Abstract
The coronavirus disease 2019 drastically impacted solid organ transplantation. Lacking scientific evidence, very stringent but presumably safer policies were imposed on organs donation and solid organ transplantation in the early stages of the pandemic. A priori policies and practices required a negative SARS-Cov-2 real-time polymerase chain reaction (PCR) of donors and recipients. Unfortunately, prolonged viral RNA shedding in candidates recovering from SARS-CoV-2 frequently hinders donation and transplantation. These a priori restrictive donation and transplant guidelines must be reevaluated and adjusted according to accumulating medical knowledge and detrimental consequences of stringency. Recent data reveal the devastating impact of stringency on waitlist time, disease progression, drop-out, and mortality. Moreover, positive PCR test results for viral genome are frequently due to non-infectious and prolonged convalescent shedding of viral genome and the cycle threshold of quantitative PCR could be leveraged to inform clinical transplant decision-making. In late 2020 we presented an evidence-adjusted significantly less restrictive policy for LT, where risk tolerance is tiered to recipient acuity. This review summarizes the evolution of policies and practices for organ recovery and transplantation in candidates recovering from COVID-2019, since then. Leniency was, subsequently, introduced into several societal and governmental recommendations for organ donation and transplantation. However, serious analytical considerations limit the use of cycle threshold to local institutional algorithms.
Keywords
SARS-CoV-2, Solid organs transplant, Polymerase chain reaction, Cycle threshold, Non-infectious shedding, Organ donation, Guidelines
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SOT: Solid Organs Transplant; COVID-19: Coronavirus Disease 2019; FDA: Food and Drug Administration; PCR: Real-time reverse transcriptase Polymerase Chain Reaction; CT: Cycle Threshold; OPTN: Organ Procurement and Transplantation Network; TTS: The Transplantation Society; ASTS: American Society of Transplant Surgeons; ISHLT: The International Society of Heart and Lung Transplantation; UNOS: United Network for Organ Sharing; AOPO: Association of Organ Procurement Organizations; AST: American Society of Transplantation
Introduction
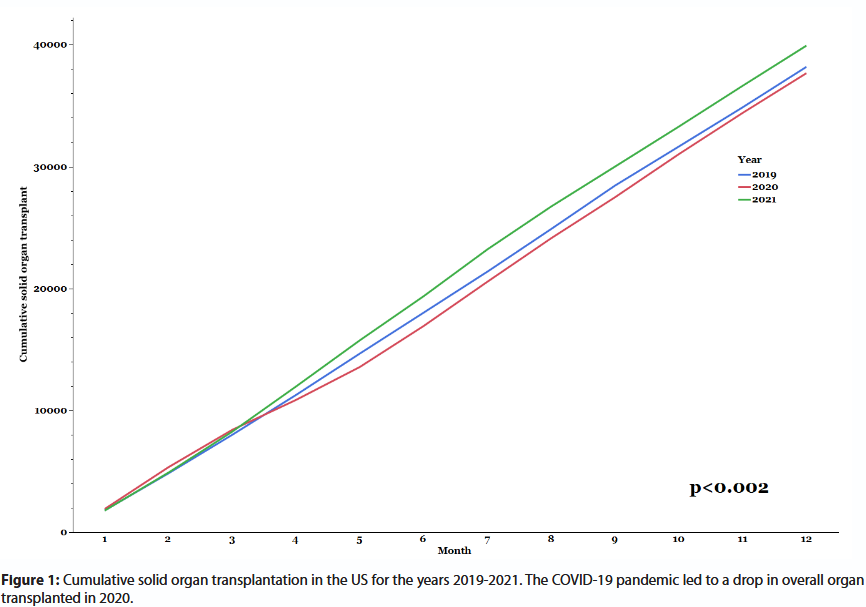
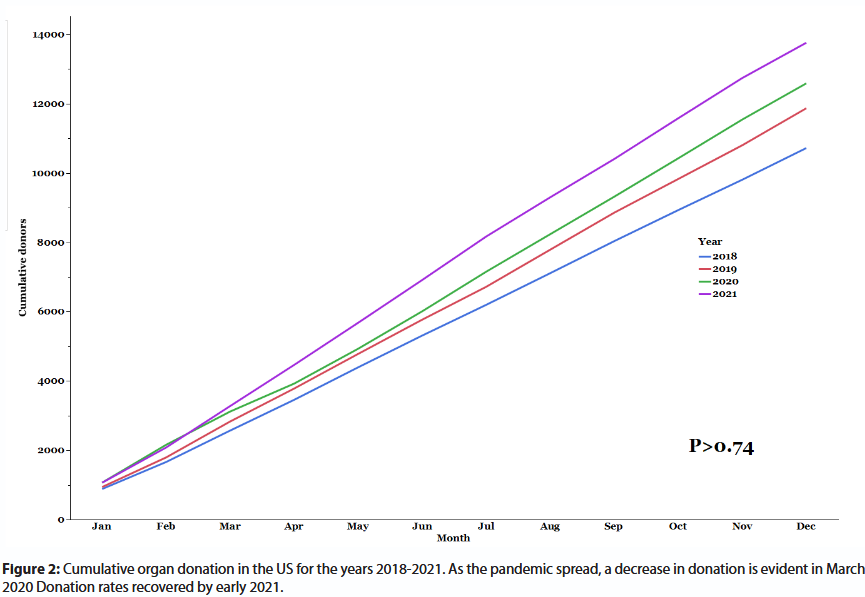
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) pandemic affected Solid-Organ transplantation (SOT) policies and practices worldwide. The medical sector had to adapt to overwhelming concerns regarding patient care, infection-control, healthcare workers’ safety, and limited healthcare resources. Transplant-specific concerns included donors and recipients with unrecognized SARS-CoV-2 infection, diminished post-transplant immunity, and infection-control of transplant candidates in the face of waitlist mortality [1]. Common adjustments included testing all recipient and donor candidates for Coronavirus