Abstract
Parkinson's Disease (PD), following Alzheimer's Disease, is the second most prevalent neurological condition globally. This progressive disorder is characterized by the loss of dopaminergic neurons in the substantia nigra, leading to motor dysfunction. Neuroinflammation, a complex process involving astrocytes and microglia, significantly contributes to PD pathogenesis. The PI3K/AKT signaling pathway, a pivotal regulator of cell survival and function, is dysregulated in PD. While it can exert neuroprotective effects, it can also be neurotoxic under certain conditions. Astrocytes, the most abundant glial cells in the central nervous system (CNS), can both promote neuronal survival by releasing neurotrophic factors and modulate synaptic activity. However, they can also contribute to neurodegeneration by releasing inflammatory mediators and reactive oxygen species (ROS). Microglia, the resident immune cells of the CNS, play a dual role. They can phagocytose damaged neurons and debris, but they can also release pro-inflammatory cytokines and chemokines, exacerbating neuroinflammation. Understanding the intricate interplay between the PI3K/AKT signaling pathway, astrocytes, and microglia in PD is crucial for developing novel therapeutic strategies.
Targeting these pathways may hold promise for mitigating neuroinflammation, enhancing neuronal survival, and improving outcomes for patients with PD.
Keywords
Parkinson disease, PD, Anti-inflammatory phenotypes, PI3K, AKT, Microglia, Astrocyte
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease, characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Clinically, it manifests with both motor symptoms including resting tremors, rigidity, and postural instability and non-motor symptoms such as autonomic, mental, sensory, and cognitive impairments [1] and a large number of people suffering from PD is increasing all over the world [2]. Despite extensive research, the precise mechanisms underlying PD pathogenesis remain elusive, limiting the development of effective treatments [3]. Recent studies have highlighted that neuroinflammation has emerged as a central player in the progression of PD, driven primarily by glial cells, particularly microglia and astrocytes. These cells exhibit a dual role: while they can promote neuronal survival under physiological conditions, their dysregulation contributes to neurodegeneration by releasing pro-inflammatory cytokines, reactive oxygen species (ROS), and other toxic mediators. The shift of microglia and astrocytes into activated, neurotoxic phenotypes (M1 and A1, respectively) exacerbates inflammation and neuronal loss. Among the factors that may lead to changes in those cells and one of the key pathways implicated in the regulation of glial cell activity and neuronal survival in PD is the PI3K/AKT signaling pathway [4].
PI3K/AKT is a critical intracellular pathway that modulates multiple cellular processes, including apoptosis, oxidative stress, and neuroinflammation. Dysregulation of this pathway has been linked to the progression of PD, as it can influence both neuroprotective and neurotoxic responses in glial cells. Notably, the activation state of microglia and astrocytes appears to be tightly regulated by PI3K/AKT signaling, making it a promising therapeutic target for mitigating inflammation and promoting neuronal survival. Phosphoinositide 3-kinases (PI3Ks) are vital for managing key processes that are central to the inflammatory response triggered by external challenges [5]. AKT, a serine/threonine-specific protein kinase, and its phosphorylated form are notably reduced in the substantia nigra pars compacta (SNpc) of PD patients. The PI3K/AKT pathway supports the survival and growth of dopamine neurons by inhibiting apoptosis, thus potentially mitigating PD symptoms. Additionally, the PI3K/AKT pathway influences oxidative stress pathways through downstream signaling molecules. The PI3K/AKT signaling pathway also interacts with other crucial cellular mechanisms, including the MAPK and NF-κB pathways. These pathways work in concert to regulate inflammation and cellular survival in PD. While the MAPK pathway primarily responds to extracellular stress signals, the NF-κB pathway is directly linked to the production of pro-inflammatory cytokines, amplifying the inflammatory response. Dysregulation of these pathways can exacerbate neuronal death in PD.
Furthermore, reactive oxygen species (ROS) play a dual role in the PI3K/AKT pathway. On one hand, ROS can activate PI3K, enhancing its neuroprotective effects. On the other hand, excessive ROS levels, commonly observed in PD, impair Akt phosphorylation, leading to oxidative stress and neuronal damage. Targeting ROS-mediated disruptions in PI3K/AKT signaling could provide a novel therapeutic approach.
Disruptions in this pathway, particularly with abnormal Parkin gene expression can lead to oxidative stress imbalances, contributing to PD [6]. In this article, we explore the intricate role of the PI3K/AKT signaling pathway in PD, with a particular focus on the dual role of microglia and astrocytes. Understanding how this pathway modulates the balance between neuroprotection and neurotoxicity in glial cells will provide new insights into therapeutic strategies aimed at slowing or preventing PD progression.
Microglia
Microglia, the resident immune cells of the central nervous system (CNS), play a dual role in neuroinflammation and neuronal survival. They are also known as "guardians" and capable of phagocytosis, chemotaxis, and cytokine production. These cells can be both beneficial and detrimental, depending on the circumstances. Understanding how microglia switch between these states is key to developing new treatments for brain diseases. Under normal conditions, they remain in a resting state, clearing cellular debris and maintaining homeostasis. However, in Parkinson's disease (PD), microglia become activated in response to pathological stimuli, such as the accumulation of α-synuclein protein [7]. Activated microglia undergo significant morphological changes, becoming larger with shorter processes and expressing new surface receptors. They engulf cellular debris and release inflammatory factors, which can either reduce or exacerbate inflammation [8]. These cells play a key role in the central nervous system (CNS) and are involved in neurodegenerative disorders like PD [9].
