Abstract
Excessive food intake leads to lipid accumulation in white adipose tissue, triggering inflammation, cellular stress, insulin resistance, and metabolic syndrome. In contrast, the dynamic energy expenditure and heat generation of brown and beige adipose tissue, driven by specialized mitochondria, render it an appealing candidate for therapeutic strategies aimed at addressing metabolic disorders. This review examines the therapeutic potential of brown and beige adipocytes for obesity and metabolic disorders, focusing on recent studies that employ optogenetics for thermogenesis control in these cells. The findings delve into the mechanisms underlying UCP1-dependent and UCP1-independent thermogenesis and how optogenetic approaches can be used to precisely modulate energy expenditure and induce thermogenesis. The convergence of adipocyte biology and optogenetics presents an exciting frontier in combating metabolic disorders and advancing our understanding of cellular regulation and energy balance.
Keywords
Brown adipocytes, Beige adipocytes, Thermogenesis, Optogenetics, Obesity, Type 2 diabetes, UCP1, bPAC, ChR2, Calcium signaling
Abbreviations
WAT: White Adipose Tissue; BAT: Brown Adipose Tissue; UCP1: Uncoupling Protein-1; Ca2+: Calcium; ChR2: Channel Rhodopsin-2; LED: Light Emitting Diode; AAV: adeno-associated Virus; EYFP: Enhanced Yellow Fluorescent Protein; TH-Cre: Tyrosine Hydroxylase-driven Cre recombinase; β3-AR: β3-adrenergic Receptor; ATP: Adenosine Triphosphate; SERCA2: Sarco/endoplasmic Reticulum Ca2+-ATPase; HFD: High-fat Diet; ms: Milliseconds; Hz: Hertz; cAMP: Cyclic Adenosine Monophosphate; PKA: cAMP-dependent Protein Kinase A; CREB: cAMP Response Element-Binding Protein; AC: Adenylyl Cyclase; bPAC: Bacterial Photoactivatable Adenylyl Cyclase; BLUF: Blue Light sensor using Flavin adenine dinucleotide; ADSCs: Adipose-derived Stem Cells; iPSCs: Induced Pluripotent Stem Cells
Unlocking the Therapeutic Potential of Brown and Beige Adipose Tissues
Extended periods of excessive food intake give rise to the accumulation of lipids within white adipose tissue (WAT), paving the way for the onset of inflammation, cellular stress, insulin resistance, and ultimately, metabolic syndrome [1]. In contrast, brown adipose tissue activation (BAT) exhibits a positive correlation with increased energy expenditure and reduced vulnerability to metabolic syndrome and cardiometabolic disorders, rendering it an enticing tissue for therapeutic intervention [2-5]. Notably, brown adipocytes possess abundant, highly specialized mitochondria that expend energy to generate heat, facilitating the maintenance of core body temperature via a process recognized as non-shivering thermogenesis [6].
BAT assumes its anatomical configuration during embryonic development, primarily located within distinct regions such as the interscapular, cervical-supraclavicular, and paravertebral zones in both rodents and human subjects [7,8]. Within these precise locations, BAT undergoes a remarkable process of extensive innervation and vascularization, becoming intricately intertwined with neural and blood vessel networks. The activation of BAT is predominantly triggered by the cold-induced release of norepinephrine originating from sympathetic nerve terminals, which subsequently binds to β-adrenergic surface receptors located on brown adipocytes [9]. Activation of β-adrenergic receptors sets in motion a cascade that initiates nutrient combustion. This entails: (1) the uptake of glucose and fatty acids/lipids from the bloodstream, coupled with the lipolysis of lipids derived from intracellular multilocular lipid droplets [10,11]; (2) the liberation of free fatty acids, priming them for subsequent β-oxidation; and (3) the creation of a proton gradient within the inner mitochondrial membrane, thereby activating the electron transport chain to facilitate ATP production [11]. Brown adipocytes exhibit a distinctive trait wherein β-adrenergic activation induces the transcription of uncoupling protein-1 (UCP1). This protein, situated on the inner mitochondrial membrane, functions to dissipate the electrochemical gradient established within the mitochondria, diverting it away from ATP synthesis. Instead, this redirection releases protons back into the mitochondrial matrix, yielding energy in the form of heat production [2]. This release in heat helps maintain core body temperature (euthermia) by warming the blood during exposure to cold temperatures.
Apart from brown adipocytes, a developmentally distinct subset of fat cells known as beige adipocytes, located within subcutaneous WAT, can experience temperature-triggered transformations from a white (UCP1-) to a brown-like (UCP1+) state [12]. Through β-adrenergic stimulation, beige adipocytes can actively participate in sustaining body temperature and enhancing resistance against obesity [13]. Furthermore, beige adipocytes are able to undergo UCP1-independent signaling, which can be mediated by futile Ca2+ signaling, a unique and finely orchestrated intracellular calcium (Ca2+) flux that culminates in thermogenesis [14].
Unfortunately, attempts to manipulate obesity and diabetes in humans via drug-induced activation of β-adrenergic receptors have yielded modest outcomes, as this approach bears the potential of elevating the risk of cardiovascular disease [15-17]. An alternative approach to chemical modulation of cell signaling is optogenetics, a state-of-the-art technique that uses light-sensitive proteins to control specific cellular processes in genetically modified cells that can be used for precise temporal, spatial and reversable control of signaling.