disease 2019 (COVID-19), utilizing virtual telemedicine platform in lieu of in-person pre- and posttransplant office visits, and most importantly reserving ongoing SOT to lifesaving procedures for candidates with life-threating end-stage organ failure [2]. The U.S. Food and Drug Administration (FDA) authorized the emergency use of SARS-CoV-2 real-time polymerase chain reaction (PCR) tests as qualitative, binary—positive / negative—assays. Accordingly, prevailing policies require a negative SARS-CoV-2 PCR of donors and recipients. As the pandemic unfolded in early 2020 the scarcity of scientific knowledge compelled transplant policymakers to impose stringent but presumably safer policies for organs retrieval and SOT [1-3]. The inevitable consequence was a decrease in SOT volume, and an ensuing increase in waitlist time, disease progression, drop-out, and mortality [4,5]. Some European countries reported as high as an 80% drop in transplant rates [6]. In a recent US survey, most transplant programs reported a decrease in SOT volume [7]. Figure 1 presents US data for SOT in the years 2019-2021. In early 2020 SOT volume exceeded that of the corresponding period in 2019. However, in the second quarter of 2020 the pandemic spread nationwide, and SOT decreased significantly, and only caught up with 2019 rates in 2021. Organ retrieval showed similar trend (Figure 2). Fortunately, our medical knowledge and understanding of COVID-19 grew rapidly; regrettably, societal and governmental agencies were slow to adjust a priori transplant policies. In late 2020, upon a thorough literature review, our group proposed an evidence-adjusted and significantly less restrictive policy for liver transplantation, where risk tolerance is tiered to recipient acuity (see Supplementary File 1). Pertinent to the proposed policy are the following premises: (i) convalescent positive PCR results are common, especially in transplant candidates with end-organ failure-associated immune dysfunction [8-10]; (ii) prolonged shedding of viral genome is frequently non-infectious; and (iii) the cycle threshold (CT) of quantitative PCR could be leveraged to go beyond the binary test results paradigm, and inform clinical transplant decision-making. The proposed leniency, consequentially, permitted reasonably safe organ retrieval and transplantation in PCR-positive candidates recovering from COVID-19. In this review we will update on evolving transplantation policies and practices since then.
Cycle Threshold
The CT value indicates the number of amplification cycles needed to reach a threshold at which a molecular diagnostic PCR test become detectable above background [11]. CT comes with several caveats: (i) CT values differ between platforms, lots of the same platform, and between SARS-Cov-2 genes; (ii) PCRs of respiratory specimens are qualitative in nature, are not calibrated by standardized controls, and lack linearity between CT values and quantity of viral genome in the specimen; lastly, (iii) CT values are poorly reproducible [12].