In the CNS, this activation leads to two distinct phenotypes: M1 and M2. The M1 phenotype is pro-inflammatory, responding as the first line of defense, while the M2 phenotype is anti-inflammatory, helping to repair and restore neurons by expressing anti-inflammatory molecules [10]. Elevated levels of inflammatory substances like TNF-α, IL-1β, and IL-6 in Parkinson's patients indicate a significant role for inflammation in the disease. The activation of microglia is associated with increased cytokine levels and occurs early in Parkinson's disease, a process known as microgliosis. M1 microglia contribute to neuron death by releasing toxic substances, while M2 microglia help repair damage by releasing anti-inflammatory substances [11]. The accumulation of α-synuclein protein activates microglia, which attempt to eliminate these toxic proteins, but this process can increase inflammation and damage nerve cells [12]. Microglia act as immune mediators in response to inflammatory molecules, initiating the inflammation pathway. They are capable of morphological changes, preserving neurons by cleaning pathogens in their physiological state or stimulating pro-inflammatory responses with their pathogenic phenotype [13]. Uncontrolled microglial activation during infection or brain damage releases neurotoxic molecules, leading to neuron death [14].
The PI3K/AKT signaling pathway is a critical regulator of microglial activation. Activation of Akt biases microglia toward the M2 phenotype, suppressing inflammation and enhancing neuroprotection. Conversely, reduced PI3K/AKT activity correlates with a shift toward the neurotoxic M1 phenotype, amplifying inflammation and neuronal damage.
Additionally, several receptors, including TLRs, CD14, TAM (Tyro3, Axl, and Mer) receptors, and TREM-2, are involved in microglial phagocytosis [15]. TLR4 plays a role in the phagocytosis of α-synuclein, and alterations in TLR4 signaling can modulate inflammatory responses and ROS production, contributing to neurodegeneration. In contrast, TREM-2 activation enhances microglial phagocytosis, reducing inflammation and clearing α-synuclein aggregates, thus exhibiting neuroprotective effects.
Collectively, the modulation of the PI3K/AKT pathway holds significant potential in shifting microglial phenotypes from M1 to M2, mitigating neuroinflammation, and enhancing dopaminergic neuron survival in PD [16].
Astrocyte
Astrocytes, the most abundant glial cells in the central nervous system (CNS) are a group of brain cells which due to their star-like shape, can connect to 90% of CNS cells. These cells regulate homeostasis and play an important role in maintaining the neurophysiological function of the CNS due to their multifaceted capabilities [17]. Based on their activation states, astrocytes are classified into two categories, neurotoxic (A1) and neuroprotective (A2) [18]. The "A1" neurotoxic astrocyte subtype is induced by microglial cytokine secretion, displaying a specific transcriptional pattern while being unable to support synapse integrity. Markers of A1 reactivity have been found in astrocytes in post-mortem samples from patients with neurodegenerative diseases such as AD, PD, HD, ALS, and MS, suggesting that neurotoxic astrocytes are present across a wide range of conditions [19]. A group of microglia that release TNF-α and IL-1α activates the A1 form of astrocytes, leading to neurotoxicity, loss of synaptogenesis, and neuronal death. This type of astrocyte can upregulate inflammatory markers like ROS, IL-1β, and TNF-α. In Parkinson's disease, there is an increase in A1 astrocytes within the substantia nigra, contributing to the degeneration of dopaminergic neurons. In contrast, A2 astrocytes enhance the expression of multiple neuroprotective factors that facilitate synaptic repair, neuronal growth, and survival. However, in Parkinson's disease, the function of A2 astrocytes is often compromised, contributing to disease progression [20].
Astrocytes and microglia form a dynamic duo in the central nervous system, collaborating to maintain brain health and respond to injury [13]. Microglia and astrocytes interact bidirectional, influencing each other's phenotypes through cytokine and chemokine signaling. Understanding the intricate relationship between astrocytes and microglia is crucial for developing effective therapeutic strategies for neurodegenerative diseases [21]. Microglia can directly influence astrocytes, as astrocytes express cytokine and chemokine receptors, allowing them to respond to inflammatory factors released by microglia. A1 astrocytes, which contribute to neurodegeneration, are highly dependent on microglial activation. Mitochondrial dysfunction in microglia can trigger astrocyte activation and the development of the A1 phenotype. GLP-1 receptor signaling in microglia can modulate both microglial and astrocyte activation [22]. The interactions between astrocytes and microglia are bidirectional, with astrocytes also influencing microglial states. Peripheral immune cells, such as CD8+ T cells, can also contribute to neurodegenerative diseases and interact with astrocytes [23].
As a result, The PI3K/AKT signaling pathway is a key regulator of astrocyte function. Activation of this pathway promotes the A2 phenotype, enhancing neuroprotection by reducing inflammatory responses and supporting neuronal repair. Conversely, reduced PI3K/AKT activity biases astrocytes toward the A1 phenotype, amplifying neurotoxicity and inflammation.