Multifaceted Applications of Optogenetics
Optogenetics integrates genetic manipulation with optical control, allowing for unparalleled precision in regulating cellular activity across both time and space. Originally conceived to manipulate neurons, its applications now extend far beyond, encompassing a wide range of cell types. This groundbreaking technique has two essential components: light-sensitive proteins and genetic engineering. The pivotal breakthrough in this field came with the identification and utilization of microbial opsin proteins, such as channelrhodopsin and halorhodopsin, which are responsive to specific wavelengths of light [18]. These opsin genes can be introduced into target cell genomes through viral vectors or transgenic approaches. Once expressed, opsins can be used to either stimulate or suppress cellular activity in response to light. Channelrhodopsin was originally discovered in certain types of green algae and when it is genetically expressed in target neurons, exposure to light causes the protein to open ion channels in the neuron's membrane, allowing positively charged ions to flow into the neuron [19,20]. This influx of ions triggers neuronal depolarization, leading to the generation of action potentials and neuron activation. Halorhodopsin, derived from halophilic archaebacteria, serves a complementary function to channelrhodopsin and can hyperpolarize neurons, effectively inhibiting their activity [21,22]. Halorhodopsin inhibits neuronal activity by pumping chloride ions into the neuron when exposed to light. This influx of negatively charged chloride ions hyperpolarizes the neuron's membrane potential, making it more difficult for the neuron to reach the threshold required for firing action potentials. As a result, neuronal firing is suppressed, and the neuron becomes less responsive to incoming signals. Together these proteins and their derivatives have provided a platform for precise control of neural function via optogenetics.
Optogenetics has had a profound impact on neuroscience. In the realm of basic research, it has empowered scientists to disentangle the functional roles of specific neuronal populations within intricate networks. By introducing light-sensitive proteins into neurons, scientists can precisely activate or inhibit these cells, leading to insights into neural circuitry and behavior, as well as neurological disorders such as Parkinson's disease, epilepsy, and depression [23-25]. Optogenetics has also been used to study the neural circuits governing memory formation and learning processes [26]. Through the manipulation of neurons implicated in memory tasks, researchers have discovered new pathways and potential therapeutic targets for conditions like Alzheimer's disease [27]. In the field of vision, optogenetics holds potential for restoring sight to individuals afflicted by degenerative eye diseases [28]. By rendering remaining retinal cells light-sensitive, this technique may provide solutions for conditions like retinitis pigmentosa [29].
Despite its role in revolutionizing neuroscience, optogenetics is not confined solely to neurons. In recent years, researchers have harnessed the technique to manipulate a diverse array of non-neuronal cells. Optogenetics has been used to manipulate immune cells, opening new avenues for controlling immune responses and advancing cancer immunotherapy [30]. In cardiac cells, the use of optogenetics allows precise control over heart rhythm, which could pave the way for therapies for arrhythmias and other heart-related conditions [31]. The expansion of optogenetic techniques into non-neuronal domains holds great promise for advancing our understanding of various biological processes and pioneering innovative therapeutic strategies.
Within the context of brown and beige adipocytes, optogenetics can be used to manipulate thermogenic activity and explore thermogenesis regulation. In this concise review, we spotlight three noteworthy studies that have harnessed optogenetics to activate thermogenesis in adipocytes and discuss the implications of this for therapeutic intervention in the ongoing battle against obesity-related diseases.
Optogenetic Activation of BAT through Sympathetic Neuromodulation via ChR2
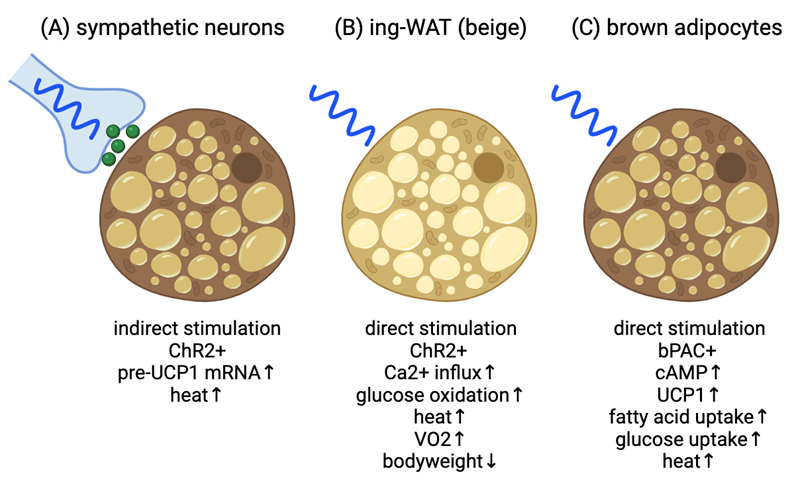
In 2019, the Bartolomucci Laboratory (Lyons et. al. [32]) published a study exploring whether selective optogenetic stimulation of sympathetic nerves innervating BAT could induce thermogenesis (Figure 1A). Two approaches were employed. The first involved modifying adeno-associated-AAV6 virus to express the blue light-sensitive cation channel ChR2 fused with enhanced yellow fluorescent protein (EYFP), controlled by the pan-neuronal human synapsin-1 promoter. Injecting this engineered AAV6 into BAT of mice led to successful transduction of TH-positive sympathetic nerves, confirming expression in the right locations. A custom made blue light LED (455nm) with a thermoprobe to allow for temperature measurements was implanted beneath the BAT through an incision on the back. Subsequently, AAV6-ChR2-EYFP-injected mice underwent a 30-minute blue LED light stimulation, resulting in significant increases in both BAT and core body temperature (as determined by rectal probe), distinct from AAV6-EYFP controls. This protocol also notably elevated transcriptional activation of Ucp1 pre-mRNA in ChR2-expressing mice compared to controls. Genetic validation followed as the second approach, employing TH-Cre X Rosa26-LSL-ChR2-YFP mice (ChR2TH-Cre+ or ChR2TH-Cre−). ChR2TH-Cre+ mice showed BAT thermogenesis upon optogenetic stimulation, evidenced by both BAT and core body temperature elevation, whereas ChR2TH-Cre− did not. The temperature elevation persisted after the LED was switched off for at least an additional 30 minutes. Moreover, increased Ucp1 pre-mRNA expression was observed in ChR2TH-Cre+ mice. These results demonstrate that optogenetic stimulation of sympathetic nerves is sufficient to induce BAT thermogenesis, indicated by increases in temperature and Ucp1 pre-mRNA. This presumably would occur due to release of norepinephrine from sympathetic nerves terminals adjacent to the brown adipocytes, however, norepinephrine secretion was not analyzed during the study. Overall, this approach holds promise for addressing obesity and diabetes through peripheral neuromodulation.