In addition to the analytical quantitation and precision limitations of qualitative PCRs, CT values are also affected by patient’s and specimen’s factors [12]. Some experts, therefore, hold that CT value should not be routinely reported, rather be provided verbally to inquiring clinicians along with precautionary discussion of implementing CT values in clinical decisions [13]. Nevertheless, low CT values frequently correlate with worse outcomes [14], while increasing CT values imply diminishing infectivity [12,15]. As a result, CT is extrapolated by many clinicians to reflect viral density, and as such, have been employed to predict disease progression, and to discriminate active viral replication and transmissibility from prolonged non-infectious shedding of viral genome [11]. The paradigm shift from the binary interpretation of PCR results was further strengthen in December 2020, when the FDA permitted the laboratory reporting of CT values for authorized molecular diagnostic SARS-CoV-2 tests—a move that likely hasten the clinical utilization of CT [16].
SOT in Candidates Convalescing from SARS-CoV-2
Recommendations of societal and governmental agencies must be evidence-based and circumspective as they establish clinical practices and entail liability. A formal shift in guidelines and policies toward leniency require incontrovertible literature to substantiate its safety. However unequivocal data that promote professional consensus are slow to form and accumulate. The ever-changing SARS-CoV-2 virulence, transmissibility, and vaccine-resistance of the rapidly evolving variants further thwarted a change in formal transplant policies. At times the unforeseen magnitude of detrimental consequences of stringency instigates and warrants a change in a priori restrictive guidelines. In Europe more than 60 thousand vulnerable patients lost transplant opportunities due to restrictive transplant policies, and inevitably suffered disease burden and unquantified excess waitlist mortality [6]. In the US, however, the heterogeneous impact of SARS-Cov-2 on waitlist mortality did not prompt for a policy change, as it varied according to the waited organ and candidate’s geography, age, sex and ethnicity [17,18]. Lacking formal guidelines for SOT in candidates with a COVID-19 positive PCR result, transplant centers had to independently balance the risk of waitlist drop-out or mortality versus transplantation on a case-by-case basis. This practical approach matches SOT risk tolerance to recipient acuity, and inexorably results in non-uniform local practices. To our best knowledge we reported the first two cases of liver transplant in SARS-CoV-2 PCR positive convalescing candidates [1]; case-reports and case-series of all abdomino-thoracic SOTs performed in PCR-positive convalescing recipients followed suit from across the globe, and usually with favorable outcomes [19-28]. In addition, larger series of SOT in SARS-CoV-2 PCR negative convalescing candidates ascertained the safety of transplantation within days or weeks after COVID-19 infection [29-33].
Organ Retrieval from Donors Convalescing from SARS-Cov-2
Due to risk of transmissibility to recipients or healthcare workers, early organ procurement organization (OPO) policies required a negative upper respiratory specimen SARS-CoV-2 PCR result within 72 hours, but preferably as close as possible to organ recovery, along with symptoms free recovery period after active infection [1,34]. PCR negative donor-derived transmission to non-lung SOT recipients was not reported despite the risk of undetected infection due to a false negative result [35]. The Organ Procurement and Transplantation Network (OPTN), a private, not-for-profit entity chartered with the operation and oversight of organ allocation and transplantation in the US, recently updated its guidelines [35]. According to the new guidelines, organ recovered from convalescing donor with PCR-positive result 21-90 days after onset of COVID-19 are unlikely to transmit infection. Infectious diseases experts can offer valuable expertise in assessing the likelihood of transmissibility. These organs are usually deemed acceptable for transplantation considering recipient risk of mortality or further complications while on the waitlist. However, long-term outcomes for organs recovered from these donors are still unknown. The safety of organs recovered from asymptomatic donors with persistence of PCR positive results within 11-20 days after mild COVID-19 is unknown. It is believed that these donors are unlikely to transmit COVID-19 and may be considered for acutely ill non-lung recipients. Safety of organs from PCR positive donor >90 days after onset is still unknown and caution should be exercised, since PCR positivity may reflect re-infection and transmissibility. Transmissibility of organ recovered from PCR-positive donors with unknown onset of COVID-19 is unknown, and infectious diseases experts should be consulted. To dates hundreds of organs from PCR positive donors were successfully grafted [35].