Astrocytes also interact dynamically with microglia to modulate neuroinflammation. Microglial activation can drive A1 astrocyte formation, while astrocytes, in turn, influence microglial states through cytokine and chemokine signaling. These bidirectional interactions, when dysregulated, perpetuate neuroinflammation and neuronal loss in PD. Targeting the PI3K/AKT pathway to shift astrocytes toward the A2 phenotype presents a promising therapeutic approach to mitigate neurodegeneration and enhance neuronal survival in PD.
PI3K/AKT Signaling Pathway
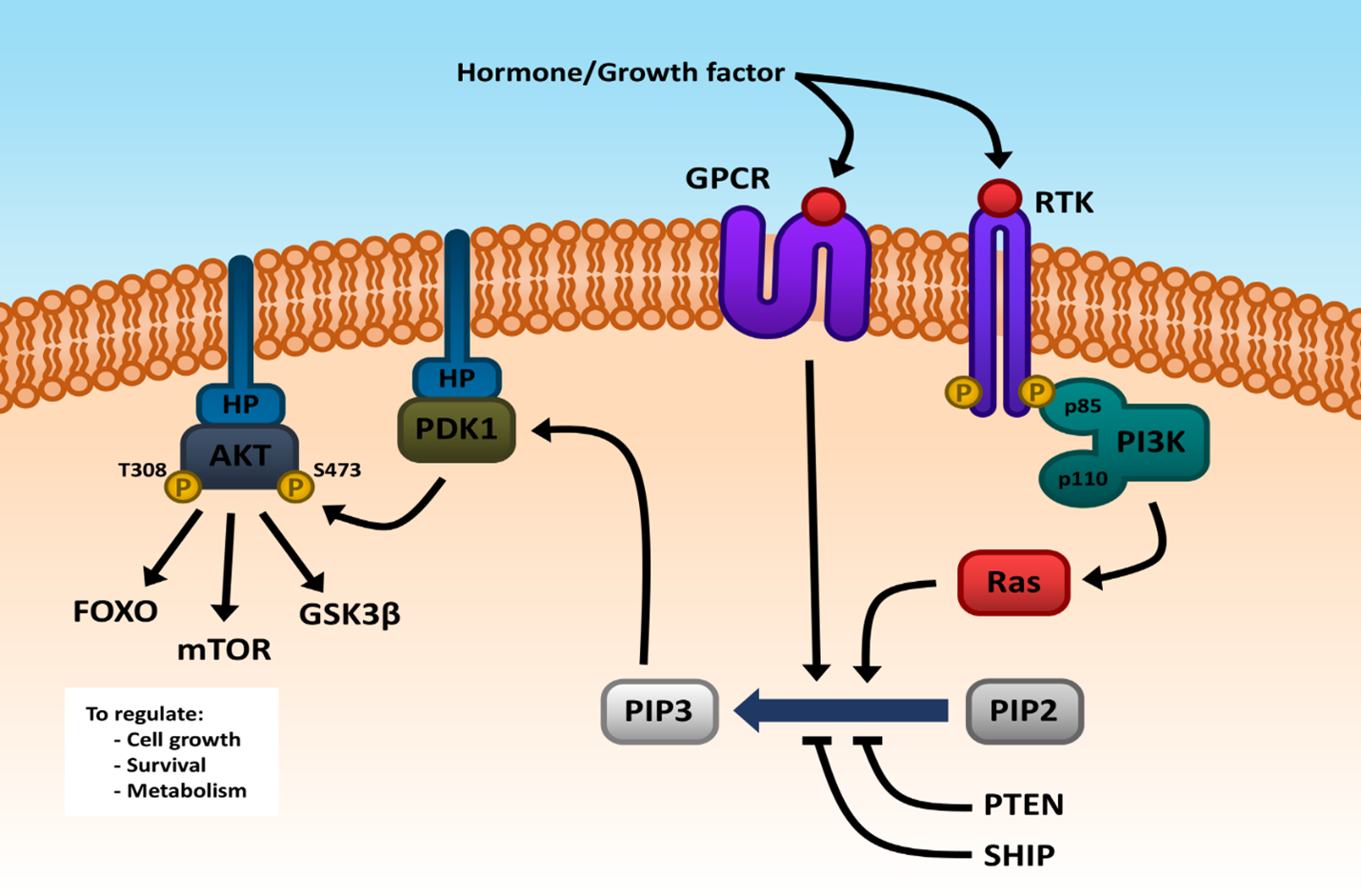
Phosphoinositide 3-kinases (PI3K) are a family of signal transducers and intracellular enzymes crucial for many cellular processes [2]. In mammalian cells, there are eight PI3Ks that are categorized into three classes (Class I, Class II, and Class III) based on their substrate specificity, structure, and regulation. PI3Ks phosphorylate inositol phospholipids at the 3-position on the inositol ring, producing vital signaling molecules like phosphatidylinositol-3,4,5-trisphosphate (PIP3), phosphatidylinositol-3,4-bisphosphate (PIP2), and phosphatidylinositol-3-phosphate (PIP) [4]. These signaling molecules regulate various cellular functions, including cell growth, apoptosis, autophagy, synaptic plasticity, and signal transduction [24]. Class I PI3Ks, are heterodimers consisting of a catalytic subunit (p110) and a regulatory subunit (p85). The p85 subunit's SH2 domain binds to phosphorylated tyrosines in the cytoplasmic domain of RTKs, which are located in the plasma membrane. Upon activation by extracellular signals such as hormones or growth factors, these receptors activate Ras, a protein that subsequently activates PI3K. PI3K then catalyzes the conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3), which acts as a docking site for the serine/threonine kinase Akt (Protein Kinase B) [25]. Akt becomes activated following phosphorylation at specific sites (T308 and S473) and then phosphorylates numerous downstream target proteins, influencing various cellular responses. Class II PI3Ks are high-molecular-weight monomers, including three types: PI3K-C2α, PI3K-C2β, and PI3K-C2γ. Class III PI3Ks, known as vacuolar protein sorting 34 (Vps34), are distinct from the other classes and are involved in different cellular processes [26].