Figure 1. Current models for optogenetic activation for adipocyte thermogenesis. (A) Blue light stimulation of ChR2+ sympathetic neurons adjacent to brown adipose tissue results in increased BAT thermogenesis and whole-body temperature. Presumptive norepinephrine secretion indicated by green spheres. (B) Blue light stimulation of ChR2+ inguinal white adipose tissue (ing-WAT) results in UCP1-independent thermogenesis via Ca2+ signaling leading to increased whole-body oxygen consumption rate and weight loss. (C) Blue light stimulation of bPAC+ brown adipocytes increase cAMP signaling leading to increased UCP1 expression, fuel uptake and heat production. Image created at BioRender.com.
Stimulation of UCP1-independent Fat Thermogenesis in Subcutaneous Adipose Tissue Using Wireless Optogenetics
Thermogenesis in adipose tissue can be stimulated by cold exposure, leading to the release of norepinephrine from the sympathetic nervous system. Norepinephrine activates the β3-adrenergic receptor (β3-AR) and its downstream signaling pathway, which triggers thermogenesis through various mechanisms, including expression and activation of UCP1. While efforts have been made to develop β3-AR agonists to promote adipose tissue thermogenesis as an anti-obesity treatment, these drugs often come with cardiovascular side effects such as high blood pressure [15,16]. A study by the Kajimura Laboratory (Tajima et. al. [33]) explored an alternative pathway to stimulate fat thermogenesis that may bypass these cardiovascular risks (Figure 1B). One such pathway is non-canonical UCP1-independent thermogenesis, previously identified in beige fat, which involves ATP-dependent calcium (Ca2+) futile cycling [14]. This particular study used a chemical stabilizer (S107) to enhance Ca2+ cycling and stimulate thermogenesis in mice, but couldn't confirm whether other tissues besides adipose tissue played a role. To address this limitation, the researchers developed a wireless optogenetics device that could be implanted adjacent to the subcutaneous adipose tissue of mice. The wireless optogenetics device consists of a small power-harvesting coil connected to a receiving circuit and a blue micro-LED to activate beige adipocytes expressing channelrhodopsin 2 (ChR2). The study optimized the wireless device's power transfer efficiency and ChR2 activation, identifying that a 5-millisecond pulse width and a 10 Hz frequency were ideal for stimulating intracellular Ca2+ influx in cultured beige adipocytes and without generating excessive heat when the device was implanted subcutaneously.
Activation of ChR2 in cultured beige adipocytes with blue light led to intracellular Ca2+influx, and this was mediated by L-type voltage-dependent Ca2+ channels. Inhibition of these channels with isradipine blocked the Ca2+ influx, confirming their involvement. Additionally, the study found that Ca2+ release from the endoplasmic reticulum via Ryanodine Receptor 2 and inositol 1,4,5-trisphosphate receptors was necessary for light-induced Ca2+ cycling. Furthermore, the activation of Ca2+ cycling in beige adipocytes was sufficient to stimulate thermogenesis, increase cellular oxygen consumption, and enhance glucose oxidation. Using Ucp1 null beige adipocytes, the researchers confirmed that this effect was UCP1-independent and primarily relied on SERCA2-mediated Ca2+ cycling.
Moving from cellular experiments to in vivo studies, the wireless optogenetics device was implanted under the skin adjacent to subcutaneous adipose tissue of adiponectin-Cre conditional mice expressing ChR2 specifically in adipocytes. Light activation of ChR2 in these mice led to a significant increase in adipose tissue temperature, demonstrating that the device could stimulate thermogenesis in vivo, even without cold exposure. The study also investigated the effects of light-induced thermogenesis on whole-body energy expenditure. Mice with the implanted device showed a significant increase in whole-body oxygen consumption without altering their physical activity, indicating enhanced thermogenesis and energy expenditure. Notably, this increase in energy expenditure was comparable to that achieved through pharmacological activation of β3-AR with CL316,243. Finally, the researchers tested whether optogenetic stimulation of thermogenesis could protect mice from diet-induced obesity. Mice with the wireless optogenetics device implanted in their adipose tissue were exposed to a high-fat diet (HFD). Light activation of thermogenesis for 10 minutes per day (10-Hz frequency and 5-ms pulse width) in these mice led to reduced body weight gain, specifically due to decreased fat mass, without affecting lean mass. The inguinal WAT of these mice was smaller, containing smaller adipocytes, and displayed no significant changes in inflammation or fibrosis markers.
