Abstract
After establishing the new standard of care of isotonic fluids for maintenance therapy, there has not been a systematic follow-up to determine whether the goal of the switch has been achieved or whether there have been any unexpected complications with the change. This is a brief review of the history of maintenance intravenous fluids and potential complications of isotonic fluid therapy spurred by a case. This article is not meant to provide evidence or practical help in clinical decision making, but to provoke a reconsideration of what, when, and how much intravenous fluids should be administered. We report a case of a 16-year-old adolescent female admitted for evaluation and management of acute gastroenteritis. We have informed consent from the patient’s family to publish this case history. In the hospital, she was treated with resuscitation fluids and a maintenance infusion with normal saline. She was diagnosed with terminal ileitis secondary to enteropathogenic E. coli/Salmonella enterocolitis. She developed new-onset hypertension within three days of admission, with an electrocardiogram demonstrating evidence of a left ventricular strain pattern. Based on the current guidelines, isotonic fluids are the preferred maintenance fluids; as such, there has been widespread use of normal saline for maintenance. Utilization of normal saline may help prevent hyponatremia but may be at the expense of new complications such as hypernatremia, hypertension, and/or hypokalemia.
Keywords
Intravenous fluids, Hypotonic, Isotonic, Hyponatremia
Abbreviations
E Coli: Escherichia Coli; NS: Normal Saline; KCl: Potassium Chloride; Cells/cu mm: Cells per cubic millimeter; Mmol/L: Mill moles per Liter; MEq/L: Mill Equivalents per Liter; Pg/ml: Pico grams per ml; Kg: Kilogram; IV/IVF: Intravenous/Intravenous Fluid; AAORCA: Anomalous Aortic Origin of the Right Coronary Artery; EKG: Electrocardiogram; CT: Computerized Tomography; SIADH: Syndrome of Inappropriate secretion of Antidiuretic Hormone
Case Report
A 16-year-old previously healthy female was admitted with flu-like symptoms. She had nausea and emesis for two days, followed by fatigue, watery diarrhea, fever, and abdominal pain. She developed syncope, dyspnea, and myalgia and was taken to the hospital. In the emergency room, physical examination was positive for fever, mild tachycardia, blood pressure of 126/75 mm Hg, and right lower quadrant tenderness. Diagnostic tests were notable for an elevated C-reactive protein level of 117 mg/l, a white blood cell count of 3,300 cells/cu mm, hemoglobin of 8.6 g/dl, and a platelet count of 149,000/microliter. Multiplex assay was negative for flu, COVID-19, and respiratory syncytial virus. The urinalysis was normal, and the urine pregnancy test was negative. The ultrasound did not visualize the appendix, a CT of the abdomen without contrast was negative for appendicitis but demonstrated thickening of the terminal ileum with surrounding lymphadenopathy. A pelvic ultrasound was negative for ovarian torsion. Due to the fever, diarrhea, and abdominal pain in the setting of the elevated inflammatory marker, the patient was admitted for further evaluation of abdominal pain and for hydration.