As a central player in the PI3K signaling cascade, Protein kinase B (PKB), more commonly referred to as Akt, is a serine/threonine kinase operating within the cell and coordinates a multitude of cellular processes. Its three isoforms named Akt1, Akt2, and Akt3 are recruited to the cell membrane via a pleckstrin homology (PH) domain and activated by phosphoinositide-dependent kinase 1 (PDK1). Once activated, Akt phosphorylates a diverse array of downstream effectors, including FOXO, mTOR, and GSK3β, to regulate cell growth, survival, and metabolism. PI3K and Akt form the central components of the PI3K/Akt signaling pathway, integral to the pathogenesis and progression of PD [27]. Upon PI3K activation, a second messenger called PIP3 is produced at the plasma membrane, interacting with intracellular Akt, leading to its activation through phosphorylation. This pathway responds to a range of signals, including growth factor receptor tyrosine kinases (RTKs) and G-protein coupled receptor (GPCR) signaling and promotes neuronal survival, enhances neurogenesis, and inhibits apoptosis triggered by neurotoxins in PD models through its influence on various proteins [28]. In addition, it plays a pivotal role in many cellular functions, including cell proliferation, adhesion, migration, invasion, and metabolism. PTEN (Phosphatase and Tensin Homolog) and SHIP (Inositol Polyphosphate-5-Phosphatase) act as negative regulators of the PI3K/AKT pathway by converting PIP3 back to PIP2, thereby reducing PI3K/AKT signaling and decreasing cell growth and survival (Figure 1) [29]. These regulatory mechanisms ensure that the pathway is tightly controlled, maintaining cellular homeostasis and preventing excessive or aberrant cell proliferation, which could lead to diseases like cancer [30].
Role of PI3K/AKT Signaling Pathway in PD
Inflammation
In neurodegenerative diseases such as PD, neuroinflammation is the initial defensive response of the brain to pathogenic agents and plays a key role in disease progression. While acute inflammation is beneficial for tissue repair, chronic inflammation can exacerbate neuronal damage [19]. In PD, persistent inflammation leads to neuronal damage, synaptic dysfunction, and ultimately apoptosis [31]. Activated microglia and astrocytes release pro-inflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin-1 β (IL-1β), and interleukin-6 (IL-6), which bind to specific receptors (TLR4) receptors and trigger inflammatory responses. This inflammation, in turn, promotes neuronal death and synaptic dysfunction, both hallmark features of PD [32]. To mitigate the detrimental effects of inflammation, it is necessary to target the underlying PI3K/AKT signaling pathway. Studies have shown that the PI3K/AKT signaling pathway serves as a crucial mediator in regulating neuroinflammation and neuronal survival in PD. Modulating PI3K/AKT signaling pathway can inhibit the expression of pro-inflammatory factors (TNF α, IL-1β, IL-6), TLR4 and thereby attenuating neuroinflammation [26]. In addition, when the Akt protein is activated, it acts as a signaling molecule that induces the translocation of FOXO1 from the nucleus to the cytoplasm. FOXO1 is a transcription factor that plays a crucial role in upregulating inflammatory genes. However, upon activation of Akt, FOXO1 is sequestered in the cytoplasm, preventing it from promoting transcription of inflammatory genes [33]. One of the key target genes of FOXO1 is TLR4. Downregulation of TLR4 leads to decreased production of inflammatory mediators, resulting in reduced inflammation [27].
Apoptosis
In addition to inflammation, apoptosis is an essential and critical mechanism and a type of programmed cell death that occurs to maintain cellular homeostasis in a normal tissue and defend cells from injury. Furthermore, apoptosis serves to maintain homeostasis by eliminating abnormal cells through immune responses, especially when cells are damaged or infected. Conversely, uncontrolled apoptosis can give rise to neurodegenerative diseases like PD [34].
The PI3K/AKT pathway plays a protective role by inhibiting apoptosis. Neurotrophic factors such as NGF and IGF-1 activate PI3K/AKT, leading to the suppression of apoptotic proteins like caspase-3 and Bax. This inhibition prevents the formation of Bax-mediated channels on the mitochondrial membrane, blocking the release of cytochrome c and promoting neuronal survival. Also, experimental studies have highlighted the critical role of the PI3K/AKT pathway in regulating apoptosis in Parkinson's disease (PD). Activation of PI3K/AKT signaling promotes the phosphorylation of pro-apoptotic factors like BAD, thereby inhibiting their activity. Concurrently, this pathway upregulates anti-apoptotic proteins such as Bcl-2 and Bcl-xL, enhancing neuronal survival. Furthermore, downstream mediators like caspase-9 and caspase-3, which play central roles in the apoptotic cascade, are directly regulated by PI3K/AKT signaling.