In summary, this study demonstrates that wireless optogenetic stimulation of thermogenesis in adipose tissue can effectively increase energy expenditure and protect against diet-induced obesity in mice. The approach primarily relies on Ca2+ cycling through SERCA2 and represents a promising avenue for developing safe and effective treatments for obesity and related metabolic disorders.
Stimulation of UCP1-dependent Thermogenesis in Brown Adipocytes Using Optogenetics
While thermogenic adipose tissue inherently possesses anti-obesity attributes, our understanding of the regulatory mechanisms underlying UCP1-dependent thermogenesis remains incomplete. The control of UCP1 encompasses various tiers, spanning from its transcriptional initiation to the turnover of its mRNA and protein. Furthermore, its activation takes place within the inner mitochondrial membrane, where it remains inactive until β-adrenergic activation [34,35]. To initiate the transcription process, β-adrenergic receptors linked with Gs-alpha subunits first trigger the activation of membrane-associated adenylyl cyclases, prompting a surge in intracellular cAMP levels [36] (Figure 2A). This heightened cAMP concentration subsequently triggers the activation of cAMP-dependent protein kinase A (PKA), leading to lipolysis and consequent release of free fatty acids—an essential prerequisite for activation of UCP1-mediated proton leakage [37]. PKA's influence extends further to the phosphorylation of CREB and activation of a cluster of thermogenic transcription factors. Ultimately, this signaling cascade causes increased mitochondrial biogenesis, amplified electron transport chain activity, and the initiation of Ucp1 transcription (Figure 2A), which collectively allow thermogenesis to take place [38].
To determine if brown adipocytes could be genetically modified for light inducible activation of UCP1-dependent thermogenesis, our laboratory took advantage of a bacterial photoactivatable adenylyl cyclase (bPAC), previously cloned from the soil bacterium Beggiatoa for chemical free stimulation of adenylyl cyclase [39,40]. bPAC is made up of two parts: an N-terminal BLUF domain (blue light sensor using flavin adenine dinucleotide) and a C-terminal class IIIb adenylyl cyclase. The active site of these cyclases is created by two cyclase domains working together as a homodimer [41]. Upon stimulation with blue light, bPAC has been shown to increase cAMP levels and alter cellular physiology in cells from numerous species including Escherichia coli, Drosophila melanogaster (nervous system), Xenopus laevis (oocytes), Mus musculus (sperm), Rattus norvegicus (pyramidal neurons) and Homo sapiens (HEK-293T cells) [39,42,43].
In our study, brown preadipocytes derived from ThermoMouse [44] expressing the blue-light-sensitive bPAC protein were generated via lentiviral transduction and used to investigate the effects of blue light stimulation on cAMP signaling and gene expression related to brown adipocyte function (Figures 1C and 2A) (Doucette et. al. [40]). Blue light stimulation, achieved through a custom microplate photo irradiation system with blue LEDs, was used to activate brown preadipocytes [45]. We found that short blue light pulses (6 seconds to 1 minute @470nm) led to significant increases in cAMP levels, comparable to the effects of forskolin, a known stimulator of endogenous adenylyl cyclase. Importantly, this effect was specific to cAMP signaling, as there was no observed cGMP production after blue light stimulation. Blue light also triggered the phosphorylation of CREB and the expression of the immediate-early CREB target gene Nr4a3, indicating that bPAC activation could lead to transcriptional responses. We also explored the effects of different patterns of blue light stimulation on Ucp1 transcription in brown adipocytes. Our results show that continuous, uninterrupted blue light exposure (up to 6 hours) led to the highest levels of Ucp1 expression. These results were comparable to brown adipocytes treated with forskolin, suggesting that bPAC is as efficient as endogenous adenylyl cyclases in stimulating Ucp1 expression. Furthermore, the expression of genes related to mitochondrial biology, including those involved in mitochondrial biogenesis, ATP synthesis, electron transport, and mitophagy were increased, suggesting that light-induced stimulation of cAMP signaling promoted mitochondrial function in brown adipocytes. In cell culture, brown adipocytes typically lose UCP1 expression over time in the absence of stimuli activating downstream cAMP signaling. We found that intermittent optogenetic stimulation with daily blue light pulses of 30 minutes could prevent loss of UCP1 expression over time. We also examined the impact of blue light stimulation on fuel uptake and thermogenesis in brown adipocytes. Activation of bPAC with blue light led to increased fatty acid and glucose uptake and microcalorimetry measurements demonstrated that bPAC+ brown adipocytes produced significantly more heat (»4-fold) in response to blue light compared to control cells. We also confirmed the UCP1-dependent nature of bPAC-induced thermogenesis using short hairpin RNA to target Ucp1 for degradation, which resulted in a lack of light-induced heat production. Overall, these findings suggest that blue light stimulation of bPAC can promote UCP1-dependent thermogenesis in brown adipocytes, providing a strategy for regulating brown adipocyte activity. Next steps will be to generate in vivo mouse models to test this system for increased energy expenditure and weight loss.