Hospital Course
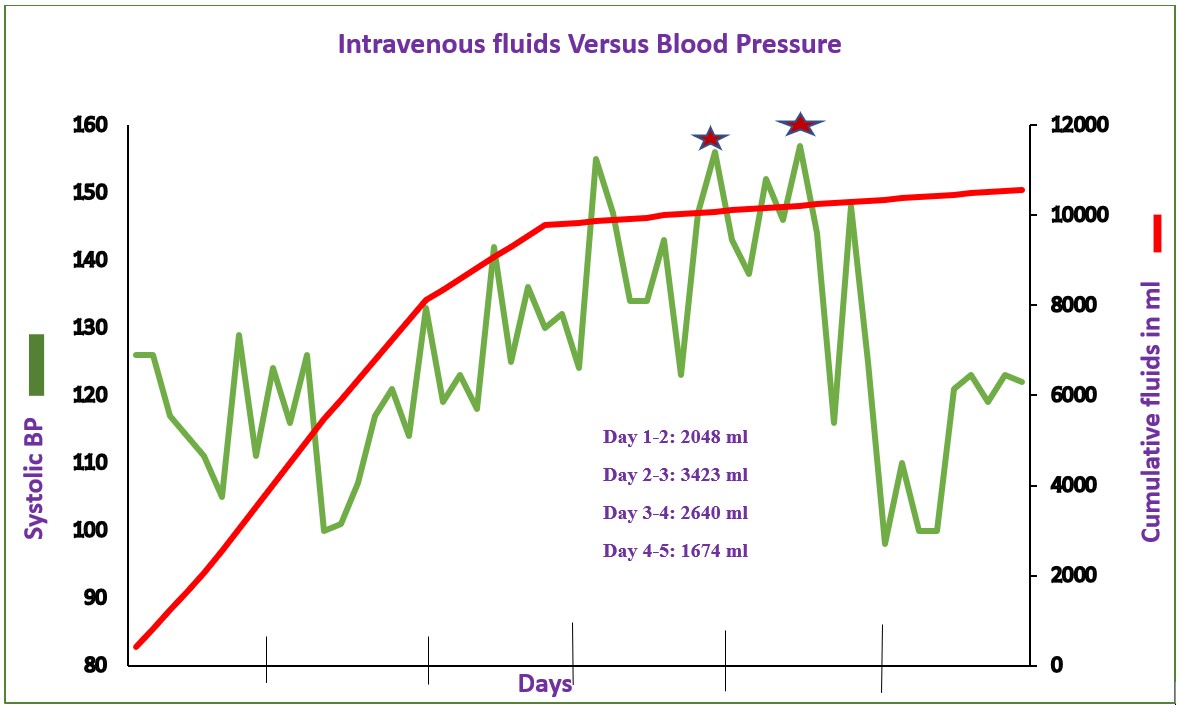
The patient weighed 70 kg and received normal saline boluses followed by maintenance infusion with D5 0.9% normal saline as well as D5 0.9% normal saline with 20 mEq of KCl per liter, totaling approximately 2 liters in the first 24 hours, 3.5 liters in the next 24 hours and 2.6 liters on the third day. The fluids were tapered to less than 1 liter. An elevation in blood pressure in the range of 144/99 to 159/107 mm of Hg occurred within 48 hours of admission. Cumulative intravenous venous fluids and the blood pressure measurements during the hospital stay are shown in Figure 1. The total fluid depicted includes both bolus and maintenance fluids. On day 2 of admission, she complained of cramping chest pain associated with left arm and abdominal pain. In addition to being given acetaminophen an electrocardiogram was done and revealed sinus rhythm, borderline T wave abnormalities in anterior leads, and a borderline prolonged QT interval. An echocardiogram two days later demonstrated an anomalous aortic origin of the right coronary artery (AAORCA) from left coronary sinus and left ventricular mass (M-Mode) Index of 59.7 g/m (2.7). A further evaluation of the hypertension included a renal ultrasound with Doppler, urine studies, serum catecholamines, TSH, T4, renin, aldosterone, lupus panel, cortisol, lipids, comprehensive metabolic panel and electrolytes and were all normal except for mild hypernatremia of 146 mEq/liter on day 1 of admission, persistent mild hyperchloremia and elevated BNP of 353 pg/ml on day 4 of admission. Isradipine 2.5 mg as needed for systolic blood pressure greater than 130 mm Hg was initiated. The hypertension resolved and isradipine was discontinued after fluids were tapered and stopped. She was noted to have Salmonella and enteropathogenic E. coli enterocolitis, which were conservatively managed.
A CT angiogram of the coronary artery was consistent with an anomalous right coronary artery with an intramural course. She subsequently underwent successful surgical repair at a later visit.
This patient had transient hypertension, which required diagnostic studies, and prolonged hospital stay secondary to maintenance fluids that led to the serendipitous discovery of an underlying congenital heart disease. This new onset hypertension, associated with intravenous fluids, completely subsided after fluids were discontinued as seen in Figure 1.
Figure 1. Systolic blood pressure increases during the hospital stay. Shown is the daily accumulated volume of IVF administered along with the days corresponding blood pressures. *Administration of 2.5 mg of Isradipine.