For instance, research has shown that activation of the PI3K/AKT pathway through pharmacological agents can suppress caspase-9 activity, reducing apoptosis in dopaminergic neurons. This mechanism is evident in models of PD where compounds such as tetrahydroxy-stilbene glucoside (TSG) mitigate neuronal loss by enhancing the Bcl-2/BAD ratio and inhibiting caspase activation [35].
By modulating both inflammation and apoptosis, the PI3K/AKT signaling pathway provides a promising therapeutic target in PD. For instance, experimental studies have shown that salidroside exerts neuroprotective effects in MPTP-induced mouse models of PD by activating the PI3K/AKT/GSK3β signaling pathway, thereby preventing the loss of dopaminergic neurons [36].
Additionally, studies have demonstrated that inhibition of the PI3K/AKT pathway induces apoptosis through p38 activation in cultured cerebellar granule neurons, suggesting a role in neuronal degeneration relevant to PD [37].
These findings substantiate the involvement of the PI3K/AKT pathway in PD pathogenesis and support its potential as a therapeutic target. Strategies aimed at enhancing Akt activity could reduce neuroinflammation, protect dopaminergic neurons, and slow disease progression [38].
Key Players in PI3K/AKT Signaling Pathway in PD
In the context of PD, where dopaminergic neurons progressively degenerate, the PI3K/AKT pathway becomes a vital focus [39]. Astrocytes, along with microglia, through their interaction with neurons and other glial cells, actively modulate this pathway. They can either support neuronal survival by enhancing PI3K/AKT activity or contribute to neurodegeneration when their regulation becomes dysregulated. Microglia, the resident immune cells of the central nervous system, also play a dual role, responding to neuroinflammation and affecting PI3K/AKT signaling. Together, astrocytes and microglia regulate key aspects of neuroprotection and neurotoxicity in PD [22].
Astrocyte Elevated Gene-1 (AEG-1), a gene present in astrocytes, plays a crucial role in neuronal survival and function. Overexpression of AEG-1 has been linked to various neurological conditions, including PD, and can contribute to cancer development. In PD, elevated AEG-1 levels can lead to neuronal death by inducing apoptosis, a process of programmed cell death. However, AEG-1 can also have neuroprotective effects by promoting astrocyte migration, which can shield neurons from further damage [40].
AEG-1's protective role is mediated through its activation of the PI3K/AKT signaling pathway. This pathway is essential for neuronal survival, and its dysregulation due to decreased AEG-1 levels can contribute to the progression of PD. The decreased expression of AEG-1, as previously discussed, contributes to neuronal loss. However, upregulation of AEG-1 alone is insufficient for neuroprotection. Also, there are different kinds of pathways which can increase the activity of the PI3K/AKT signaling pathway, thereby preventing oxidative stress and neuronal damage [41]. In summary, AEG-1 and the PI3K/AKT pathway are interdependent, and maintaining adequate levels of AEG-1 is crucial for neuronal survival and preventing the progression of PD. The cited study indicates a direct correlation between AEG-1 expression and the PI3K/AKT signaling pathway. However, AEG-1 is not solely responsible for regulating this pathway in PD; multiple other mechanisms are likely at play [42].
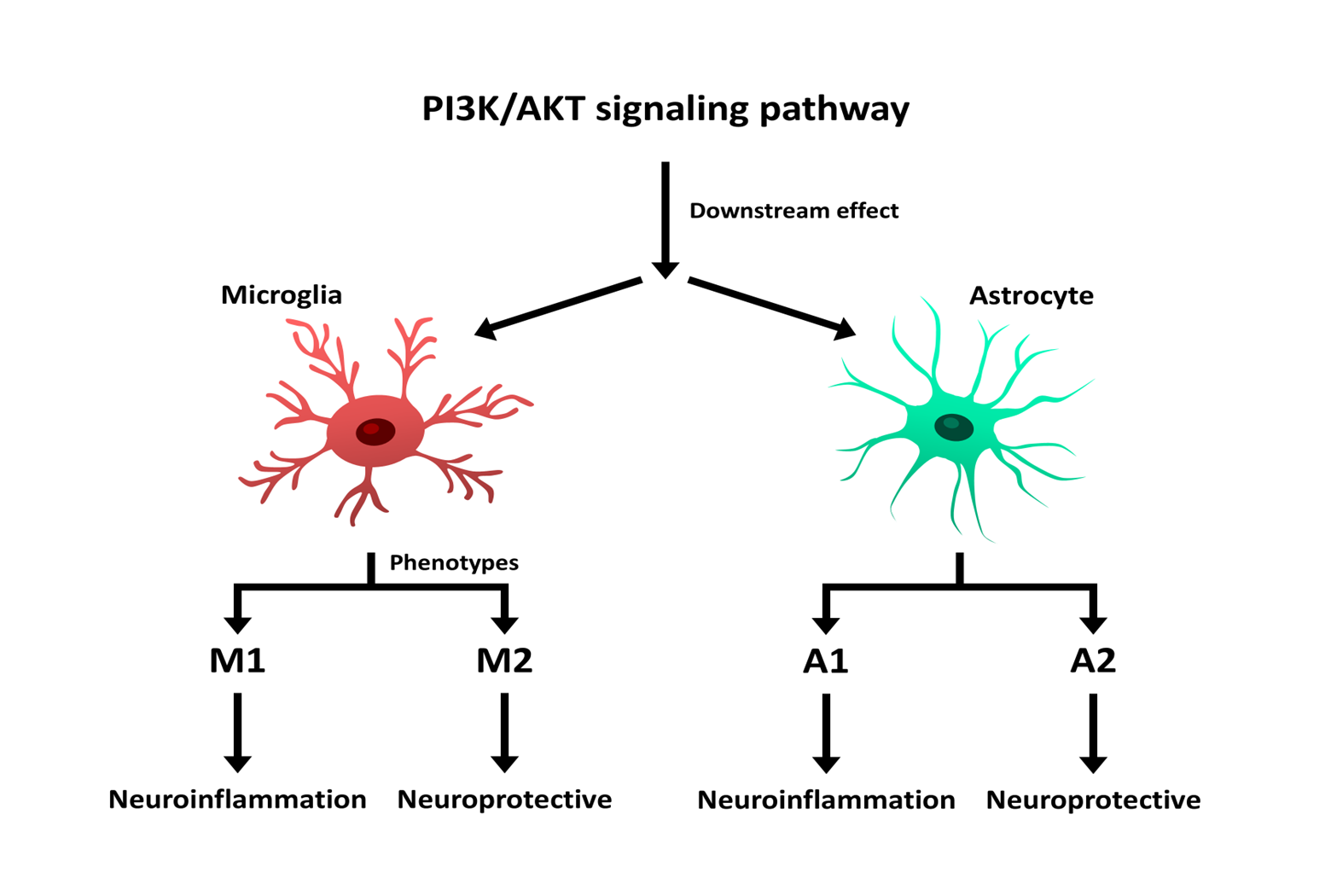