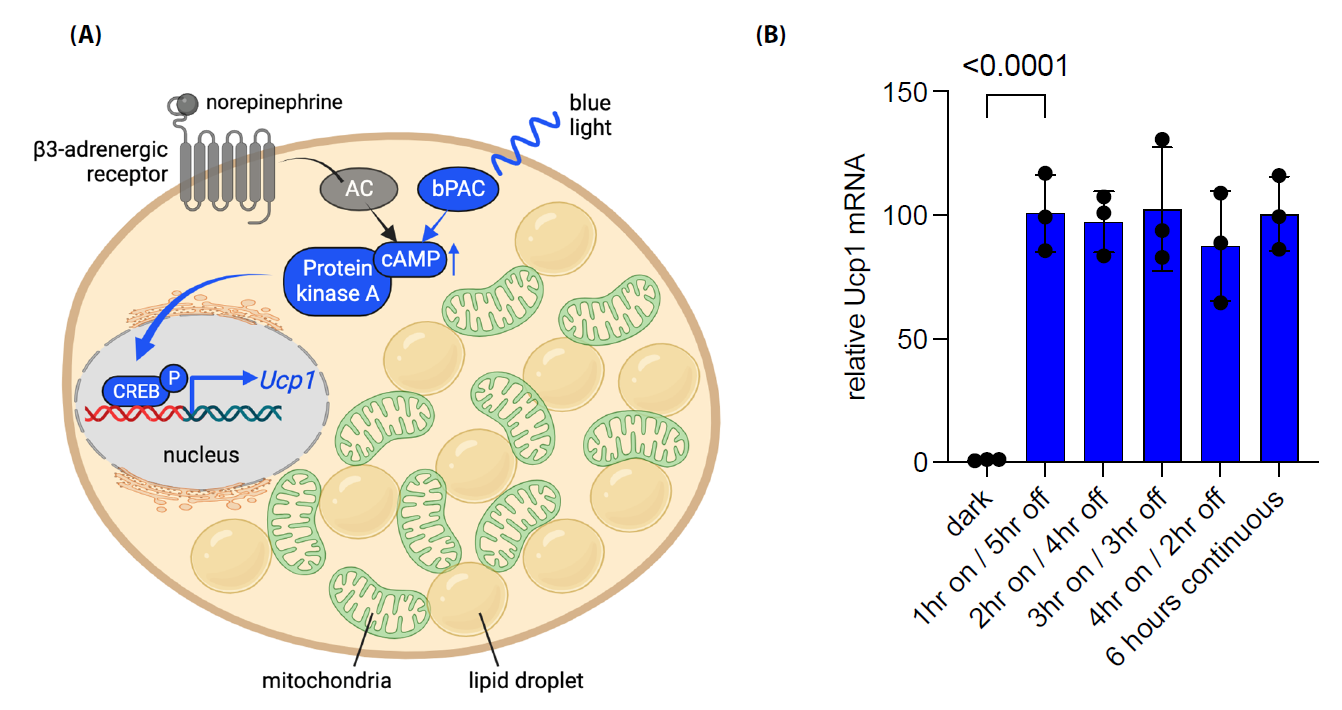
One lingering question regarding the use of bPAC-expressing brown adipocytes for weight loss pertains to the kinetics of brown adipocyte activation. This is particularly relevant as the induction of thermogenesis in bPAC+ brown adipocytes require longer pulses of blue light compared to the UCP1-independent ChR2 studies mentioned above. Prolonged exposure to blue light pulses to sustain Ucp1 expression could potentially lead to excessive heat generation via a wireless blue light LED, compromising cellular behavior or promoting tissue damage. An alternative hypothesis revolves around the idea that brown adipocytes might need continuous cAMP signaling to uphold thermogenesis in comparison to beige adipocytes. Recent experiments conducted in our laboratory on beige adipocytes transduced with bPAC have shed some light on this matter. One experiment revealed that Ucp1 expression reached its peak at the one-hour mark and remained elevated for an additional five hours in the absence of light (Figure 2B). This level of expression was similar to what was observed with six hours of uninterrupted blue light exposure in the brown adipocytes. This finding raises the possibility that beige adipocytes may have a lower activation threshold for UCP1-dependent thermogenesis through bPAC stimulation, although this hypothesis will require further validation. Future investigations will be performed to determine the minimal amount of blue light (<1 hour) required to maintain Ucp1 expression and sustain thermogenesis in beige adipocytes effectively. Additionally, exploring further modifications of PACs or discovering new PACs that exhibit heightened sensitivity may hold promise for achieving a more rapid induction of UCP1-dependent thermogenesis.
Figure 2. Blue light stimulation of bPAC leads to increased expression of Ucp1 in beige adipocytes. (A) Illustrative depiction of the process by which optogenetic stimulation of bPAC results in elevated Ucp1 expression in beige or brown adipocytes. Blue light initiates the activation of cytoplasmic bPAC, bypassing the necessity for norepinephrine-induced adipocyte stimulation via the b3-adrenergic receptor and its subsequent activation of endogenous adenylyl cyclase (AC). The bPAC signaling cascade elevates intracellular cAMP levels, leading to the activation of protein kinase A. This, in turn, facilitates the phosphorylation of CREB and its translocation into the nucleus, where it collaborates with other thermogenic transcription factors (not shown) to boost Ucp1 transcription. (B) SV40 large T antigen immortalized preadipocytes derived from mouse inguinal adipose tissue (Kerafast, Inc. Cat# EVC005) were differentiated into mature beige adipocytes for 9 days and pulsed with blue light at time zero for the indicated times and harvested at 6 hours as previously described [40,54]. qPCR data indicates that beige adipocytes induce and maintain Ucp1 expression for at least 5 hours with a single 1-hour pulse of blue light (470nm). Data are normalized to b-actin expression. Error bars represent ± standard deviation with one-way ANOVA analysis p< 0.0001 indicated. Figure A created at BioRender.com and modified from Doucette et al. [40].