Discussion
Children with AAORCA are often asymptomatic. Adolescents typically present with chest pain and ischemic changes after exercise, but unfortunately, 50% of them present with cardiac arrest; AAORCA is the second most common cause of sudden cardiac death during sporting activities [1]. According to Binka et al. ischemia may be induced by exercise in a subject with myocardium supplied by the right coronary artery that can be caused by “slit-like shape of the coronary origin, intramural course, length of intramural segment or due to interatrial course between aorta and pulmonary artery” [2]. We hypothesize that this patient had chest pain and ischemic changes in her electrocardiogram due to volume overload from the intravenous fluids resulting in increased myocardial work. Intravenous fluid therapy has a long history, starting from the early 19th century. Fluid therapy can be classified as: resuscitation, maintenance, and replacement. The resuscitation phase indications are altered mental status, persistent vomiting, shock, severe dehydration, acute abdomen, bloody diarrhea, respiratory distress, and dyselectrolytemia. Replacement of the deficit should consider urinary losses, sweat water and electrolyte losses, and insensible water losses. The composition and volume of maintenance fluids should provide water and electrolytes to maintain adequate tissue perfusion without causing complications such as volume overload, depletion, and dyselectrolytemia [3]. Malcolm Holliday and William Segar published the original study on maintenance intravenous fluids [4]. They derived the formula for providing total maintenance fluid in 24 hours of 100 ml/kg for infants from 3.5 kg to 10 kg, 1000 ml + 50 ml/kg from 11-20 kg for every kg over 10, and 1500 ml + 20 ml/kg for every kg over 20 kg up to a maximum of 2400 ml over the entire pediatric age range excluding neonates, known as the 100/50/20 rule. The sodium, chloride, and potassium concentrations were recommended to be 3, 2, and 2 mEq/100 kcal/day, respectively. These were calculated based on energy expenditure, weight, body surface area, and urine solutes, along with recommendations of Darrow, Wallace, and Talbot [5]. Also, in 1957, the first cases of the syndrome of inappropriate antidiuretic hormone (SIADH) in 2 patients with bronchogenic carcinoma were described [6]. SIADH is a disorder that can lead to hyponatremia due to the non-physiologic secretion of ADH, leading to free water retention followed by a natriuresis that maintains fluid balance at the expense of serum osmolality. Despite this, since 1957, hypotonic maintenance intravenous fluids were the standard of care.
Adverse events associated with intravenous fluid therapy were recognized and since 1992, there have been several studies demonstrating a high rate of hyponatremia associated with hypotonic maintenance fluids (Table 1). The prospective and retrospective analysis by Arieff et al. [7], the meta-analysis by Choong et al. [8] and [Foster et al. [9], and randomized trials by Neville et al. [10], McNab et al. [11], and Moghtaderi et al. [12] demonstrated that hypotonic fluids are associated with hyponatremia.
|
Year |
Name/Publication |
Type of Study |
Findings |
Recommendations |
Limitation |
|
1992 |
Arieff et al. [7] |
Prospective of 16 previously healthy children with symptomatic hyponatremia from multiple hospitals over 6 years and a retrospective analysis of 24,412 surgical cases from single tertiary hospital over 3 years |
15/16 died to postoperative hyponatremia. 83 hyponatremia and 7 deaths in the retrospective study |
Avoid intravenous hypotonic fluids |
Patients received hypotonic fluids not only for maintenance but also for deficit correction and replacement of losses |
|
2003 |
Mortiz et al., [32] |
Review article |
|
Holliday & Segar guidelines apply only for healthy children; others need isotonic fluids |
Review article |
|
2006 |
Choong et al. [8] |
Meta-Analysis from Medline (1966–2006), Embase, the Cochrane Library. Six studies that compared hypotonic to isotonic maintenance solutions in children |
Hypotonic fluids increase the risk of hyponatremia with an odds ratio of 17 |
Hypotonic solutions have potential harm, and isotonic or near-isotonic are physiologic. |
