unfolding and inactivation of proteins by counterions in protein-nanoparticles interaction. Colloids and Surfaces B: Biointerfaces. 2016 Sep 1;145:194-200.
72. Ghosh G, Mukherjee A, Bhilwade HN, Gupta A, Korde A, Mukhopadhyaya R. Selective Cytotoxicity of Counterion-Conjugated Charged Iron Oxide Nanoparticles: A Study with Lymphoblastoid Raji Cells. J. Adv. Nanomaterials. 2018 Dec;3(4):45-56.
73. Ghosh G, Panicker L. Protein–nanoparticle interactions and a new insight. Soft Matter. 2021;17(14):3855-75.