Navigating the Challenges of Optogenetics-based Therapies and Obesity Management
Technical challenges must be overcome to transition optogenetics from a research tool to a viable therapeutic approach for human diseases. Currently, only four early-phase clinical trials are in progress, all focused on treating retinitis pigmentosa [46]. The available data, though limited, indicates favorable tolerability of the optogenetic gene therapy [46,47]. The accessibility of the eye to light makes optogenetics promising for visual conditions, but trials for other conditions may require years for the development of safe implantable light sources. The success of optogenetics in humans depends on optimizing gene delivery methods, particularly viral vectors like adeno-associated viruses (AAVs), while addressing concerns about immune reactions, delivery precision, and safety [48]. One primary challenge is delivering light in a manner suitable for human-scale tissue volumes. Enhancements in optogenetic tools, including increased sensitivity to light and the use of far-red light to minimize tissue damage, offer potential solutions [48]. Optogenetic techniques may have unintended off-target effects on neighboring cells or tissues, and achieving precise targeting of specific cells remains an ongoing challenge. Blue light, commonly used for optogenetic activation, has limited tissue penetration, and requires transplantation of blue-light transmitters to target internal tissues [33,49]. The development of far-red optogenetic proteins may eliminate the need for wireless transmitters since an external source of red light can penetrate deep into tissues [48,50]. However, the long-term stability and viability of optogenetic tools within the body are not understood, posing a concern for therapeutic applications. Manipulating cellular processes can lead to unpredictable consequences, and the potential side effects and unintended outcomes must be addressed. The transition from experimental use in animals to clinical applications in humans will involve regulatory challenges, safety testing, and ethical considerations, making clinical translation a complex and time-consuming process [48]. As optogenetic methods advance, therapeutic applications should gradually make their way into clinical trials.
The biology of adipose tissue could help circumvent numerous challenges associated with optogenetic therapies in other organ systems and tissues. Unlike the targeting of native cells, such as specific neuronal subsets in the spinal cord or brain, brown and beige adipose tissue has the potential to be transplanted to facilitate weight loss. Patient-specific beige adipose tissue can be generated in the laboratory from adipose-derived stem cells (ADSCs) that are obtainable through tissue biopsy or liposuction [51,52]. Transgenic modification of ADSCs with optogenetic genes and their subsequent differentiation into beige adipocytes could be performed in the laboratory prior to reintroduction into patients. Studies confirm the successful vascularization of human-derived beige adipose tissue, which has been shown to enhance systemic glucose tolerance when transplanted in mice [53]. One drawback to the use of ADSCs derived from lipoaspirates is that they might have limited expansion and differentiation capacity, especially when derived from patients with metabolic disease [54]. Patient-specific induced pluripotent stem cells (iPSCs) provide an alternative source for brown and beige adipocytes, ensuring a replenishable, unlimited supply of material for weight loss applications [54-56]. Furthermore, recently developed non-immunogenic iPSCs may mitigate concerns regarding immunogenicity related to the display of optogenetic peptides on transplanted cells, while eliminating the need for patient-specific derivation [57]. These adipose tissue sources offer solutions to challenges posed by transgene delivery via AAVs, which may result in unintended effects on adjacent cell types or tissues. Estimations suggest that 50 grams of activated brown adipose tissue can potentially burn up to 500 calories daily, implying that the amount of material needed for transplantation may be relatively modest [58]. Moreover, subcutaneous transplantation offers the advantage of minimal invasiveness, with the possibility of repeated tissue replacement over time. While adipose tissue may offer certain advantages over the targeting other disease-related tissues with optogenetics technology, the challenges associated with its clinical translation remain significant and necessitate thorough consideration.
Summary and Future Perspectives
The innate thermogenic capabilities and resistance to metabolic syndrome conferred by brown and beige adipose tissues make them suitable candidates for therapies for combating obesity and related metabolic disorders. Our understanding of brown and beige adipocyte signaling pathways, particularly UCP1-dependent and UCP1-independent mechanisms, sheds light on the potential for precise modulation of energy expenditure. The emergence of optogenetics as a revolutionary tool for cellular control adds an exciting dimension to this quest. Its ability to harness light-sensitive proteins for temporal and spatial control offers new avenues for manipulating brown and beige adipocytes. The studies highlighted here demonstrate the feasibility of inducing thermogenesis in these cells using optogenetic techniques, offering insights into signaling mechanisms and promising strategies for weight management and metabolic health. However, challenges remain, including optimizing the kinetics of brown adipocyte activation and the discovery of new, highly sensitive optogenetic proteins that can be used to sustain thermogenesis. These questions underscore the need for further research and refinement of optogenetic approaches. The future holds exciting possibilities as we continue to unlock the therapeutic potential of these remarkable tissues and cutting-edge optogenetics, however, more research is needed to translate this technology into clinical application. Overall, optogenetics offers a promising prospect of personalized interventions for individuals struggling with obesity while expanding our understanding of cellular regulation and energy balance in adipose tissues.