Most studies were heterogeneous in design, small, of variable quality, did not allow for confounding factors, and focused on a limited pediatric population. Authors commented practitioners used hypotonic fluids for deficit replacement, perioperative fluids & ongoing losses |
|
2007 |
Holliday et al. [30] |
Review article |
|
Harm associated with 0.9% saline compared with balanced electrolyte solutions such as Lactated Ringer |
Review article |
|
2010 |
Neville et al. [10] |
Prospective ADH levels, plasma and urinary chemistry in children undergoing surgery randomized to 1 of 4 fluid regimens: 0.9% or 0.45% saline solution at either 100% or 50% maintenance fluid rate. Randomized, Nonblinded, at Sydney Children's Hospital between August 2005 and December 2007 |
Plasma sodium concentrations fell in both hypotonic groups at 8th hour with hyponatremia more common than in the NS groups |
Concluded that fluid type and not rate caused hyponatremia, thus isotonic saline decreased the risk of hyponatremia |
Only 124 children in the study |
|
2014 |
Foster et al. [9] |
Systematic review & Meta-analysis from MEDLINE, the Cochrane Central Registry, Cumulative Index for Nursing and Allied Health Literature, and Pediatric Academic Societies abstracts using a predefined protocol. |
Hypotonic maintenance fluids increase the risk of hyponatremia compared to isotonic fluids. |
Relative risk of hyponatremia 2.37 with hypotonic fluids, hypernatremia 0.81 with hypotonic fluids. |
They studied the risk of hypernatremia among those who received hypotonic fluids and not isotonic fluids. Commented that the included studies were not powered to detect difference in incidences of hypernatremia, neurologic sequel or hypertension |
|
2015 |
McNab et al. [11] |
A Randomized double blinded controlled trial on 690 patients at the Royal Children’s Hospital Australia, randomly assigned (1:1) to receive either isotonic fluid containing Na140 or hypotonic with Na77 for 72 h |
Isotonic fluid with 140 mmol/L had a lower risk of developing hyponatremia than hypotonic fluids |
Isotonic fluid should be used as intravenous fluid for maintenance hydration in children. |
8 patients in the Na140 group and 4 in the Na77 group developed serious adverse events, 2 patients receiving Na140 had episodes in which over hydration contributed to clinical deterioration, studies on balanced solutions and not isotonic saline |
|
2015 |
Moritz et al. [3] |
Review article |
Hypotonic maintenance fluids are associated with the development of hospital-acquired hyponatremia as well as deaths. |
Isotonic saline for maintenance therapy, volume adjustment in oligoanuric and edematous states |
Review article |
|
2016 |
Moghtaderi et al. [12] |
Prospective Randomized 190 children after surgery. Randomly divided: One group received 50 mEq/L sodium and 20 mEq/L potassium in D/W 5% and second group received 154 mEq/l sodium and 20 mEq/L potassium in D/W 5% at the maintenance dose for a period of 6 hours following the surgery. |
The incidence of hyponatremia before and after maintenance therapy was 9.5% and 36%, respectively. After the therapy, the incidence of hyponatremia was 54% and 17% in hypotonic and isotonic groups, respectively |
Isotonic maintenance infusions after surgery reduce incidence of hyponatremia. |
Study compared hypotonic fluids to normal saline, with hypotonic at 50 mEq/l versus isotonic at 154 mEq/l that contain both sodium and potassium. Besides, there is limited power on 190 patients. |
|
2018 |
AAP [13] |
Review article |
28 days to 18 years of age requiring maintenance IVFs should receive isotonic fluids |
Review article, |
|
|
2021 |
Lehtiranta et al. [16] |
Unblinded randomized trial on 614 acutely ill children, either sodium 140 or potassium 5 in 5% dextrose or sodium 80 and potassium 20 in 5% dextrose. |
Clinically significant dyselectrolytemia more common in children receiving plasma like isotonic fluid therapy |
Isotonic fluid may be unsuitable for fluid therapy in acutely ill children unless extra potassium is added |
|
As a result of the growing recognition of complications associated with hypotonic fluids, in 2015, isotonic fluids were recommended for maintenance therapy in a landmark article [3] and by the American Academy of Pediatrics in 2018 [13]. Since the new recommendations, no studies have been performed to evaluate if there was a change in practice towards utilizing isotonic fluid therapy or if the issue of hyponatremia associated with maintenance fluids resolved.