Societal and Governmental Guidelines for SOT in Candidates Recovering from COVID-19
Matching transplant urgency of a critically ill candidate convalescing from a recent SARS-Cov-2 infection and the risk of residual active infection always entails intimate knowledge of candidate’s illness, and therefore can only be determined locally by candidate’s health care providers. As a result, detailed general recommendations for SOT in PCR-positive candidates are not practical. Additionally, the implementation of CT values in clinical guidelines necessitate delineation of cut-off values above which the patient may be deemed non-infectious. Since CT cut-off values differ between lots, platforms and viral genes, non-local guidelines usually refrain from including CT in clinical transplant recommendations. As a result, current leniency in societal and governmental guidelines for SOT, rarely refer to CT values. Familiarly with institutional PCR platform, however, allows for implementation of CT in local clinical decision-making algorithms. Supplementary file 2 presents current COVID-19 guidelines of the Miami Transplant Institute and exemplifies the practicality of inclusion of CT values in local institutional clinical algorithms.
The World Health Organization provides recommendations for vaccination in immunocompromised persons, but otherwise remains silent in regard to SOT [36]. The International Liver Transplant Society (“ILTS”) and The European Society for Organ Transplant (“ESOT”) also refrain from providing SOT guidelines, but hold interval educational webinars with updates and controversies concerning organ donation and transplantation and COVID-19 [37].
The Transplantation Society (TTS) and the American Society of Transplant Surgeons (ASTS) updated their transplantation guidelines most recently in June 2020 [38,39]. Therefore, current TTS and ASTS guidelines still reflects transplant practices of the early stages of the pandemic. Accordingly, these societies recommend that in an ill candidate with SARS-CoV-2, transplant should be deferred until clinical improvement and no virus detected (ideally with two negative PCR results 24 hours apart, 10-14 days after symptom onset and only once symptoms have resolved), unless transplantation is urgent.
The International Society of Heart and Lung Transplantation (ISHLT) revised its guidelines in February 2021, and like our algorithm, recommends a case-by-case approach that balances the risks of viral transmission and waitlist mortality. CT values play no practical role in ISHLT’s recommendations [40].
The Disease Control and Prevention (“CDC”) and FDA currently and unequivocally recommend against the use of CT values for assessment of an individual’s degree of infectivity or risk for disease severity [35].
The OPTN updated its guidelines for testing and organ recovery from donor candidates on January 2022 in a summary of current evidence composed by its Ad Hoc Disease Transmission Advisory Committee (“DTAC”) [35]. The update reflects the pertinent considerations and leniencies in our original algorithm [1]. A summary of evidence for the safety of SOT in a PCR-positive recipients recovering from COVID-19 is not available.
The National Institutes of Health refers guidance for SOT to transplant professional organizations, while it recommends that organ recovery should be deferred if infection is strongly suspected [35].
The United Network for Organ Sharing (UNOS)—a private, non-profit organization under federal contract to operate OPTN—holds webinars and town hall meetings regarding COVID-19, but refers to the Association of Organ Procurement Organizations (AOPO) ’s bulletin, and to the American Society of Transplantation (AST) for transplantation and donation guidelines, respectively [41]. Last updated on July 2020, the AOPO bulletin still present the stringent donor guidelines of the early pandemic [42]. The AST’s updated guidelines for donation (July 2021) and transplantation (January 2022) are similar to those of the ISHLT [43,44]. Professional organizations recommendation is summarized in Table 1.