Meanwhile, microglia are also key players in PI3K/AKT signaling pathway. Microglia act as a double-edged sword, capable of both inducing neurodegeneration by activating neuroinflammatory pathways and promoting neuroprotection through the reduction of inflammation [43]. As previously mentioned, microglia exhibit two primary phenotypes: M1 and M2. The activation of microglia, as well as the activity of each phenotype, is closely linked to PI3K/AKT signaling pathway and tightly regulated by it. Under the influence of this signaling pathway, microglia differentiate into the corresponding phenotype. Specifically, the M1 phenotype is activated by a decrease in signaling pathway activity, whereas the M2 phenotype is activated by an increase in signaling pathway activity [44]. Findings have indicated that The PI3K/Akt signaling pathway has been identified as a critical regulator of microglial function, often promoting neuroprotective outcomes. PI3K/AKT signaling pathway often biases microglia towards the M2 phenotype due to the anti-inflammatory effects of Akt modulators and this result in induces anti-inflammatory phenotypes. Furthermore, activated microglia can increase the production of pro-inflammatory factors through PI3K/AKT signaling pathway. Conversely, microglial activation itself, can stimulate this signaling pathway and leading to neuroinflammation (Figure 2) [45].
Conclusion
This review underscores the crucial role of the PI3K/AKT signaling pathway in regulating neuroinflammation, apoptosis, and neuronal survival in Parkinson's disease (PD). The intricate interplay between astrocytes and microglia, modulated by PI3k/AKT determines whether these glial cells promote neuroprotection or contribute to neurodegeneration. The study suggests that glial cells, including astrocytes and microglia, can influence neuronal survival or contribute to neurodegeneration depending on their activation states. While A2 astrocytes and M2 microglia exert neuroprotective effects, A1 astrocytes and M1 microglia exacerbate neuroinflammation and neuronal death, further implicating PI3K/AKT as a potential therapeutic target.
Targeting the PI3K/AKT pathway to shift glial phenotypes from neurotoxic (A1 and M1) to neuroprotective (A2 and M2) offers a promising strategy for mitigating PD progression. Additionally, the study highlights the role of Astrocyte Elevated Gene-1 (AEG-1) in enhancing neuronal survival through the PI3K/AKT pathway. However, upregulating AEG-1 alone may not be sufficient to prevent PD progression. Given the dual role of astrocytes and microglia in PI3K/AKT signaling, a promising therapeutic approach could involve targeting the shift between glial phenotypes. Future research should explore agents that can promote the conversion of A1 astrocytes to A2 and M1 microglia to M2 by specifically modulating the PI3K/AKT pathway. While significant progress has been made in understanding the PI3K/AKT signaling pathway and its role in Parkinson's disease (PD), several gaps in knowledge remain. For instance, the precise molecular mechanisms governing the crosstalk between PI3K/AKT and other pathways, such as MAPK and NF-κB, require further exploration. Investigating how these interactions influence glial cell phenotypes could uncover novel targets for therapeutic intervention.
Additionally, the development of selective PI3K/AKT modulators that can specifically target dopaminergic neurons or glial cells without affecting systemic functions remains a major challenge. Preclinical studies using advanced models, such as organoids or single-cell sequencing, could provide deeper insights into cell-specific effects of pathway modulation.
Future research should also focus on combining PI3K/AKT modulators with therapies targeting other pathological processes, such as protein aggregation or mitochondrial dysfunction, to create more comprehensive treatment strategies. These combined approaches may offer enhanced neuroprotection and improved clinical outcomes for PD patients.
Additionally, investigating AEG-1's role in facilitating these transitions could provide valuable insights into the balance between neuroprotection and neurotoxicity.