There was a recent national QI project [14] done in collaboration with AAP, where interventions led to a 5% increase in the exclusive isotonic fluid use. It concluded that the intervention bundle significantly improved the use of isotonic maintenance IVF without a concomitant increase in adverse events or electrolyte testing. The study did not report adverse events such as hypertension or electrolyte imbalance after isotonic fluid use. As nephrologists, we do recommend only isotonic fluids for maintenance therapy, but we prefer balanced salt solutions such as Ringer lactate or Plasmalyte for maintenance therapy rather than normal saline infusion. As pointed out in multiple studies in pediatric critical care medicine [15], hyperchloremia and metabolic acidosis which can ultimately lead to organ dysfunction unless recognized and treated is a consequence of maintenance saline infusion and not from balanced salt solutions. While normal saline contains 154 mEq/L each of sodium and chloride, Ringer’s lactate contains 130 mEq/L of sodium and 109 mEq/L of chloride and Plasmalyte contains 140 mEq/L sodium and 98 mEq/L chloride. Sodium content is only 90 mEq/L and the chloride strength is 80 mEq/L in Oral Rehydration solution. A sodium concentration of 154 mmol/L in 0.9% solution is actually supra-physiological and also contains supra-physiological amounts of chloride.
Guyton and Coleman [16] proposed a mechanism of pressure natriuresis that enhances sodium and water excretion when arterial pressure is elevated, to return blood volume to normal and reduce the blood pressure, suggesting a dominant role for the kidneys in hypertension. But recent studies have demonstrated that endothelial dysfunction also contributes to hypertension from excess salt through interstitial non-osmotic sodium storage [17], besides, the blood pressure increases occurring through an increased arterial wall tension through shear stress [18]. Salt loading has been shown to impair vascular endothelial function, increase microvascular dysfunction, and impair left ventricular mechanical relaxation even in young healthy normotensives [19].
Due to different population’s genetic differences, there are a range of responses to salt loading, with various effects on the vessel wall and the ability to excrete the excess load. Despite these differences, a modest reduction in a population salt consumption over a 4-week period can result in a significant reduction in blood pressure and could lower the incidence of strokes, heart attacks and heart failure. Furthermore, the lower the salt intake, the lower was the blood pressure based on a meta-analysis [20]. While dietary sodium has not been shown to directly affect the endothelial layer, rapid microvascular rarefaction causing reduced oxygen delivery to the tissues and elevated total peripheral resistance have been demonstrated secondary to salt intake in rats [22]. This causes reduced arterial vasodilator capacity [23] and potentiation of local vasoconstrictive effectors [24]. In humans, intravenous sodium loading has been shown to disturb the endothelial surface even in young normotensives, increasing microvascular permeability to albumin independent of blood pressure [21]. Thus, even a modest decrease in sodium concentration of the isotonic solutions may reduce harm in the hypertensive pediatric population.
Perhaps these effects on the vessel wall explain the new onset of chest pain, ischemic changes, and ECG changes in our patient after normal saline. Our patient did not have any evidence of renal dysfunction, but her ability to excrete sodium may have been diminished by her bacterial gastroenteritis causing dehydration. Up-regulation of the sympathetic nervous system and renin angiotensin axis from dehydration may have caused a cycle of vasoconstriction, inflammation, vascular damage, and ischemia and thus intensifying the hypertension [25].
Another dilemma with isotonic fluid therapy is illustrated in Table 2. The amount of sodium in isotonic saline is approximately three-times the recommended daily allowance of dietary sodium. A randomized clinical trial from 2020 also demonstrated that isotonic fluids are associated with dyselectrolytemia [27]. The higher chloride in saline solutions can cause hyperchloremic metabolic acidosis in addition to vasoconstriction of the afferent arterioles in the kidneys reducing glomerular filtration rate. This can lead to fluid overload, coagulopathy, and hypertension [28]. In a study of Pizzaro et al. [29], oral rehydration therapy with 80 mEq/L of sodium chloride did not cause hyponatremia, while effectively correcting the dehydration [30].