| Organretrieval | |
| OPTN | Non-lung organ recovery from convalescing donor with PCR-positive result 21-90 days after onset of COVID-19 is acceptable |
| Safety of organs recovered from asymptomatic donors with persistence of PCR positive results within 11-20 days after mild COVID-19 is unknown. It is believed that these donors are unlikely to transmit COVID-19 and may be considered for acutely ill non-lung recipients | |
| Safety of organs from PCR positive donor >90 days after onset is unknown and caution should be exercised | |
| Organ recovered from PCR-positive donors with unknown onset of COVID-19 is unknown, and infectious diseases experts should be consulted | |
| NIH | Organ recovery should be deferred if infection is strongly suspected |
| Solid organ transplantation | |
| WHO | No recommendations regarding solid organ transplantation |
| ESOT/ILTS | No specific recommendations, but hold educational webinars |
| TTS | Transplant should be deferred until clinically improved with no detectable virus. Documentation of negative PCR testing at least 24 hours apart is recommended before a candidate should be cleared for transplant unless the need for transplant is urgent |
| ASTS | PCR testing immediately (within 12 hours) prior to organ transplantation; if positive, the procedure should be postponed until/if the recipient clears the virus |
| ISHLT/AST | Case-by-case approach that balances the risks of viral transmission and waitlist mortality |
| NIH | As per transplant professional organizations |
| UNOS | As per transplant professional organizations, but holds webinars and town hall meetings |
| Local | CT value may be implemented by infectious disease experts to imply diminishing infectivity. Risk tolerance is tiered to recipient acuity. |
OPTN: Organ Procurement and Transplantation Network; NIH: National Institutes of Health; ESOT: European Society for Organ Transplant; ILTS: International Liver Transplant Society; TTS: The Transplantation Society; ASTS: American Society of Transplant Surgeons; ISHLT: The International Society of Heart and Lung Transplantation; AST: American Society of Transplantation; UNOS: United Network for Organ Sharing
Table 1: Organ recovery and transplantation in candidates recovering from COVID-19.
Clinical Applicability of New Knowledge
The OPTN along with relevant stakeholders recently provided a practical summary for non-lung organ retrieval from PCR positive donors. Accordingly, a PCR positive asymptomatic donor or a symptomatic candidate more than 10 days after onset and with resolution of symptoms may be considered, as per recipient acuity and infectious disease consultation.
Guidelines for transplantation of a PCR positive recipient vary considerably between professional societies and transplant centers. Some guidelines require a negative PCR prior to transplantation. However, they all agree with a case-by-case approach that balances the risks of viral transmission and waitlist mortality.
Summary
The early stages of SARS-Cov-2 pandemic brought forth unprecedented challenges to SOT across the globe. The limited and inevitably very restrictive guidance offered by societal and governing agencies severely incapacitated SOT. Accumulated medical knowledge regarding the minimal risk of transmissibility in SARS-CoV-2 PCR positive convalescing patients, enabled a gradual and cautious leniency in guidelines. Due to significant limitations of qualitative PCRs, CT values appear to continue play a role only in local institutional clinical algorithms; non-local guidelines usually refrain from or advise against the inclusion of CT in clinical decision-making. In patients recovering from a recent COVID-19 infection, detailed guidelines are available for donor candidates, while only general recommendations are made for recipient candidates. Some major transplant professional societies still refrain from providing SARS-Cov-2 recommendations.
Funding Information
No funding has been received for this study.
Conflict of Interest
The authors have no conflict of interest to disclose.
References
2. Ritschl PV, Nevermann N, Wiering L, et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: A By-proxy Society Recommendation Consensus approach. American Journal of Transplantation. 2020;20(7):1826-1836.
3. Di Maira T, Berenguer M. COVID-19 and liver transplantation. Nat Rev Gastroenterol Hepatol. Sep 2020;17(9):526-528. Ahn C, Amer H, Anglicheau D, Ascher N, Baan C, Bat-Ireedui B, et al. Global Transplantation COVID Report March 2020. Transplantation. Oct 2020;104(10):1974-1983.
4. Vitale G, Gitto S, Marra F, Morelli MC. From advanced disease to transplantation: an overview of the liver at the time of COVID-19 pandemic. Intern Emerg Med. Jan 2022;17(1):15-24.
5. EU National Competent Authorities on Organ donation and transplantation. Organ Donation and Transplantation and the COVID-19 pandemic. Updated 03/07/2020. Accessed 04/07/2022, 2022. https://ec.europa.eu/health/sites/default/files/blood_tissues_organs/docs/organs_ncastatement_covid19_en.pdf
6. Gonzalez AJ, Kapila N, Thomas E, Pinna A, Tzakis A, Zervos XB. Managing liver transplantation during the COVID-19 pandemic: A survey among transplant centers in the Southeast United States. World J Hepatol. Dec 27 2021;13(12):2161-2167.
7. Albillos A, Lario M, Alvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. Dec 2014;61(6):1385-96.
8. Bhat TA, Panzica L, Kalathil SG, Thanavala Y. Immune Dysfunction in Patients with Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. Nov 2015;12 Suppl 2(Suppl 2):S169-75.
9. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. Sep 2008;3(5):1526-33.
10. Raveh Y, Simkins J, Nicolau-Raducu R. Liver transplantation in COVID-19 positive patients. American Journal of Transplantation. 2020 Nov 1.
11. Infectious Diseases Society of America. IDSA and AMP joint statement on the use of SARS-CoV-2 PCR cycle threshold (Ct) values for clinical decision-making. Updated 03/12/2021. Accessed 04/04/2022, 2022. https://www.idsociety.org/globalassets/idsa/public-health/covid-19/idsa-amp-statement.pdf
12. Binnicker MJ. Challenges and Controversies to Testing for COVID-19. J Clin Microbiol. Oct 21 2020;58(11):e01695-20.
13. Rao SN, Manissero D, Steele VR, Pareja J. A Systematic Review of the Clinical Utility of Cycle Threshold Values in the Context of COVID-19. Infect Dis Ther. Sep 2020;9(3):573-586.
14. Public Health Ontario. An Overview of Cycle Threshold Values and their Role in SARS-CoV-2 Real-Time PCR Test Interpretation. Updated 09/17/2020. Accessed 04/04/2022, https://www.publichealthontario.ca/-/media/documents/ncov/main/2020/09/cycle-threshold-values-sars-cov2-pcr.pdf?la=en
15. U.S. Food and Drug Administration. COVID-19 Test Uses: FAQs on Testing for SARS-CoV-2. Updated 02/24/2022. Accessed 04/04/2022, 2022. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/covid-19-test-uses-faqs-testing-sars-cov-2
16. Miller J, Wey A, Musgrove D, Son Ahn Y, Hart A, Kasiske BL,et al. Mortality among solid organ waitlist candidates during COVID-19 in the United States. Am J Transplant. Jun 2021;21(6):2262-2268.
17. Ashfaq A, Gray GM, Carapellucci J, Amankwah EK, Ahumada LM, Rehman M, et al. Impact of Coronavirus-2019 On Pediatric and Adult Heart Transplantation Waitlist Activity and Mortality in The United States: A Descriptive Approach. Lancet Reg Health Am. Nov 2021;3:100060.
18. Hogan J, Kwon T, Paye-Jaouen A, Fait C, Cointe A, Baudouin V. Kidney Transplantation in a COVID-19-positive Pediatric Recipient. Transplantation. Jul 1 2021;105(7):e74-e75.
19. Johnstad CM, Murray D, Dhingra R, Smith JW, Fiedler AG. Successful heart transplantation in a patient who recovered from COVID-19. J Card Surg. Mar 2021;36(3):1148-1149.
20. Kulkarni AV, Parthasarathy K, Kumar P, et al. Early liver transplantation after COVID-19 infection: The first report. Am J Transplant. Jun 2021;21(6):2279-2284.
21. Kute V, Meshram HS, Fleetwood VA, Chauhan S, Lentine KL. Solid Organ Transplantation in SARS-CoV-2 Recovered Transplant Candidates: a Comprehensive Review of Recent Literature. Curr Transplant Rep. Mar 9 2022:1-13.
22. Lang C, Jaksch P, Hoda MA, Lang G, Staudinger T, Tschernko E, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. Oct 2020;8(10):1057-1060.
23. Manzia TM, Gazia C, Lenci I, Angelico R, Toti L, Monaco A, et al. Liver transplantation performed in a SARS-CoV-2 positive hospitalized recipient using a SARS-CoV-2 infected donor. Am J Transplant. Jul 2021;21(7):2600-2604.
24. Murad H, Dubberke E, Mattu M, Parikh B, Wellen J, Alhamad T. Repeat SARS-CoV-2 testing after recovery. Is a pretransplant PCR necessary? Am J Transplant. Sep 2021;21(9):3206-3207.
25. Natori Y, Anjan S, Martin EF, Selvagi G, Villavicencio A, Coro A, et al. When is it safe to perform abdominal transplantation in patients with prior SARS-CoV-2 infection: A case series. Clin Transplant. Dec 2021;35(12):e14370.
26. Okubo K, Iqbal S, Lizaola-Mayo B, Aqel B, Graf EH, Banacloche JC, et al. Liver Transplant in a Polymerase Chain Reaction-Positive COVID-19 Recipient: A Case Report. Transplant Proc. Oct 2021;53(8):2490-2494.