Conflicts of Interest
There are no conflicts of interest.
Funding Statement
This work was supported by NIH COBRE award P20GM121301 (A. Brown, L. Liaw, and C.J. Rosen) and NIDDK award 1R01DK124261 (A. Brown). The project utilized services of Core Facilities funded by NIH COBRE award P20GM106391 (R. Friesel, PI) and NIH award U54GM115516 (C.J Rosen, PI).
Acknowledgments
I kindly thank Julieta Martino for assistance and expertise in reviewing and providing constructive feedback on this review article.
References
2. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nature Medicine. 2013 Oct;19(10):1252-63.
3. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009 Apr 9;360(15):1509-17.
4. Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown?. Genes & Development. 2013 Feb 1;27(3):234-50.
5. Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, et al. Brown adipose tissue is associated with cardiometabolic health. Nature Medicine. 2021 Jan;27(1):58-65.
6. Cannon B, Nedergaard JA. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277-359.
7. Zhang F, Hao G, Shao M, Nham K, An Y, Wang Q, et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metabolism. 2018 Jan 9;27(1):252-62e3.
8. Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. American Journal of Physiology-Endocrinology and Metabolism. 2014 Apr 15;306(8):E945-64.
9. Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002 Aug 2;297(5582):843-5.
10. Wade G, McGahee A, Ntambi JM, Simcox J. Lipid transport in brown adipocyte thermogenesis. Frontiers in Physiology. 2021 Dec 23;12:787535.
11. Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Hormone Molecular Biology and Clinical Investigation. 2014 Jul 1;19(1):25-37.
12. Roh HC, Tsai LT, Shao M, Tenen D, Shen Y, Kumari M, et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metabolism. 2018 May 1;27(5):1121-37e5.
13. Cheng L, Wang J, Dai H, Duan Y, An Y, Shi L, et al. Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus. Adipocyte. 2021 Jan 1;10(1):48-65.
14. Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature Medicine. 2017 Dec 1;23(12):1454-65.
15. Vosselman MJ, Van der Lans AA, Brans B, Wierts R, Van Baak MA, Schrauwen P, et al. Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012 Dec 1;61(12):3106-13.
16. Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metabolism. 2015 Jan 6;21(1):33-8.
17. Sui W, Li H, Yang Y, Jing X, Xue F, Cheng J, et al. Bladder drug mirabegron exacerbates atherosclerosis through activation of brown fat-mediated lipolysis. Proceedings of the National Academy of Sciences. 2019 May 28;116(22):10937-42.
18. Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, et al. The microbial opsin family of optogenetic tools. Cell. 2011 Dec 23;147(7):1446-57.
19. Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences. 2003 Nov 25;100(24):13940-5.
20. Berlin S, Isacoff EY. Synapses in the spotlight with synthetic optogenetics. EMBO Reports. 2017 May;18(5):677-92.
21. Lanyi JK. Halorhodopsin: a light-driven chloride ion pump. Annual Review of Biophysics and Biophysical Chemistry. 1986 Jun;15(1):11-28.
22. Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PloS One. 2007 Mar 21;2(3):e299.
23. Chen Y, Xiong M, Zhang SC. Illuminating Parkinson's therapy with optogenetics. Nature Biotechnology. 2015 Feb;33(2):149-50.
24. Paz JT, Huguenard JR. Optogenetics and epilepsy: past, present and future: shedding light on seizure mechanisms and potential treatments. Epilepsy Currents. 2015 Jan;15(1):34-8.
25. Muir J, Lopez J, Bagot RC. Wiring the depressed brain: optogenetic and chemogenetic circuit interrogation in animal models of depression. Neuropsychopharmacology. 2019 May;44(6):1013-26.
26. Ehmann N, Pauls D. Optogenetics: Illuminating neuronal circuits of memory formation. Journal of Neurogenetics. 2020 Jan 2;34(1):47-54.
27. Mirzayi P, Shobeiri P, Kalantari A, Perry G, Rezaei N. Optogenetics: Implications for Alzheimer's disease research and therapy. Molecular Brain. 2022 Dec;15(1):20.
28. Sakai D, Tomita H, Maeda A. Optogenetic therapy for visual restoration. International Journal of Molecular Sciences. 2022 Nov 30;23(23):15041.
29. Yan B, Viswanathan S, Brodie SE, Deng WT, Coleman KE, Hauswirth WW, Nirenberg S. A clinically viable approach to restoring visual function using optogenetic gene therapy. Molecular Therapy-Methods & Clinical Development. 2023 Jun 8;29:406-17.
30. Tan P, He L, Han G, Zhou Y. Optogenetic immunomodulation: shedding light on antitumor immunity. Trends in Biotechnology. 2017 Mar 1;35(3):215-26.
31. Sasse P, Funken M, Beiert T, Bruegmann T. Optogenetic termination of cardiac arrhythmia: mechanistic enlightenment and therapeutic application?. Frontiers in Physiology. 2019 Jun 6;10:675.
32. Lyons C, Razzoli M, Larson E, Svedberg D, Frontini A, Cinti S, et al. Optogenetic-induced sympathetic neuromodulation of brown adipose tissue thermogenesis. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2020 Feb;34(2):2765.
33. Tajima K, Ikeda K, Tanabe Y, Thomson EA, Yoneshiro T, Oguri Y, et al. Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis. Nature Communications. 2020 Apr 7;11(1):1730.