|
Weight |
Daily volume |
Dietary sodium |
Half saline |
Saline |
|
5 kg |
600 ml |
4 mEq * |
46 mEq |
92 mEq |
|
10 kg |
1000 ml |
52 mEq ** |
77 mEq |
154 mEq |
|
20 kg |
1500 ml |
65 mEq ** |
115 mEq |
231 mEq |
|
50 kg |
2100 ml |
100 mEq ** |
162 mEq |
323 mEq |
|
(dietaryguidelines.gov) [26] * Breast milk content ** Recommended Daily Allowance |
||||
Given that the recommended intravenous sodium content is greater than the RDA, greater than sodium content of oral rehydration therapy, and greater than the normal plasma sodium, it may be time to reconsider the sodium content of maintenance intravenous fluids especially if complications arise from its use. In the pediatric age group, total body fluid is directly correlated with body weight. Therefore, monitoring fluid status for complications should include daily intake/output, daily weight, and blood pressure, as in this index patient during parenteral therapy. In our patient, the combination of fluid volume and the fluid shifts resulting from the hypertonicity of the intravenous fluids led to transient hypertension. While there is general agreement with Holliday and Segar’s formula for maintenance fluid volume, there is a need for rationale guidelines regarding the type of isotonic fluids indicated for maintenance therapy in children.
In this era of precision medicine, in the future, a genomic approach may be utilized to determine the most accurate IV fluid therapy for each child based on genotype rather than volume status alone. We may have a personalized drug treatment based on genotype, proteome and metabolome [31]. The patient’s response to each fluid type can be predicted and thus the provider can generate a tailored intravenous fluid plan. But for the present time, it is ideal to have isotonic fluids that match human physiology especially in the pediatric population.
Conclusion
Studies done before and after the new guidelines recommended in 2015 were underpowered to detect complications following parenteral fluid therapy in the real world. Presently there are ongoing studies and research into long term effects of various IV fluid management strategies. But these are not enough to make specific alternative suggestions. This is a limitation of our review and further studies may be required to conclusively provide alternative recommendations. But it may be time to re-evaluate guidelines on the type of isotonic fluids for maintenance therapy, in pediatric patients who are brought to the Emergency Department or admitted to hospital.
References
2. Binka E, Zhao N, Wood S, Zimmerman SL, Thompson WR. Exercise-Induced Abnormalities of Regional Myocardial Deformation in Anomalous Aortic Origin of the Right Coronary Artery. World J Pediatr Congenit Heart Surg. 2020 Nov;11(6):712-9.
3. Moritz ML, Ayus JC. Maintenance Intravenous Fluids in Acutely Ill Patients. N Engl J Med. 2015 Oct;373(14):1350-60.
4. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957 May;19(5):823-32.
5. DARROW DC, PRATT EL. Fluid therapy; relation to tissue composition and the expenditure of water and electrolyte. J Am Med Assoc. 1950 May 27;143(4):365-73.
6. SCHWARTZ WB, BENNETT W, CURELOP S, BARTTER FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am J Med. 1957 Oct;23(4):529-42.
7. Arieff AI, Ayus JC, Fraser CL. Hyponatraemia and death or permanent brain damage in healthy children. BMJ. 1992 May 9;304(6836):1218-22.
8. Choong K, Kho ME, Menon K, Bohn D. Hypotonic versus isotonic saline in hospitalised children: a systematic review. Arch Dis Child. 2006 Oct;91(10):828-35.
9. Foster BA, Tom D, Hill V. Hypotonic versus isotonic fluids in hospitalized children: a systematic review and meta-analysis. J Pediatr. 2014 Jul;165(1):163-9.e2.
10. Neville KA, Sandeman DJ, Rubinstein A, Henry GM, McGlynn M, Walker JL. Prevention of hyponatremia during maintenance intravenous fluid administration: a prospective randomized study of fluid type versus fluid rate. J Pediatr. 2010 Feb;156(2):313-9.e1-2.
11. McNab S, Duke T, South M, Babl FE, Lee KJ, Arnup SJ, et al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. Lancet. 2015 Mar 28;385(9974):1190-7.
12. Moghtaderi M, Kajbafzadeh A, Arshadi H, Gorji M, AleEsmail A, Mahboobi A, et al. Comparison of the Incidence of Postoperative Hyponatremia after Infusion of Hypotonic Versus Isotonic Intravenous Solutions in Children. J Ped Nephrol. 2016 Dec 22;4(3):92-6.