In light of its critical role in regulating neuroinflammation and apoptosis, the PI3K/AKT pathway offers multiple therapeutic opportunities. For instance, GSK-3β inhibitors have shown promise in reducing tau hyperphosphorylation and enhancing neuronal survival. Similarly, mTOR activators can promote autophagy, aiding in the clearance of misfolded proteins like α-synuclein, a hallmark of Parkinson's disease. These approaches not only mitigate neuronal damage but also address the underlying mechanisms driving disease progression.
Future studies should investigate the synergistic effects of combining PI3K/AKT modulators with other therapeutic agents, such as anti-inflammatory drugs or antioxidants, to achieve more comprehensive neuroprotection. Additionally, exploring the role of PI3K/AKT crosstalk with pathways like MAPK and NF-κB may uncover novel strategies to fine-tune neuroinflammatory and neuroprotective responses.
Further research should focus on localized therapeutic strategies that specifically target dopaminergic regions in the substantia nigra to maximize neuroprotection while minimizing systemic effects. Such approaches hold promise for developing innovative therapies that modulate neuroinflammation and glial cell states in PD.
Conflict of Interest
There is no conflict of interest in this article.
Funding Statement
There is no funding for this article.
Acknowledgments
None.
References
2. Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer's Disease and Parkinson's Disease. Front Pharmacol. 2021 Apr 15;12:648636.
3. Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020 Apr 1;10(1):54.
4. Goyal A, Agrawal A, Verma A, Dubey N. The PI3K-AKT pathway: A plausible therapeutic target in Parkinson's disease. Exp Mol Pathol. 2023 Feb;129:104846.
5. Isik S, Yeman Kiyak B, Akbayir R, Seyhali R, Arpaci T. Microglia Mediated Neuroinflammation in Parkinson's Disease. Cells. 2023 Mar 25;12(7):1012.
6. He X, Li Y, Deng B, Lin A, Zhang G, et al. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022 Sep;55(9):e13275.
7. Wolf SA, Boddeke HW, Kettenmann H. Microglia in Physiology and Disease. Annu Rev Physiol. 2017 Feb 10;79:619-43.
8. Poppell M, Hammel G, Ren Y. Immune Regulatory Functions of Macrophages and Microglia in Central Nervous System Diseases. Int J Mol Sci. 2023 Mar 21;24(6):5925.
9. Wang Z, Wang Q, Li S, Li XJ, Yang W, He D. Microglial autophagy in Alzheimer's disease and Parkinson's disease. Front Aging Neurosci. 2023 Jan 10;14:1065183.
10. Yu H, Chang Q, Sun T, He X, Wen L, An J, et al. Metabolic reprogramming and polarization of microglia in Parkinson's disease: Role of inflammasome and iron. Ageing Res Rev. 2023 Sep;90:102032.
11. Rentsch P, Egan T, Kuriakose A, Stayte S, Vissel B. The ratio of M1 to M2 microglia in the striatum determines the severity of L-Dopa-induced dyskinesias. J Neurochem. 2023 Dec;167(5):633-47.
12. Lv QK, Tao KX, Wang XB, Yao XY, Pang MZ, Liu JY, et al. Role of α-synuclein in microglia: autophagy and phagocytosis balance neuroinflammation in Parkinson's disease. Inflamm Res. 2023 Mar;72(3):443-62.
13. Gao C, Jiang J, Tan Y, Chen S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct Target Ther. 2023 Sep 22;8(1):359.
14. Badanjak K, Fixemer S, Smajić S, Skupin A, Grünewald A. The Contribution of Microglia to Neuroinflammation in Parkinson's Disease. Int J Mol Sci. 2021 Apr 28;22(9):4676.
15. Li XX, Zhang F. Targeting TREM2 for Parkinson's Disease: Where to Go? Front Immunol. 2021 Dec 24;12:795036.
16. Butler CA, Popescu AS, Kitchener EJA, Allendorf DH, Puigdellívol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021 Aug;158(3):621-39.
17. Matejuk A, Ransohoff RM. Crosstalk Between Astrocytes and Microglia: An Overview. Front Immunol. 2020 Jul 16;11:1416.
18. Wang T, Sun Y, Dettmer U. Astrocytes in Parkinson's Disease: From Role to Possible Intervention. Cells. 2023 Sep 22;12(19):2336.
19. Zhang N, Yan Z, Xin H, Shao S, Xue S, Cespuglio R, et al. Relationship among α‑synuclein, aging and inflammation in Parkinson's disease (Review). Exp Ther Med. 2023 Nov 21;27(1):23.
20. Ding ZB, Song LJ, Wang Q, Kumar G, Yan YQ, Ma CG. Astrocytes: a double-edged sword in neurodegenerative diseases. Neural Regen Res. 2021 Sep;16(9):1702-10.
21. Gotoh M, Miyamoto Y, Ikeshima-Kataoka H. Astrocytic Neuroimmunological Roles Interacting with Microglial Cells in Neurodegenerative Diseases. Int J Mol Sci. 2023 Jan 13;24(2):1599.
22. Kam TI, Hinkle JT, Dawson TM, Dawson VL. Microglia and astrocyte dysfunction in parkinson's disease. Neurobiol Dis. 2020 Oct;144:105028.