34. Oeckl J, Bast-Habersbrunner A, Fromme T, Klingenspor M, Li Y. Isolation, culture, and functional analysis of murine thermogenic adipocytes. STAR Protocols. 2020 Dec 18;1(3):100118.
35. Li Y, Fromme T. Uncoupling protein 1 does not produce heat without activation. International Journal of Molecular Sciences. 2022 Feb 22;23(5):2406.
36. Dickson LM, Gandhi S, Layden BT, Cohen RN, Wicksteed B. Protein kinase A induces UCP1 expression in specific adipose depots to increase energy expenditure and improve metabolic health. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2016 Jul 1;311(1):R79-88.
37. Evans BA, Merlin J, Bengtsson T, Hutchinson DS. Adrenoceptors in white, brown, and brite adipocytes. British Journal of Pharmacology. 2019 Jul;176(14):2416-32.
38. Tong T, Shen Y, Lee HW, Yu R, Park T. Adenylyl cyclase 3 haploinsufficiency confers susceptibility to diet-induced obesity and insulin resistance in mice. Scientific Reports. 2016 Sep 28;6(1):34179.
39. Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, et al. Light Modulation of Cellular cAMP by a Small Bacterial Photoactivated Adenylyl Cyclase, bPAC, of the Soil Bacterium Beggiatoa*♦. Journal of Biological Chemistry. 2011 Jan 14;286(2):1181-8.
40. Doucette CC, Nguyen DC, Barteselli D, Blanchard S, Pelletier M, Kesharwani D, et al. Optogenetic activation of UCP1-dependent thermogenesis in brown adipocytes. Iscience. 2023 Apr 21;26(4):106560.
41. Stierl M, Penzkofer A, Kennis JT, Hegemann P, Mathes T. Key residues for the light regulation of the blue light-activated adenylyl cyclase from Beggiatoa sp. Biochemistry. 2014 Aug 12;53(31):5121-30.
42. Jansen V, Alvarez L, Balbach M, Strünker T, Hegemann P, Kaupp UB, et al. Controlling fertilization and cAMP signaling in sperm by optogenetics. Elife. 2015 Jan 20;4:e05161.
43. Tanwar M, Khera L, Haokip N, Kaul R, Naorem A, Kateriya S. Modulation of cyclic nucleotide-mediated cellular signaling and gene expression using photoactivated adenylyl cyclase as an optogenetic tool. Scientific Reports. 2017 Sep 21;7(1):12048.
44. Galmozzi A, Sonne SB, Altshuler-Keylin S, Hasegawa Y, Shinoda K, Luijten IH, Chang JW, Sharp LZ, Cravatt BF, Saez E, Kajimura S. ThermoMouse: an in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Reports. 2014 Dec 11;9(5):1584-93.
45. Katz S, Backeris P, Merck C, Suprun M, D'Souza S, Bishop DF, et al. Design and validation of an open-source modular Microplate Photoirradiation System for high-throughput photobiology experiments. PLoS One. 2018 Oct 5;13(10):e0203597.
46. White M, Whittaker RG. Post-Trial Considerations for an Early Phase Optogenetic Trial in the Human Brain. Open Access Journal of Clinical Trials. 2022;14:1-9.
47. Sahel JA, Boulanger-Scemama E, Pagot C, Arleo A, Galluppi F, Martel JN, et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nature Medicine. 2021 Jul;27(7):1223-1229. 48.
48. Shen Y, Campbell RE, Cote DC, Paquet ME. Challenges for Therapeutic Applications of Opsin-Based Optogenetic Tools in Humans. Frontiers in Neural Circuits. 2020 Jul;14:41.
49. Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nature Methods. 2015 Oct;12(10):969-74.
50. Ye H, Fussenegger M. Optogenetic Medicine: Synthetic Therapeutic Solutions Precision-Guided by Light, Cold Spring Harbor Perspectives in Medicine. 2019 Sep 3;9(9):a034371.
51. Singh AM, Zhang L, Avery J, Yin A, Du Y, Wang H, et al. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nature Communications. 2020 Jun 2;11(1):2758.
52. Gonzalez Porras MA, Stojkova K, Acosta FM, Rathbone CR, Brey EM. Engineering Human Beige Adipose Tissue. Frontiers in Bioengineering and Biotechnology. 2022 Jul 1;10:906395.
53. Min SY, Kady J, Nam M, Rojas-Rodriguez R, Berkenwald A, Kim JH, et al. Human 'brite/beige' adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nature Medicine. 2016 Mar;22(3):312-8.
54. Su S, Guntur AR, Nguyen DC, Fakory SS, Doucette CC, Leech C, et al. A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome. Cell Reports. 2018 Dec 11;25(11):3215-3228.e9.
55. Brown AC. Brown adipocytes from induced pluripotent stem cells-how far have we come? Annals of the New York Academy of Sciences. 2020 Mar;1463(1):9-22.
56. Butts JC, Martino J, Brown AC. Insights into current models for developing brown adipocytes from induced pluripotent stem cells. Advances in Stem Cell Biology. 2021;10:95-115.
57. Shani T, Hanna JH. Universally non-immunogenic iPSCs. Nature Biomedical Engineering. 2019 May;3(5):337-8.
58. Yang JP, Anderson AE, McCartney A, Ory X, Ma G, Pappalardo E, et al. Metabolically Active Three-Dimensional Brown Adipose Tissue Engineered from White Adipose-Derived Stem Cells. Tissue Engineering Part A. 2017 Apr;23(7-8):253-62.