13. Feld LG, Neuspiel DR, Foster BA, Leu MG, Garber MD, Austin K, et al. Clinical Practice Guideline: Maintenance Intravenous Fluids in Children. Pediatrics. 2018 Dec;142(6):e20183083.
14. Rooholamini SN, Jennings B, Zhou C, Kaiser SV, Garber MD, Tchou MJ, et al. Effect of a Quality Improvement Bundle to Standardize the Use of Intravenous Fluids for Hospitalized Pediatric Patients: A Stepped-Wedge, Cluster Randomized Clinical Trial. JAMA Pediatr. 2022 Jan 1;176(1):26-33.
15. Bulfon AF, Alomani HL, Anton N, Comrie BT, Rochwerg B, Stef SA, et al. Intravenous Fluid Prescription Practices in Critically Ill Children: A Shift in Focus from Natremia to Chloremia? J Pediatr Intensive Care. 2019 Dec;8(4):218-25.
16. Hall JE, Guyton AC, Coleman TG, Mizelle HL, Woods LL. Regulation of arterial pressure: role of pressure natriuresis and diuresis. Fed Proc. 1986 Dec;45(13):2897-903.
17. Olde Engberink RHG, Selvarajah V, Vogt L. Clinical impact of tissue sodium storage. Pediatr Nephrol. 2020 Aug;35(8):1373-80.
18. Marketou ME, Maragkoudakis S, Anastasiou I, Nakou H, Plataki M, Vardas PE, Parthenakis FI. Salt-induced effects on microvascular function: A critical factor in hypertension mediated organ damage. J Clin Hypertens (Greenwich). 2019 Jun;21(6):749-57.
19. Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005 Oct;46(4):725-31.
20. He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013 Apr 30;(4):CD004937.
21. Rorije NMG, Olde Engberink RHG, Chahid Y, van Vlies N, van Straalen JP, van den Born BH, et al. Microvascular Permeability after an Acute and Chronic Salt Load in Healthy Subjects: A Randomized Open-label Crossover Intervention Study. Anesthesiology. 2018 Feb;128(2):352-60.
22. Hansen-Smith FM, Morris LW, Greene AS, Lombard JH. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ Res. 1996 Aug;79(2):324-30.
23. Frisbee JC, Lombard JH. Development and reversibility of altered skeletal muscle arteriolar structure and reactivity with high salt diet and reduced renal mass hypertension. Microcirculation. 1999 Sep;6(3):215-25.
24. Lukaszewicz KM, Falck JR, Manthati VL, Lombard JH. Introgression of Brown Norway CYP4A genes on to the Dahl salt-sensitive background restores vascular function in SS-5(BN) consomic rats. Clin Sci (Lond). 2013 Mar;124(5):333-42.
25. Satou R, Penrose H, Navar LG. Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr Hypertens Rep. 2018 Oct 5;20(12):100.
26. https://www.dietaryguidelines.gov/
27. Lehtiranta S, Honkila M, Kallio M, Paalanne N, Peltoniemi O, Pokka T, et al. Risk of Electrolyte Disorders in Acutely Ill Children Receiving Commercially Available Plasmalike Isotonic Fluids: A Randomized Clinical Trial. JAMA Pediatr. 2021 Jan 1;175(1):28-35.
28. Hayes W. Ab-normal saline in abnormal kidney function: risks and alternatives. Pediatr Nephrol. 2019 Jul;34(7):1191-9.
29. Pizarro D, Posada G, Villavicencio N, Mohs E, Levine MM. Oral rehydration in hypernatremic and hyponatremic diarrheal dehydration. Am J Dis Child. 1983 Aug;137(8):730-4.
30. Holliday MA, Ray PE, Friedman AL. Fluid therapy for children: facts, fashions and questions. Arch Dis Child. 2007 Jun;92(6):546-50.
31. Su J, Yang L, Sun Z, Zhan X. Personalized Drug Therapy: Innovative Concept Guided With Proteoformics. Mol Cell Proteomics. 2024 Mar;23(3):100737.
32. Moritz ML, Ayus JC. Prevention of Hospital-Acquired Hyponatremia: A Case for Using Isotonic Saline. Pediatrics. February 2003;111(2):227-30.