23. Chen K, Wang H, Ilyas I, Mahmood A, Hou L. Microglia and Astrocytes Dysfunction and Key Neuroinflammation-Based Biomarkers in Parkinson's Disease. Brain Sci. 2023 Apr 7;13(4):634.
24. Lu R, Yang Z, Xu G, Yu S. miR-338 modulates proliferation and autophagy by PI3K/AKT/mTOR signaling pathway in cervical cancer. Biomed Pharmacother. 2018 Sep;105:633-44.
25. Shi X, Wang J, Lei Y, Cong C, Tan D, Zhou X. Research progress on the PI3K/AKT signaling pathway in gynecological cancer (Review). Mol Med Rep. 2019 Jun;19(6):4529-35.
26. Cianciulli A, Porro C, Calvello R, Trotta T, Lofrumento DD, Panaro MA. Microglia Mediated Neuroinflammation: Focus on PI3K Modulation. Biomolecules. 2020 Jan 14;10(1):137.
27. Rai SN, Dilnashin H, Birla H, Singh SS, Zahra W, Rathore AS, et al. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox Res. 2019 Apr;35(3):775-95.
28. Sengupta P, Das R, Majumder P, Mukhopadhyay D. Connecting the ends: signaling via receptor tyrosine kinases and cytoskeletal degradation in neurodegeneration. Explor Neurosci. 2024 Feb 20;3(1):1-26.
29. Keshri PK, Singh SP. Unraveling the AKT/ERK cascade and its role in Parkinson disease. Arch Toxicol. 2024 Oct;98(10):3169-90.
30. Iranpanah A, Kooshki L, Moradi SZ, Saso L, Fakhri S, Khan H. The Exosome-Mediated PI3K/Akt/mTOR Signaling Pathway in Neurological Diseases. Pharmaceutics. 2023 Mar 21;15(3):1006.
31. Jin X, Dong W, Chang K, Yan Y. Research on the signaling pathways related to the intervention of traditional Chinese medicine in Parkinson's disease:A literature review. J Ethnopharmacol. 2024 May 23;326:117850.
32. Qu Y, Li J, Qin Q, Wang D, Zhao J, An K, et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson's disease. NPJ Parkinsons Dis. 2023 Feb 4;9(1):18.
33. Farid HA, Sayed RH, El-Shamarka ME, Abdel-Salam OME, El Sayed NS. PI3K/AKT signaling activation by roflumilast ameliorates rotenone-induced Parkinson's disease in rats. Inflammopharmacology. 2024 Apr;32(2):1421-37.
34. Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024 Jan 18;187(2):235-56.
35. Jha SK, Jha NK, Kar R, Ambasta RK, Kumar P. p38 MAPK and PI3K/AKT Signalling Cascades inParkinson's Disease. Int J Mol Cell Med. 2015 Spring;4(2):67-86.
36. Zhang W, He H, Song H, Zhao J, Li T, Wu L, et al. Neuroprotective Effects of Salidroside in the MPTP Mouse Model of Parkinson's Disease: Involvement of the PI3K/Akt/GSK3β Pathway. Parkinsons Dis. 2016;2016:9450137.
37. Vázquez de la Torre A, Junyent F, Folch J, Pelegrí C, Vilaplana J, Auladell C, et al. PI3 k/akt inhibition induces apoptosis through p38 activation in neurons. Pharmacol Res. 2013 Apr;70(1):116-25.
38. Erekat NS. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin Anat. 2022 Jan;35(1):65-78.
39. Xiao CL, Yin WC, Zhong YC, Luo JQ, Liu LL, Liu WY, et al. The role of PI3K/Akt signalling pathway in spinal cord injury. Biomed Pharmacother. 2022 Dec;156:113881.
40. Leem E, Kim SR. Limited therapeutic potential of astrocyte elevated gene-1 transduction in an animal model of Parkinson's disease. Neural Regen Res. 2020 Oct;15(10):1850-1.
41. Leem E, Kim HJ, Choi M, Kim S, Oh YS, Lee KJ, et al. Upregulation of neuronal astrocyte elevated gene-1 protects nigral dopaminergic neurons in vivo. Cell Death Dis. 2018 May 1;9(5):449.
42. Wang CY, Qiu ZJ, Zhang P, Tang XQ. Differentiated Embryo-Chondrocyte Expressed Gene1 and Parkinson's Disease: New Insights and Therapeutic Perspectives. Curr Neuropharmacol. 2023;21(11):2251-65.
43. Thomas SD, Abdalla S, Eissa N, Akour A, Jha NK, Ojha S, et al. Targeting Microglia in Neuroinflammation: H3 Receptor Antagonists as a Novel Therapeutic Approach for Alzheimer's Disease, Parkinson's Disease, and Autism Spectrum Disorder. Pharmaceuticals (Basel). 2024 Jun 25;17(7):831.
44. Rauf A, Badoni H, Abu-Izneid T, Olatunde A, Rahman MM, Painuli S, et al. Neuroinflammatory Markers: Key Indicators in the Pathology of Neurodegenerative Diseases. Molecules. 2022 May 17;27(10):3194.
45. Wang Y, Ge X, Yu S, Cheng Q. Achyranthes bidentata polypeptide alleviates neurotoxicity of lipopolysaccharide-activated microglia via PI3K/Akt dependent NOX2/ROS pathway. Ann Transl Med. 2021 Oct;9(20):1522.