27. Rouphael C, D’Amico G, Ricci K, Cywinski J, Miranda C, Koval C, et al. Successful orthotopic liver transplantation in a patient with a positive SARS-CoV2 test and acute liver failure secondary to acetaminophen overdose. Am J Transplant. Mar 2021;21(3):1312-1316.
28. Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon Jr R, Kim S, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. May 2021;9(5):487-497.
29. Bharat A, Querrey M, Markov NS, Kim S, Kurihara C, Garza-Castillon R, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. Dec 16 2020;12(574):eabe4282.
30. Kute VB, Fleetwood VA, Meshram HS, Guenette A, Lentine KL. Use of Organs from SARS-CoV-2 Infected Donors: Is It Safe? A Contemporary Review. Curr Transplant Rep. 2021/12/01 2021;8(4):281-292.
31. Kute VB, Godara S, Guleria S, Ray DS, Aziz F, Hegde U, et al. Is it Safe to Be Transplanted From Living Donors Who Recovered From COVID-19? Experience of 31 Kidney Transplants in a Multicenter Cohort Study From India. Transplantation. Apr 1 2021;105(4):842-850.
32. Santeusanio AD, Bhansali A, Rana M, Lerner S, Shapiro R. Kidney transplantation in patients with prior coronavirus disease 2019 (COVID-19). Clin Transplant. Jun 2021;35(6):e14288.
33. Agrawal D, Saigal S. Utilization of SARS-COV-2 positive donors and recipients for Liver transplantation in the pandemic era – An evidence-based review. Journal of Liver Transplantation. 2022 Mar 11;2022:100081.
34. Organ Procurement and Transplantation Network (OPTN). Summary of Current Evidence and Information– Donor SARS-CoV-2 Testing & Organ Recovery from Donors with a History of COVID-19. Updated 01/21/2022. Accessed 04/05/2022, 2022. https://optn.transplant.hrsa.gov/media/kkhnlwah/sars-cov-2-summary-of-evidence.pdf
35. Organization WH. Interim recommendations for an extended primary series with an additional vaccine dose for COVID-19 vaccination in immunocompromised persons. Updated 26 October 2021. Accessed 04/01/2022, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-immunocompromised-persons
36. International Liver Transplant Society (ILTS). COVID-19 Organ Donation & Transplant Town Hall #5 – COVID Updates and Controversies. Updated 10/04/2021. Accessed 04/01/2022, 2022. https://ilts.org/education/webinars/covid-19-updates-controversies/
37. The Transplantation Society. Guidance on Coronavirus Disease 2019 (COVID-19) for Transplant Clinicians. The Transplantation Society. Updated 8 June 2020. Accessed 08/11/2020, 2020. https://tts.org/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians
38. American Society of Transplant surgeons (ASTS). COVID-19 Resources. Updated June 2020. Accessed 04/13/2022, 2022. https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/transplant-capacity-and-testing#retrieval
39. International Society of Heart and Lung Transplantation (ISHLT). Guidance regarding the SARS CoV-2 pandemic. Updated 02/01/2021. Accessed 04/07/2022, 2022. https://ishlt.org/ishlt/media/documents/SARS-CoV-2_Guidance-for-Cardiothoracic-Transplant-and-VAD-center.pdf
40. United Network for Organ Sharing. COVID-19 and solid organ transplant. Accessed 04/07/2022, 2022. https://unos.org/covid/
41. The Association of Organ Procurement Organizations. COVID-19 (coronavirus) bulletin. Accessed 04/07/2022, 2022. https://www.aopo.org/information-about-covid-19-coronavirus-is-being-released-rapidly-we-will-post-updates-as-we-receive-them/
42. The American Society of Transplantation. FAQs for Organ Transplantation. Updated 01/29/2022. Accessed 04/07/2022, 2022. https://www.myast.org/sites/default/files/2022_Jan_29.%20Clean_FAQ_COVIDUpdates.pdf
43. The American Society of Transplantation. Recommendations and Guidance for Organ Donor Testing. Updated 07/07/2021. Accessed 04/07/2022, 2022. https://www.myast.org/sites/default/files/Donor%20Testing%20Document_07.07.21.pdf